Abstract
An effective disease-control strategy should protect the host from the major economically important and geographically widespread variants of a pathogen. Plum pox virus (PPV) is the causal agent of sharka, the most devastating viral disease of Prunus species. We have shown previously that the hairpin RNA expression driven by h-UTR/P1, h-P1/HCPro, h-HCPro and h-HCPro/P3 constructs, derived from the PPV-M ISPaVe44 isolate, confers resistance to the homologous virus in Nicotiana benthamiana plants. Since the production of transgenic stone fruits and their evaluation for PPV resistance would take several years, the ISPaVe44-resistant plant lines were used to evaluate which construct would be the best candidate to be transferred to Prunus elite cultivars. To do that, nine PPV isolates of the D, M, Rec, EA and C strains originally collected from five Prunus species in different geographical areas, were typed by sequencing and used to challenge the transgenic N. benthamiana lines; 464 out of 464 virus-inoculated plants of lines h-UTR/P1, h-HCPro and h-HCPro/P3 showed complete and long-lasting resistance to the seven PPV isolates of D, M and Rec strains. Moreover, the h-UTR/P1 plants were also fully resistant to PPV-C and -EA isolates. Our data suggest that the h-UTR/P1 construct is of particular practical interest to obtain stone fruit plants resistant to the sharka disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sharka is the most detrimental viral disease of stone fruits causing important economic losses mainly on plum, apricot and peach crops (Cambra et al. 2006; Barba et al. 2010). The aetiological agent of sharka is Plum pox virus (PPV), which is a member of the genus Potyvirus. PPV is transmitted by several species of aphids in a non-persistent manner as well as by infected propagative material. Although in many countries the virus has quarantine status and considerable efforts have been made to eradicate it or to contain its spreading, PPV is widespread in several of the most important Prunus-producing areas worldwide (García and Cambra 2007; Barba et al. 2010).
PPV isolates can differ in pathogenicity, host range and aphid transmissibility (Candresse and Cambra 2006; Barba et al. 2010). On the basis of serological and molecular properties PPV isolates have been classified into six strains: D, M, Rec, EA, C and W (Candresse et al. 1998; Nemchinov et al. 1998; Glasa et al. 2004; James and Varga 2005). D, M and Rec are the most widespread and economically important strains whereas EA, C and W are minor and highly divergent ones. In particular, PPV-EA is confined in Egypt, PPV-C is a cherry-adapted strain and reported mainly from Europe and PPV-W was declared eradicated, after its initial discovery at a private residence in Canada (NAPPO Phytosanitary Alert System, http://www.pestalert.org/). Recently, a new strain, named T, has been proposed to group together closely related viral isolates collected in a Turkish province (Serçe et al. 2009). PPV-Rec and PPV-T are characterised by recombination events between PPV-M and PPV-D genomes.
PPV genome is a single stranded positive sense RNA molecule of about 9.700 nucleotides in length that has the VPg protein covalently attached to its 5′-end. It contains a long open reading frame that is translated from the second AUG codon (nt 147–149) as a polyprotein which is cleaved into mature proteins by viral proteinases, two of which are P1 and HC-Pro (Lopez-Moya et al. 2000; García and Cambra 2007).
The actual strategies for mitigating sharka disease are based on the use of certified virus-tested plant materials, the eradication of diseased trees and the reduction of aphid populations by regular treatment with insecticides, although this last strategy is not effective to control non-persistent viruses. The development of resistant Prunus cultivars remains the best solution to control sharka. Unfortunately, very few sources of resistance have been identified in Prunus species. Moreover, the selection process is difficult and slow because of the undesirable characteristics carried by resistant plants and others constraints of fruit trees species such as long biological cycle with long juvenile period of seedlings and high degree of heterozygosis (Kegler et al. 1998; Decroocq et al. 2005; Ion-Nagy et al. 2006; Karayiannis et al. 2008).
A promising alternative to obtain PPV resistant plants is to transform virus-susceptible elite cultivars with the appropriate PPV resistance gene. Because transformation, regeneration and resistant tests of fruit trees are demanding and time consuming (Petri and Burgos 2005), the ability of a particular transgene in conferring resistance has been evaluated in herbaceous model plants (Ravelonandro et al. 1993; Guo and Garcia 1997; Wittner et al. 1998; Jacquet et al. 1998; Guo et al. 1998; Pandolfini et al. 2003; Barajas et al. 2004; Di Nicola-Negri et al. 2005; Hily et al. 2007; Liu et al. 2007). From the 1990s up to the discovery of RNA silencing phenomenon, and its potential use as a tool to obtain virus-resistant plants (Smith et al. 2000), the leading idea to induce PPV resistance was to express in plants PPV-derived proteins (Lopez-Moya et al. 2000). However, only a minor fraction of plants transformed to express PPV-derived proteins showed resistance. Retrospectively, it is now possible to explain most of those resistances as result of the unwanted and unpredictable activation of the degradative RNA silencing pathway (Guo and Garcia 1997; Guo et al. 1998; Jacquet et al. 1998; Guo et al. 1999; Scorza et al. 2001; Barajas et al. 2004; Hily et al. 2005). The principal limitation of the previous PPV-resistance strategies being the impossibility to transfer, in a predictable way, the resistance trait to the same or different species using the same molecular construct.
RNA silencing is a sequence-specific RNA degradation process induced by double stranded RNA (dsRNA). The dsRNA is diced into small interfering RNAs (siRNAs) of about 21–26 nt. siRNAs act as a guide to recognise complementary RNAs for their degradation. (Waterhouse et al. 2001; Ruiz-Ferrer and Voinnet 2009). In plants RNA silencing, among other functions, has evolved as an antiviral defence mechanism (Waterhouse et al. 2001; Voinnet 2005; Ruiz-Ferrer and Voinnet 2009).
In order to develop reliable tools for inducing PPV resistance, that are not highly dependent on a particular event of transformation, distinct laboratories have expressed in plants PPV-derived dsRNA sequences (Pandolfini et al. 2003; Di Nicola-Negri et al. 2005; Hily et al. 2007; Liu et al. 2007). In particular, the hairpin constructs were developed using isolates of M (Di Nicola-Negri et al. 2005) or D strains (Pandolfini et al. 2003; Hily et al. 2007; Liu et al. 2007). In this context, we have shown previously that ectopic expression of self-complementary hairpin RNAs (h-UTR/P1, h-P1/HCPro, h-HCPro and h-HCPro/P3) derived from PPV ISPaVe44, an Italian PPV-M isolate that induces severe symptoms on peach, is able to confer efficient and predictable resistance to ISPaVe44 (Di Nicola-Negri et al. 2005). However, as the RNA silencing operates in a sequence-specific fashion, the real capability of a particular dsRNA construct to control sharka disease will be directly proportional to the breadth of PPV resistance spectrum that it is able to confer. Until now the available data on PPV resistance spectrum conferred by the dsRNA expressing constructs are scarce. In fact, transgenic plants were only challenged with either the same viral isolate (Di Nicola-Negri et al. 2005) or with an isolate of the same PPV strain utilised to build the hairpin construct (Pandolfini et al. 2003; Hily et al. 2007; Liu et al. 2007).
In order to select the best hairpin construct candidate for conferring sharka resistance in Prunus spp. we selected and molecularly typed nine different PPV isolates originally collected from five stone fruit species and used them to challenge transgenic N. benthamiana h-UTR/P1, h-P1/HCPro, h-HCPro and h-HCPro/P3 plants. In this paper we show that the h-UTR/P1 construct is able to confer complete resistance to a broad range of PPV strains. The broader PPV resistance spectrum conferred by h-UTR/P1 is discussed in the light of the potential capability of different hairpin constructs to produce effective siRNAs homologous to isolates of PPV-EA and -C strains.
Materials and methods
Plant material and viruses
Transgenic N. benthamiana h-UTR/P1 line 6, h-P1/HCPro lines 72, 79 and 87, h-HCPro line 101 and h-HCPro/P3 lines 162 and 163 were previously described (Di Nicola-Negri et al. 2005). Except for lines 79 and 87 all others are single locus transgenic lines (Di Nicola-Negri et al. 2005; Ilardi et al. 2007). The four PPV sequences used to build up the hairpin constructs derive from the ISPaVe44 isolate (accession #AY917135). UTR/P1 sequence starts at the 5′ end of PPV genome and includes a part of the P1 gene (nt 1–733), P1/HCPro covers the 3′ end of P1 gene and partially covers the HC-Pro gene (nt 954–1,603), HCPro covers the central portion of HC-Pro (nt 1,680–2,386) and the HCPro/P3 covers the 3′ end of HC-Pro gene and a part of P3 gene (nt 1,935–2,613; Di Nicola-Negri et al. 2005).
PPV ISPaVe11, ISPaVe17, ISPaVe18, ISPaVe39, ISPaVe40 and ISPaVe46 were from CRA-PAV collection (www.cra-pav.it) while, PPV Pa1, BR and SwC isolates were kindly provided by L. Palkovics, A. Bazzoni and D. Boscia, respectively. Potato virus Y NTN (PVYNTN) isolate Pa21 was kindly provided by M. Turina.
CRA-PAV PPV isolates were maintained under greenhouse conditions in the woody peach host GF305. PPV Pa1, BR, SwC and PVY Pa21 were inoculated on the herbaceous host N. benthamiana and virus-infected leaves were collected, lyophilised and used as pre-inoculum source.
Virus resistance tests
Seeds from the T0 N. benthamiana lines were germinated and the T1 transgenic plants were identified by PCR using primers (35SDAG5′:5′TTCGCAAGACCCTTCCTCTA3′ and 35SDAG3′: 5′CGTACCTGAGTCCACTTCGT3′) spanning from the 35S promoter to the DAG1 intron (accession # NC_003074) separating the inverted repeats. For each construct a specific fragment was amplified: h-UTR/P1, 849 nt; h-P1/HCPro, 761 nt; h-HCPro, 823 nt; and h-HCPro/P3, 813 nt.
Resistance tests were performed on transgenic, non-transgenic segregant and untransformed N. benthamiana plants using PPV infected N. benthamiana fresh leaves as inoculum source. Plants, at the 5–7 true-leaf stage, were mechanically inoculated on all leaves with 1:10 w/v of PPV infected leaves ground in 0.1 M phosphate buffer pH 7.2.
PPV infection was evaluated by visual symptom observations on both inoculated and upper non-inoculated leaves between 11 and 17 days post inoculation (dpi) and also by Double Antibody Sandwich Indirect ELISA (DASI-ELISA) (Agritest, Bari, Italy). Successively DASI-ELISA was repeated on the upper new emerging leaves at about 28, 56 and 72 dpi. Immunocapture RT–PCR (IC-RT–PCR) of DASI-ELISA negative plants was performed using the polyclonal anti-PPV-antiserum aISPaVe21 at 1:500 dilution (kindly provided by G. Pasquini) and the PPV coat protein gene specific primers P1–P2 (Wetzel et al. 1991) and/or 5′PPV-291 (5′TGGTATGAAGGAGTTAACG3′) and 3′PPV-291 (5′TTGCGCTGAATTCCATACCT3′). The 5′PPV-291 and 3′PPV-291 primers were designed in this investigation to allow the amplification of a PPV coat protein gene fragment of 291 nucleotides in length.
Viral cDNA synthesis, PCR amplification and sequencing
Total RNAs were extracted from PPV- and PVY-infected N. benthamiana plants as previously described (Ilardi et al. 1995). Primers were designed (see supplementary material) to generate overlapping cDNA fragments spanning from the 5′ untranslated genomic region to the first third of P3 gene (about 2,613 nt). Oligonucleotide primers sequences were based on the online available PPV and PVY sequences (http://www.ncbi.nlm.nih.gov). When necessary, strain-specific primers were designed. The first-strand cDNAs were synthesised using Superscript II or III (Invitrogen) and PCR amplifications were performed with the proofreading Pfu DNA polymerase (Stratagene) as recommended by the suppliers. PCR products were purified by “GFX PCR DNA and Gel Band Purification Kit” (GE Healthcare) and then sequenced (Genechron–Ylichron, Rome, Italy) using the respective PCR primers. Nucleotide sequences accession numbers of viral isolates used in the resistance tests are reported in Table 1.
Nucleotide sequence and phylogenetic analyses
Nucleotide sequence analysis and comparisons were performed using EMBOSS align and ClustalW programmes (http://www.ebi.ac.uk). Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 4 (Tamura et al. 2007). The nucleotide sequences used were those obtained in this paper (Table 1) and those of fully sequenced PPV isolates available in GenBank. To avoid phylogenetic tree overloading only 10 out of 21 closely related sequences of North American PPV-D isolates available in GenBank were used. PPV-D isolates Dideron (X16415), NATa (NC_001445), NATb (D13751), 48922 (AY912058), Cdn3 (AY953262), Vulcani (AY912057), Cdn1 (AY953261), PENN3 (DQ465242), PENN4 (DQ465243), PENN6 (EF640934), PENN2 (AF401296), PENN1 (AF401295) and PENN9 (EF640937); PPV-M isolates PS (AJ243957), SK 68 (M92280) and GR0019 (FM955843); PPV-Rec isolates BOR-3 (AY028309) and J4c (EU117116); PPV-T isolate Ab-TK (EU734794); PPV-EA isolates (AM157175) and (DQ431465); PPV-C isolates SwC (Y09851) and SoC (AY184478).
In silico prediction of potentially effective ISPaVe44 siRNAs
In silico prediction of potentially effective ISPaVe44 siRNAs was conducted with two siRNAs prediction tools, siRNA design (http://jura.wi.mit.edu/bioc/siRNAext/) and Block-iTTM RNAi designer (https://rnaidesigner.invitrogen.com/rnaiexpress/). The search was conducted using 5 siRNA pattern masks for each tool, two of which were in common. Block-iTTM RNAi designer masks: (1) AAN19TT (http://www.rockefeller.edu/labheads/tuschl/sirna.html); (2) NAN21; (3) NA(A/G)N17(C/T)N2; (4) NAN18(T/C)N2 (http://www.rockefeller.edu/labheads/tuschl/sirna.html); and (5) Block-ITTM proprietary pattern. siRNA design masks: (1) AAN19TT; (2) NAN21; (6) N2(C/G)N8(A/U/T)N8(A/U/T)N2 (Pei and Tuschl 2006); (7) N4AN6TN2HN5WN2 (Reynolds et al. 2004); and (8) N2SN17WN2 (Ui-Tei et al. 2004). Default parameters were used with each siRNA prediction tools except that, in siRNA design tool, the parameter “end your siRNAs with” was set to NN. In the case of the siRNA design tool only siRNAs with negative energy difference (difference of 5′ end sense energy and 5′ end antisense energy) were taken in consideration. The ISPaVe44 sequences used to predict the presence of effective siRNAs were those present in the four hairpin constructs that had at least 21 bases of identity in a continuous stretch with either PPV-EA ISPaVe39 or PPV-C SwC or PVYNTN Pa21. The ISPaVe44 sequences analysed were: (a) UTR/P1-27-SwC AAATTCTCTACCAACTTTACTGCAAGTCAAGATGTCAACCATTGTATTTGGCTCATTCACTTGCCACCTCGATGCAGCTATCCACCA; (b) UTR/P1-23-SwC AGTAACGTGCATCTACTGTGCCGAAGAGCGGCAAAAAGTCTCATAAATACATATGAGAGTGCAACAGCTAGTGCTTGGAAAGG; (c) UTR/P1-30-SwC TGGAAAGGCCTGGAGGAGAAGTTGCAACCCATGTTTGCTAAGCGAGAGTTTAGCAAAACAGTCACAAAGAGAAAAGGGCTTCGGTGCTTC; (d) P1/HC-22-SwC CGTGATAGATTTTCGACAGTAATTGAGCAGCATGAGAAAACAGTGATGGATAACTATCCACAGTTTTCACATGTTCTTGCTT; (e) UTR/P1-26-ISPaVe39 AATGCAGACAGATTGGCAAAGGCCTGGACCCGTCCAGAGAACCGCCAAGTCAGTAACGTGCATCTACTGTGCCGAAGAGCGGCAAA; (f) HC-23-ISPaVe39 and HC/P3-23-ISPaVe39 CCAACAAAGAATCACTTAGTTGTTGGTAACTCAGGTGATTCGAAGTATGTGGATTTGCCCACAGCAAAGGGAGGTGCAATGTT; (g) HC-25-PVYNTNPa21 and HC/P3-25-PVYNTNPa21 ATAATCAACTTGATACTAATGGCAATTTCGTGTGGGGACAGAGAGAATATCATGCCAAACGCTTCTTTAGGAATTATTTCGATGT.
The sequences in bold indicate stretch of continuous nucleotide identity between the hairpin construct and the respective viral genomic sequence.
Results and discussion
Molecular characterization of PPV isolates utilised in the resistance tests
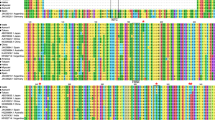
To have a good representation of the genetic variability present in PPV species, virus isolates originally collected from different geographical area and in distinct fruit tree hosts were selected for virus resistance tests (Table 1). PPV isolates collected from Prunus species considered resistant to sharka disease, like cherry and almond (Németh 1994; Rubio et al. 2003), were also included. The serotype of each isolate was confirmed by using strain-specific monoclonal antibody (Table 1). To unambiguously assign each viral isolate to the respective PPV strain (M, D, Rec, EA and C) and to know their phylogenetic relationship the nucleotide sequence of the first 2,613 nt of the PPV genome of ISPaVe17, ISPaVe46, Pa1, ISPaVe11, ISPaVe40, BR, ISPaVe39 and SwC isolates was determined. The sequenced region overlaps the four PPV ISPaVe44 fragments utilised in the construction of the hairpin constructs. Nucleotide sequence identity between ISPaVe44 and all other isolates ranged from 76.1 to 98.5% (Table 1). As expected, the lowest identities were found between ISPaVe44 and the ISPaVe39 (PPV-EA) and SwC (PPV-C) isolates. Figure 1 shows a phylogenetic tree reconstructed from thirty-two PPV 5′ genomic sequences. It shows that PPV isolates selected for the resistance test were correctly allocated within the respective strains and that indeed they well represent the genetic variability of PPV.
Phylogenetic tree of PPV isolates reconstructed using the genomic sequence spanning from nucleotide 27–2,613. The sequences used were either retrieved from the databases or determined in this work (Table 1); squares indicate PPV isolates used in the resistance tests; triangle indicates the ISPaVe44 utilised for the production of the four hairpin constructs; all the other PPV isolates are identified by their accession numbers. The evolutionary history was inferred using the neighbour-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches. Only bootstrap values >75% are shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. Bar 0.05 substitutions per site
The four hairpin constructs confer resistance to PPV isolates of M, D and Rec strains
We have previously shown that the h-UTR/P1, h-P1/HCPro, h-HCPro and h-HCPro/P3 transgenic T1 plants were fully resistant to the PPV-M ISPaVe44 isolate (Di Nicola-Negri et al. 2005). In the present study, to evaluate broadness, robustness and technological exploitability of the RNA silencing-mediated PPV resistance conferred by the four hairpin constructs, single locus T1 plants of lines 6 (h-UTR/P1), 72 (h-P1/HCPro), 101 (h-HCPro), 162 and 163 (h-HCPro/P3) were challenged with PPV-D isolates ISPaVe17, ISPaVe46 and Pa1, PPV-M isolates ISPaVe11 and ISPaVe40 and finally with two PPV-Rec isolates PPV-BR and ISPaVe18.
All PPV-inoculated wild-type (wt) and non-transgenic segregants showed typical systemic symptoms within 10 dpi and were positive in the serological assays. Conversely, none of the seven PPV isolates accumulated at detectable levels, in T1 transgenic plants of lines 6, 101, 162 and 163 at any of the time points analysed up to 72 dpi (Table 2). Moreover, all transgenic plants challenged with an inoculum containing both ISPaVe17 and ISPaVe18 remained PPV-free. For each pair of plant line/PPV isolate, at least one DASI-ELISA negative plant was further evaluated for PPV presence by IC-RT–PCR and back-inoculation on wt N. benthamiana plants. All tested plants were PPV-free according to the two additional assays (data not shown). In line 72 (h-P1/HCPro) PPV Pa1 accumulated in 2 out of 16 transgenic plants at 12 dpi. However, the two Pa1-infected plants recovered at 56 dpi (Table 2). Two additional h-P1/HCPro lines, the 79 and 87, were challenged with PPV Pa1. None of the eleven transgenic plants of line 79 accumulated detectable amount of virus, whereas one plant out of twelve of line 87 h-P1/HCPro was positive in DASI-ELISA. Altogether, of 616 transgenic plants challenged with PPV isolates of the M, D and Rec strains only 3 were infected, 2 of which showed a recovery phenotype. The three susceptible plants harboured the h-P1/HCPro construct. Nucleotide sequences comparison of the four hairpin constructs with the respective genomic regions of the PPV-M, -D and -Rec isolates used in the resistance tests revealed an overall nucleotide identity, for each pair analysed, higher than 94% with the presence of several long stretches of continuous nucleotide homology, the longest (206 nt long) being detected between PPV-BR and h-UTR/P1. The observed PPV resistance spectrum is in accord with previous data showing that for RNA silencing-mediated potyvirus resistance the threshold similarities between the transgene and the infecting virus was around 90% (Nomura et al. 2004; Missiou et al. 2004; Xu et al. 2009). However, our data differ from those of Guo et al. (1998) who reported that the protection was less effective against a PPV-M isolate than against the PPV-D isolate from which the transgene was derived. Similarly, the highly PPV-D resistant C5 plum clone was shown to permit, at some extent, replication and diffusion of a PPV-Rec isolate (Polak et al. 2008). The difference between our resistance data and those of Guo et al. (1998) and Polak et al. (2008) could be due to the type of molecular construct used, hairpin versus sense construct and/or to the PPV genomic regions selected to construct the transgenes.
Collectively, the infection tests indicate that the h-UTR/P1, h-HCPro and h-HCPro/P3 hairpin constructs conferred full resistance to viral isolates of the most widespread and economically important PPV strains the D and M, and to the natural recombinant PPV-Rec. It should be noticed that resistance was obtained even against the PPV-D Pa1 isolate that is able to naturally infect almond plants (Salamon and Palkovics 2002).
The h-UTR/P1 construct confers resistance to isolates of PPV-EA and PPV-C strains that are distantly related to ISPaVe44
PPV-M, PPV-D, PPV-Rec and the recently proposed PPV-T strain are phylogenetically more closely related to each other than to PPV-EA and PPV-C strains (Fig. 1; Fanigliulo et al. 2003; Serçe et al. 2009). In order to get further insight into the breadth of resistance, T1 plants of lines 6 (h-UTR/P1), 72 (h-P1/HCPro), 101 (h-HCPro), and 162 (h-HCPro/P3) were challenged with the PPV-EA isolate ISPaVe39 and the PPV-C isolate SwC. In addition, plants were also tested against a distinct Potyvirus the PVY Pa21. PPV ISPaVe39- and SwC-inoculated wt and non-transgenic segregants showed systemic symptoms within 7 dpi and were positive by DASI-ELISA. All transgenic plants of lines 72, 101 and 162 challenged with PPV SwC were already infected at the first time point analysed. A similar susceptibility was also observed when the same lines were inoculated with PPV ISPaVe39 with the exception of 10% of transgenic plants of lines 101 and 162 that remained virus free till the end of the experiment (Table 3). Conversely, neither PPV SwC nor PPV ISPaVe39 was detected by DASI-ELISA in transgenic plants of line 6 h-UTR/P1 at any time points analysed up to 72 dpi. At least one DASI-ELISA negative plant or more for each viral infection were further evaluated for PPV presence by IC-RT–PCR and back-inoculation on wt N. benthamiana plants. The two additional assays confirmed that tested plants of line 6 h-UTR/P1 were SwC and ISPaVe39 free (data not shown). The infection test was repeated a third time only on plants of line 6 h-UTR/P1. All transgenic plants were PPV-free as evaluated by DASI-ELISA and IC-RT–PCR (Table 3 and data not shown). In contrast, none of the four transgenic lines was resistant to PVY NTN Pa21 infection. Nucleotide sequences comparison of the four hairpin constructs with the respective genomic regions of the PPV ISPaVe39 and PPV SwC revealed an overall nucleotide identity, for each pair analysed, lower than 83%. The lowest overall nucleotide identity was found with the h-UTR/P1 construct being 77.8% and 71.2% in ISPaVe39/h-UTR/P1 and SwC/h-UTR/P1 pairs, respectively. The spectrum of virus resistance mediated by the RNA silencing is generally restricted to viral strains with about 90% sequence identity to the introduced transgenes (Maki-Valkama et al. 2000; Bau et al. 2003; Xu et al. 2009). However, it has been shown previously that RNA silencing can be achieved in plants through expression of short hairpin RNAs indicating that 21 nt homology between the hairpin construct and the RNA target are sufficient to knock-down gene expression (Lu et al. 2004). A clear-cut relationship between the ability of h-UTR/P1 in conferring resistance to ISPaVe39 and SwC and the preferential presence of stretches of continuous nucleotide homology longer than or equal to 21 nucleotides between h-UTR/P1 and the two viral genome sequences was not found (Table 4). However, since not all siRNAs are equally effective in knocking-down the expression of the homologous RNA target (Pei and Tuschl 2006) we used two siRNAs prediction tools, siRNA design (Yuan et al. 2004), and Block-iTTM RNA designer to look for whether the stretches of homology present between ISPaVe39 or SwC and the hairpin constructs were also sites for the production of potentially effective siRNAs. Five siRNA motif patterns were utilised with each siRNA prediction tool two of which were in common between the two tools. Interestingly, in accord with the resistance data, six out of the eight siRNA patterns identified h-UTR/P1 but not the h-P1/HC as the construct capable of producing potentially effective siRNAs homologous to PPV Swc (Table 4); the best predicted siRNA target region overlapped with the PPV polyprotein translational initiation site. Moreover, no effective siRNAs were identified in the region of homology between h-HC or h-HC/P3 and PVYNTN Pa1 (Table 4). However, only siRNA pattern 5 was able to predict in h-UTR/P1 but not in h-HC and h-HC/P3 constructs the presence of effective siRNAs homologous to ISPaVe39. Recently, it has been shown that the hairpin derived SULFUR (SUL) siRNAs mediate translational repression in addition to mRNA degradation of the endogenous SUL gene and that translational inhibition could be indeed a widespread mechanism of action of plant miRNA and siRNA (Brodersen et al. 2008). Thus, the broad spectrum of PPV resistance conferred by the h-UTR-P1 construct could be mediated by both slicing and translational inhibition of viral genomic sequences. The presence of effective h-UTR/P1 derived siRNAs partially homologous (1–2 mismatch) to ISPaVe39 and SwC sequences (data not shown) could give an additional contribution to the resistance phenomenon. However, we do not know the biological relevance of the predicted siRNAs and this aspect lies outside the scope of the present work. Moreover, the efficacy of RNA silencing depends not only on the nature of siRNAs but also on the accessibility of the target RNA (Shao et al. 2007). Nevertheless, the above data could suggest a potential link between ISPaVe39 and SwC resistance and in silico predicted h-UTR/P1 derived effective siRNAs.
Conclusion
Here, we systematically analysed the capability of four hairpin constructs to confer resistance to all known PPV strains. The data presented show that all (225 out of 225) h-UTR/P1 N. benthamiana plants challenged with nine PPV isolates of the M, D, Rec, EA and C strains (Fig. 1) originally collected from peach, plum, apricot, almond and sweet cherry in different geographical areas (Table 1) were resistant to viral infection (Tables 2, 3). Moreover, the resistance was maintained over prolonged growing periods (72 dpi). The above evidence together with the previous findings (Di Nicola-Negri et al. 2005) that a high proportion (90%) of the transformed plants were resistant to PPV and that hairpin mediated RNA silencing was active in controlling viral infection in Prunus spp. (Hily et al. 2007) make the h-UTR/P1 construct of particular practical interest to obtain transgenic stone fruit plants resistant to the devastating sharka disease. It remains to be established, however, the possible influence of mixed viral infections (Missiou et al. 2004; Lennefors et al. 2008) on the h-UTR/P1 RNA silencing-mediated PPV resistance.
References
Barajas D, Tenllado F, Gonzales-Jara P, Martinez-Garcia B, Atencio FA, Diaz-Ruiz JR (2004) Resistance to Plum pox virus (PPV) in Nicotiana benthamiana plants transformed with the PPV HC-Pro silencing suppressor gene. J Plant Pathol 86:239–248
Barba M, Hadidi A, Candresse T, Cambra M (2010) Plum pox virus. In: Hadidi A, Barba M, Candresse T, Jelkmann W (eds) Virus and virus-like diseases of pome and stone fruits. The American Phytopathological Society Press, St Paul, MN, USA (in press)
Bau HJ, Cheng YH, Yu TA, Yang JS, Yeh SD (2003) Broad-spectrum resistance to different geographic strains of Papaya ringspot virus in coat protein gene transgenic papaya. Phytopathol 93:112–120
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O (2008) Widespread translational inhibition by plant miRNAs and siRNAs. Science 32:1185–1190
Cambra M, Capote N, Myrta A, Llácer G (2006) Plum pox virus and the estimated costs associated with sharka disease. EPPO Bull 36:202–204
Candresse T, Cambra M (2006) Causal agent of sharka disease: historical perspective and current status of Plum pox virus strains. EPPO Bull 36:239–246
Candresse T, Cambra M, Dallot S, Lanneau M, Asensio M, Gorris MT, Revers F, Macquaire G, Olmos A, Boscia D, Quiot JB, Dunez J (1998) Comparison of monoclonal antibodies and polymerase chain reaction assays for the typing of isolates belonging to the D and M serotypes of plum pox potyvirus. Phytopathol 88:198–204
Decroocq V, Foulongne M, Lambert P, Le Gall O, Mantin C, Pascal T, Schurdi-Levraud V, Kervella J (2005) Analogues of virus resistance genes map to QTLs for resistance to sharka disease in Prunus davidiana. Mol Gen Genome 272:680–689
Di Nicola-Negri E, Brunetti A, Tavazza M, Ilardi V (2005) Hairpin RNA-mediated silencing of Plum pox virus P1 and HC-Pro genes for efficient and predictable resistance to the virus. Transgenic Res 14:989–994
Fanigliulo A, Comes S, Maiss E, Piazzolla P, Crescenzi A (2003) The complete nucleotide sequence of Plum pox virus isolates from sweet (PPV-SwC) and sour (PPV-SoC) cherry and their taxonomic relationships within the species. Arch Virol 148:2137–2153
García JA, Cambra M (2007) Plum pox virus and sharka disease. Plant Viruses 1:69–79
Glasa M, Palkovics L, Komínek P, Labonne G, Pittnerová S, Kúdela O, Candresse T, Subr Z (2004) Geographically and temporally distant natural recombinant isolates of Plum pox virus are genetically very similar and form a unique PPV subgroup. J Gen Virol 85:2671–2681
Guo HS, Garcia JA (1997) Delayed resistance to Plum pox potyvirus mediated by a mutated RNA replicase gene: involvement of a gene-silencing mechanism. Mol Plant Microbe Interact 10:160–170
Guo HS, Cervera MT, Garcia JA (1998) Plum pox poptyvirus resistance associated to transgene silencing that can be stabilized after different number of plant generations. Gene 206:263–272
Guo HS, Lopez-Moya JJ, Garcia JA (1999) Mitotic stability of infection-induced resistance to Plum pox potyvirus associated with transgene silencing and DNA methylation. Mol Plant Microbe Interact 12:103–111
Hily JM, Scorza R, Webb K, Ravelonandro M (2005) Accumulation of the long class of siRNA is associated with resistance to Plum pox virus in transgenic woody perennial plum tree. Mol Plant Microbe Interact 18:794–799
Hily JM, Ravelonandro M, Damsteegt V, Bassett C, Petri C, Liu Z, Scorza R (2007) Plum pox virus coat protein gene intron hairpin RNA construct provides resistance to Plum pox virus in Nicotiana benthamiana and Prunus domestica. J Am Soc Hort Sci 132:850–858
Ilardi V, Mazzei M, Loreti S, Tomassoli L, Barba M (1995) Biomolecular and serological methods to identify CMV strains on tomato. EPPO Bull 25:321–327
Ilardi V, Di Nicola-Negri E, Brunetti A, Gentile A, Monticelli S, Damiano C (2007) RNA interference for sharka disease resistance. Acta Hort 738:593–599
Ion-Nagy L, Lansac M, Eyquard JP, Salvador B, Garcia JA, Le Gall O, Hernould M, Schurdi-Levraud V, Decroocq V (2006) PPV long-distance movement is occasionally permitted in resistant apricot hosts. Virus Res 120:70–78
Jacquet C, Ravelonandro M, Bachelier JC, Dunez J (1998) High resistance to Plum pox virus (PPV) in transgenic plants containing modified and truncated form of PPV coat protein gene. Transgenic Res 7:29–39
James D, Varga A (2005) Nucleotide sequence analysis of Plum pox virus isolate W3174: evidence of a new strain. Virus Res 110:143–150
Karayiannis I, Thomidis T, Tsaftaris A (2008) Inheritance of resistance to Plum pox virus in apricot (Prunus armeniaca L). Tree Genet Genome 4:143–148
Kegler H, Fuchs E, Gruntzig M, Schwarz S (1998) Some results of 50 years’ research on resistance to Plum pox potyvirus. Arch Phytopath Pflanz 31:479–506
Lennefors BL, Van Roggen PM, Yndgaard F, Savenkov EI, Valkonen JPT (2008) Efficient dsRNA-mediated transgenic resistance to Beet necrotic yellow vein virus in sugar beets is not affected by other soilborne and aphid-transmitted viruses. Transgenic Res 17:219–228
Liu Z, Scorza R, Hily J-M, Scott SW, James D (2007) Engineering resistance to multiple Prunus fruit viruses through expression of chimeric hairpins. J Am Soc Hort Sci 132:407–414
Lopez-Moya JJ, Fernandez-Fernandez MR, Cambra M, Garcia JA (2000) Biotechnological aspects of Plum pox virus. J Biotech 76:121–136
Lu S, Shi R, Tsao C, Yi X, Li L, Chiang VL (2004) RNA silencing in plants by the expression of siRNA duplexes. Nucleic Acids Res 32(21):e171. doi:10.1093/nar/gnh170
Maki-Valkama T, Valkonen JPT, Kreuze JF, Pehu E (2000) Transgenic resistance to PVYO associated with post-transcriptional silencing of P1 transgene is overcome by PVYN strains that carry highly homologous P1 sequences and recover transgene expression at infection. Mol Plant Microbe Interact 13:66–373
Missiou A, Kalantidis K, Boutla A, Tzortzakaki S, Tabler M, Tsagris M (2004) Generation of transgenic potato plants highly resistant to Potato virus Y (PVY) through RNA silencing. Mol Breed 14:185–197
Nemchinov L, Crescenzi A, Hadidi A, Piazzolla P, Verderevskaya T (1998) Present status of the new cherry subgroup of Plum pox virus (PPV-C). In: Hadidi A, Khetarpal RK, Koganezawa H (eds) Plant virus disease control. APS Press, St Paul, Minnesota, pp 629–638
Németh M (1994) History and importance of plum pox in stone-fruit production. EPPO Bull 24:525–536
Nomura K, Ohshima K, Anai T, Uekusa H, Kita N (2004) RNA silencing of the introduced coat protein gene of Turnip mosaic virus confers broad-spectrum resistance in transgenic Arabidopsis. Phytopathol 94:730–736
Pandolfini T, Molesini B, Avesani L, Spena A, Polverari A (2003) Expression of self-complementary hairpin RNA under the control of the rolC promoter confers systemic disease resistance to Plum pox virus without preventing local infection. BMC Biotechnol 3:7
Pei Y, Tuschl T (2006) On the art of identifying effective and specific siRNAs. Nat Methods 3:670–676
Petri C, Burgos L (2005) Transformation of fruit trees. Useful breeding tool or continued future prospect? Transgenic Res 14:15–26
Polak J, Pivalova J, Kundu J, Jokes M, Scorza R, Ravelonandro R (2008) Behaviour of transgenic Plum pox virus-resistant Prunus domestica L clone C-5 grown in the open field under a high and permanent infection pressure of the PPV-Rec strain. J Plant Pathol 90:S133–S136
Ravelonandro M, Monsion M, Delbos R, Dunez J (1993) Variable resistance to Plum pox virus and potato virus Y infection in transgenic plants expressing Plum pox virus coat protein. Plant Sci 91:157–169
Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A (2004) Rational siRNA design for RNA interference. Nat Biotech 22:326–330
Rubio M, Martínez-Gómez P, Dicenta F (2003) Resistance of almond cultivars to Plum pox virus (sharka). Plant Breed 122:462–464
Ruiz-Ferrer V, Voinnet O (2009) Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 60:485–510
Salamon P, Palkovics L (2002) Characterization of Plum pox virus PPV-BT-H isolated from naturally infected blackthorn (Prunus spinosa L) in Hungary. Eur J Plant Pathol 108:903–907
Scorza R, Callahan A, Levy L, Damsteegt V, Webb K, Ravelonandro M (2001) Post-transcriptional gene silencing in Plum pox virus resistant transgenic European plum containing the Plum pox potyvirus coat protein gene. Trasgenic Res 10:201–209
Serçe CU, Candresse T, Svanella-Dumas L, Krizbai L, Gazel M, Caglayan K (2009) Further characterization of a new recombinant group of Plum pox virus isolates, PPV-T, found in orchards in the Ankara province of Turkey. Virus Res 142:121–126
Shao Y, Chan CY, Maliyekkel A, Lawrence CE, Roninson IB, Ding Y (2007) Effect of target secondary structure on RNAi efficiency. RNA 13:1631–1640
Smith NA, Singh SP, Wang MB, Stoutjesdijk PA, Green AG, Waterhouse PM (2000) Total silencing by intron-spliced hairpin RNAs. Nature 407:319–320
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Ui-Tei K, Naito Y, Takahashi F, Haraguchi T, Ohki-Hamazaki H, Juni A, Ueda R, Saigo K (2004) Guidelines for the selection of highly efficient siRNA sequences for mammalian and chick RNA interference. Nucl Acids Res 32:936–948
Voinnet O (2005) Induction and suppression of RNA silencing: insights from viral infections. Nat Rev Genet 6:206–220
Waterhouse PM, Wang MB, Lought T (2001) Gene silencing as an adaptive defence against viruses. Nature 411:834–842
Wetzel T, Candresse T, Ravelonandro M, Dunez J (1991) A polymerase chain reaction assay adapted to Plum pox virus detection. J Virol Methods 33:355–365
Wittner A, Palkovics L, Balazs E (1998) Nicotiana benthamiana plants transformed with the Plum pox virus helicase gene are resistant to virus infection. Virus Res 53:97–103
Xu L, Song YZ, Zhu JH, Guo XQ, Zhu CX, Wen FJ (2009) Conserved sequences of replicase gene-mediated resistance to Potyvirus through RNA silencing. J Plant Biol 52:550–559
Yuan B, Latek R, Hossbach M, Tuschl T, Lewitter F (2004) siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucl Acids Res 32:130–134
Acknowledgments
We are grateful to Andrea Iazzoni for his technical support. The study was supported by Research Program MiPAAF—CIPE FRU.MED., Project ProViSUD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Register.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Di Nicola-Negri, E., Tavazza, M., Salandri, L. et al. Silencing of Plum pox virus 5′UTR/P1 sequence confers resistance to a wide range of PPV strains. Plant Cell Rep 29, 1435–1444 (2010). https://doi.org/10.1007/s00299-010-0933-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0933-6