Abstract
Amenability to tissue culture stages required for gene transfer, selection and plant regeneration are the main determinants of genetic transformation efficiency via particle bombardment into sugarcane. The technique is moving from the experimental phase, where it is sufficient to work in a few amenable genotypes, to practical application in a diverse and changing set of elite cultivars. Therefore, we investigated the response to callus initiation, proliferation, regeneration and selection steps required for microprojectile-mediated transformation, in a diverse set of Australian sugarcane cultivars. 12 of 16 tested cultivars were sufficiently amenable to existing routine tissue-culture conditions for practical genetic transformation. Three cultivars required adjustments to 2,4-D levels during callus proliferation, geneticin concentration during selection, and/or light intensity during regeneration. One cultivar gave an extreme necrotic response in leaf spindle explants and produced no callus tissue under the tested culture conditions. It was helpful to obtain spindle explants for tissue culture from plants with good water supply for growth, especially for genotypes that were harder to culture. It was generally possible to obtain several independent transgenic plants per bombardment, with time in callus culture limited to 11–15 weeks. A caution with this efficient transformation system is that separate shoots arose from different primary transformed cells in more than half of tested calli after selection for geneticin resistance. The results across this diverse cultivar set are likely to be a useful guide to key variables for rapid optimisation of tissue culture conditions for efficient genetic transformation of other sugarcane cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane is a genetically complex crop with a long (10–15 year) conventional breeding and selection cycle, and vegetative propagation of resulting cultivars (James 2004). Therefore, sugarcane improvement via genetic engineering depends on the ability to transform elite cultivars without adversely changing their existing commercial characteristics.
Direct gene transfer into sugarcane through particle bombardment is routine in cultivars amenable to embryogenic callus culture. The process is very efficient, producing tens of independent transgenic plant lines per bombardment (Bower et al. 1996). Bombardment directly into meristems or other excised tissues, followed by shoot proliferation or regeneration via a callus stage has been inefficient or unsuccessful for production of non-chimaeric transgenic plants (Gambley et al. 1994; Snyman et al. 2006). By comparison with more recalcitrant related species such as sweet sorghum (Raghuwanshi and Birch 2010), the surface layers of sugarcane embryogenic callus evidently have a higher proportion of cells that are able to proliferate and regenerate under conditions that permit the selection of transformed plantlets. Callus formation and plant regeneration vary with sugarcane genotype, explant type, culture conditions and combinations of these factors (Chen et al. 1988; Fitch and Moore 1993; Gandonou et al. 2005; Heinz and Mee 1969; Kresovich et al. 1985; Liu 1983; Snyman et al. 2006).
Agrobacterium-mediated gene transfer into sugarcane is so far more genotype- and batch-dependent, with a lower absolute transformation efficiency (Arencibia and Carmona 2006; Joyce et al. 2010). Embryogenic callus is a preferred tissue for co-cultivation, so we anticipate that the tissue-culture considerations for particle bombardment will be a subset of those important for use with Agrobacterium.
In general, it is considered that the incidence of undesired somaclonal variation is likely to increase with time in culture (Burner and Grisham 1995; Kaeppler et al. 2000). One advantage of the use of sugarcane furled leaf and sheath tissue for embryonic callus initiation is that it is relatively easy to arrange year-round availability of this explant type from field-grown plants, so that fresh callus batches can be regularly initiated to minimise time in culture for gene transfer. However, the effect of growing season or physiological status of the source plant on callus initiation has not been reported.
Various transgene-encoded traits of potential commercial value have been experimentally demonstrated in sugarcane genotypes amenable to transformation (Arencibia et al. 1997; Butterfield et al. 2002; GalloMeagher and Irvine 1996; Hamerli and Birch 2010; Petrasovits et al. 2007; Rossouw et al. 2010; Vickers et al. 2005; Wang et al. 2005; Wu and Birch 2007; Zhang et al. 1999, 2006). It is important to ensure diversity of genotypes across an industry to minimise disease risk. Furthermore, ingress of a pathogen can necessitate a rapid change in approved cultivars (Croft et al. 2008). Therefore, we investigated tissue culture response of diverse Australian sugarcane genotypes. This paper describes broad applicability of routine conditions, and adjustments that proved effective for efficient production of transgenic plant lines in elite recipient cultivars that were initially recalcitrant.
Materials and methods
Plant material and callus induction
Sixteen Australian sugarcane (Saccharum hybrid) cultivars (Table 1) were investigated for their ability to form regenerable embryogenic callus from unexpanded leaf and sheath tissues. Source plants were typically 6–12 months old in field plots at Kalamia in North Queensland. Cane tops were collected by cutting near the top visible dewlap and the first exposed internode. The outer leaf sheaths were peeled off and the tops were trimmed to 12–15 cm in length, including several young internodes, the apical meristem region and up to seven furled leaves and sheaths. These shoot tops were swabbed using 95% ethanol and outer sheaths were removed aseptically to obtain from each stalk an uncontaminated inner spindle of about 1 cm diameter. Transverse sections about 1–3 mm thick were then cut from the zone up to 4 cm above the apical meristem and placed onto culture medium.
Culture media and conditions
Callus was initiated and proliferated on Murashige and Skoog basal medium supplemented with 3 mg l−1 (13.6 μM) 2,4-dichlorophenoxy acetic acid (2,4-D), 500 mg l−1 casein hydrolysate, 15 g l−1 sucrose, 0.1 mg l−1 thiamine-HCl, 0.5 mg l−1 pyridoxine–HCl, 0.5 mg l−1 nicotinic acid, 2 mg l−1 glycine, 100 mg l−1 myo-inositol, 9 g l−1 agar and 10% v/v coconut water (MSC3) (Heinz and Mee 1969; Liu 1983; Murashige and Skoog 1962). Unless specified otherwise, all tissue culture was conducted at 28°C in darkness with fortnightly subculture. Callus quality was assessed visually and embryogenic callus abundance was scored 6 weeks after initiation. To obtain the preferred callus type for gene transfer, nodular embryogenic callus was selectively transferred at each subculture.
To induce plant regeneration and rooting, embryogenic callus was transferred to medium without 2,4-D (MSC0) and incubated under a 16 h photoperiod of approximately 1,000 Lux provided by fluorescent ‘daylight’ lamps. As Q208 initially regenerated albino shoots, it was tested for callus formation on lower 2,4-D levels (1, 1.5 and 2 mg l−1) for 5–6 weeks before transfer to MSC0. In a further variation, embryogenic callus formed by Q117, Q138, Q208 and KQ228 on medium containing 1.5 mg l−1 2,4-D was tested for regeneration on MSC0 under bright (1,000 Lux) or dim (300 Lux) light.
Regenerated plantlets with robust root systems were selected for transfer to peat pots containing a 1:1 peat and sand mixture. To avoid microbial growth, the roots were washed to remove any attached medium prior to potting. For initial acclimatization in the containment glasshouse under natural light at 28°C, the peat pots on trays were covered with transparent humidity domes and watered once daily. After a week, vents were first opened then the cover was removed to reduce humidity progressively over several days. The plants were grown for 3–4 weeks before transfer into larger pots.
For shoot proliferation from single excised plantlets, we tested MSC0 medium supplemented with various combinations of 6-benzyladenine (BA) and 6-furfurylaminopurine (Kinetin), based on previous studies of shoot proliferation from excised meristems (Ramgareeb et al. 2010). The media tested were MP1 (0.1 mg l−1 BA; 0.015 mg l−1 Kinetin), MP2 (0.15 mg l−1 BA; 0.0225 mg l−1 Kinetin), MP3 (0.2 mg l−1 BA; 0.03 mg l−1 Kinetin), MP4 (0.1 mg l−1 BA; 0.0225 mg l−1 Kinetin) and MP5 (0.2 mg l−1 BA; 0.015 mg l−1 Kinetin).
Transformation by particle bombardment
Pre-embryogenic or embryogenic callus (3–5 weeks old) was treated on osmotic medium for 4 h before and after microprojectile bombardment using selectable marker construct pUbiKn and co-precipitated reporter constructs (Bower et al. 1996). Selection commenced at 48 h on MSC3 medium containing geneticin at 50 mg l−1. After 5–6 weeks, rapidly growing embryogenic calli were transferred to MSC0 containing geneticin at 50 mg l−1 and incubated in the light for plant regeneration. Selection on MSC3 may be extended to obtain more transgenic lines, but because of the high transformation efficiency in sugarcane, our routine emphasis has been on minimal time in culture. Geneticin was used at 25 mg l−1 for KQ228 as described below.
Molecular analysis of transgenic lines
Genomic DNA was prepared from young leaves of greenhouse-grown plants using the ISOLATE Plant DNA mini kit (Bioline). Copy number was indicated by qRT-PCR undertaken on 100 ng of genomic DNA using primers designed to amplify a 97 bp amplicon at the 3′ end of the luc coding sequence. The PCR used FastStart Universal SYBR Green Master (Roche Diagnostics) in a Rotorgene 3000 thermocycler (Corbett Research). After denaturation at 95°C for 10 min, there were 45 cycles of denaturation at 95°C for 15 s followed by annealing/extension at 62°C for 60 s. Glyceraldehyde 3-phosphate dehyrogenase (GAPDH) was used as a reference gene to normalise between PCR reactions, using the primers previously found suitable for this purpose in sugarcane (Iskandar et al. 2004). No template reactions were included as negative controls.
Calli were checked for LUC activity using a low-light camera (Mudge and Birch 1998; Raghuwanshi and Birch 2010). Luminometer assays (Luehrsen and Walbot 1993) were used to quantify LUC activity in extracts from young leaves of greenhouse-grown plants.
Effect of time in culture on vigour
Single-budded setts from glasshouse-grown transgenic lines produced after various periods in callus culture, and from field-grown stalks of recipient genotype Q127 were planted into replicated single-row plots in a randomised block design in the field in May 2006. Stalk height (base to the top visible dewlap) and stalk diameter (at the centre of the stalk) were measured in two stalks per plot at 9 months.
Statistical analysis
Statistical analysis was carried out using analysis of variance and Dunnett’s multiple comparison test (GraphPad Prism).
Results
Embryogenic callus formation
Sugarcane cultivars varied in the rate and quality of callus formed on MSC3 from tissue discs cut from the furled leaf and sheath region immediately above the stalk apical meristem (Table 1). Callus generally became visible on the cut surfaces within 5–7 days and initiation generally decreased with distance beyond 4 cm above the meristem. There were differences between individual stalks of the same genotype, but amenability to embryogenic callus initiation and proliferation was broadly consistent between batches in the cultivars that were tested on multiple occasions using batches of about 10–20 cane tops.
Browning of explants and production of diffusible phenolic material was observed in most cultivars, with highest levels from KQ228 and Q208. The incidence seemed higher in explants obtained during water-limited seasons such as late spring and early winter. Adverse effects were minimised by shortening the interval between subcultures in proportion to the intensity of browning, which generally subsided after several subcultures.
As noted previously from sugarcane spindle leaf explants (Chagvardieff et al. 1981; Chen et al. 1988; Ho and Vasil 1983; Liu 1983), the first visible callus commonly appears on phloem tissue, has a mucilaginous surface appearance and may develop a nodular organisation. As this tissue grows, several sugarcane callus types can be distinguished based on appearance and texture during handling for subculture. We distinguished:
-
compact (comprised of hard, smooth-surfaced nodules and generally opaque),
-
friable (crumbling easily into smaller cell clumps, with a less-smooth surface and generally semi-translucent),
-
soft (soft texture, amorphous structure and generally translucent) or
-
sticky (as for soft callus plus mucilaginous in appearance and adhering to implements).
The initial abundance of different callus types varied between genotypes (Table 1), and then also with culture medium, age and selective subculturing.
Some cultivars produced mainly embryogenic callus on MSC3. By 6 weeks, cultivars Q117, Q135, Q157, Q158, Q185, Q186, Q208 and Q209 had a high proportion of yellow, compact, embryogenic callus. They yielded approximately 35–40 g of this material per initial tissue disc, within 10 weeks, when subcultured fortnightly on MSC3.
Q138, Q170, Q183 and KQ228 produced mainly non-embryogenic yellow or white soft or sticky callus with few embryogenic areas. Q174 and Tellus produced a large amount of friable callus. For all of these genotypes, selective subculturing of somatic embryo clusters on MSC3 at 2-week intervals resulted in predominantly embryogenic callus cultures that were readily maintained for 4–6 months. Longer periods between subcultures on MSC3 led to various changes in callus morphology including initiation of plant regeneration, attributed to gradual depletion of 2,4-D in the medium. For cultivars Q135 and Q209, greater caution was required to avoid exposure to light as this triggered the initiation of shoots on embryogenic callus. Immature leaf explants from Q204 became necrotic on MSC3 medium and did not recover or produce any visible callus.
KQ228 repeatedly showed a seasonal influence on embryogenic callus initiation. Explants obtained during dry periods in late Spring and early Summer produced less than 1.5 g of callus/explant over a period of 10 weeks, whereas explants obtained in early Autumn during a period of rapid cane growth after rain produced more than 35 g of embryogenic callus/explant.
With selective subculturing under the routine culture conditions, 15 of the 16 tested cultivars yielded regenerable embryogenic callus suitable for genetic transformation, at least to the level of 16 g of pre-embryogenic and embryogenic callus within 4 weeks from five cane tops. This is sufficient for at least 25 bombardments, which from experience using Q117 would be anticipated to yield about 200 independent transgenic plant lines. However, as noted below, some genotypes required adjustments to the routine conditions during callus production in order to achieve efficient regeneration of normal plants after the period required for gene transfer and selection.
Plant regeneration
Callus texture and morphology (up to 12 weeks after initiation) were good indicators of regeneration ability in the light after subculture of callus pieces about 5 mm in diameter onto MSC0. With the exceptions noted below, green shoots were produced within 2–3 weeks from 50–100% of hard callus pieces with smooth-surfaced nodular appearance, whereas non-embryogenic callus types tended to proliferate further amorphous callus under these conditions. This applied equally to compact embryogenic callus derived by selective subculture from an initial stage of predominantly friable, soft or sticky callus (Table 1).
Cultivars differed in the rate of plant regeneration on MSC0, but with the exceptions noted below all yielded plantlets with robust root systems within 8–12 weeks on MSC0. Cultivars also differed in the culture period during which they retained regenerability, an extreme case being Q200 for which embryogenic calli were able to regenerate plants 5–6 weeks after initiation but regenerability declined steeply after 7–8 weeks.
Q208 and KQ228 showed slow shoot initiation accompanied by proliferation of non-embryogenic material, from 30–40% of embryogenic calli incubated on MSC0 under bright light (1,000 Lux). Regeneration of these cultivars was improved by incubation under dim light (300 Lux) for the first 3 weeks on MSC0 (Fig. 1). Other genotypes such as Q117 and Q138 were more tolerant of dim or bright light at the start of regeneration (Fig. 1).
Influence of light intensity on regeneration from calli of Q117, Q138, KQ228 and Q208. Results are percentage of embryogenic calli (5 mm in diameter) that regenerated green or albino shoots (>5 mm in height) within the indicated times on MSC0 medium at 28°C under 1,000 Lux (filled circle) or 300 Lux (open circle)
Of the shoots regenerated from MSC3-grown callus of Q208 and KQ228, 14–55% were albino and could only be maintained on media containing sucrose. In initial tests using Q208, reduction of 2,4-D concentration during callus production improved the proportion of green shoots. However, substantially less callus was produced on 1.0 mg l−l 2,4-D. Relative to performance on MSC3, regenerable callus production per starting explant was slightly lower at 1.5 mg l−l 2,4-D and the proportion of albino shoots subsequently regenerated was much lower (Table 2). Medium containing 1.5 mg l−1 2,4-D also proved suitable for production of callus that regenerated mainly green shoots from KQ228 (data not shown).
Q138 was unusual in that 6% of plants regenerated from MSC3-grown callus had proliferating axillary buds with premature sprouting. This effect was eliminated in plants regenerated from callus produced on medium containing 1.5 mg l−1 2,4-D.
Q170 produced clusters of many green shoots within 4 weeks on MSC0 medium. To achieve rooted plants suitable for potting in 10–12 weeks, it was necessary to selectively subculture the larger shoots from each cluster.
Selection of transgenic lines
The routine parameters for microprojectile-mediated gene transfer into embryogenic callus (Bower et al. 1996), followed by selection for geneticin resistance conferred by selectable marker Ubi-Kn, were suitable for all 15 tested sugarcane cultivars. The transformation efficiency, judged by the formation of discrete geneticin-resistant calli, was 12–15% of bombarded embryogenic callus pieces (equivalent to 7–12 discrete geneticin-resistant calli per 3.5 cm diameter target area). The frequency of co-expression of a co-precipitated, non-selected, Ubi-driven reporter gene was close to 80% as described previously for easily cultured sugarcane cultivars (Bower et al. 1996). This indicates that 50 mg l−1 geneticin in MSC3 provided effective selection against non-transformed material for all 15 tested sugarcane genotypes.
The procedure was accelerated to accommodate the shorter period of regenerability noted above for Q200. For this cultivar, embryogenic callus was bombarded 3–4 weeks after initiation, and calli were transferred onto regeneration medium 3–4 weeks after bombardment. To reduce the risk of chimaeric regenerants including non-transformed sectors (Bower et al. 1996), selection on geneticin-containing medium was maintained until potting.
KQ228 was unusual in regenerating plants from only 30% of transformed calli on MSC0 containing 50 mg l−1 geneticin, even under the low-light conditions described above. This was increased to 72–82% (in repeated tests) by decreasing the geneticin concentration to 25 mg l−1 throughout the callus selection and regeneration stages.
Molecular analysis of transgenic lines to detect chimaeric-transformed calli
To test the frequency with which distinct transformation events occur within the same piece of callus after geneticin selection, we analysed 4–9 plants regenerated from each of 33 transgenic LUC+ calli, using qRTPCR to indicate the relative copy number of a co-bombarded luc transgene. The tested callus lines were sampled at random from multiple bombardments into recipient genotype Q117. The efficiencies of transformation (4–10 separate calli per bombardment after selection, depending on bombarded callus quality), and co-expression (80–90% positive for non-selected, Ubi-driven LUC activity) were typical of the efficient sugarcane transformation system (Bower et al. 1996). More than half of the tested transgenic callus lines yielded plants with different luc copy numbers. Figure 2a shows an example of aligned qRTPCR curves, indicating plants originating from two luc copy-number classes.
Example of detection of plants arising from different primary transformed cells in a single selected callus. a qRT-PCR on equal quantities of genomic DNA from five individual plants, indicating a higher luc copy number in plants 1 and 3 than in plants 2, 4 and 5. GAPDH was used as a reference gene for normalisation. Negative controls showed no non-specific amplification. b LUC activity was high in plants 1 and 3 (the mean with standard error from these plants is shown), and undetectable in plants 2, 4 and 5 (likely due to integration of incomplete luc sequence fragments that include the 97 bp template region for the qRT-PCR)
Limited Southern analyses to date indicate that most transgene integration patterns are stable in sugarcane (Bower et al. 1996). Therefore, plants with different transgene copy numbers most likely arise from different primary transformed cells. The PCR analyses of plants indicated that it was common for discrete geneticin-resistant calli to be composites arising from at least two (16/33 callus lines) or three (2/33 callus lines) separate transformed cells. These different transformation events commonly gave rise to different levels of LUC reporter activity. Figure 2b shows an example of LUC+ and LUC− plant lines (with different copy numbers of the amplified region of luc) from the same callus. Plants arising from different primary transformation events can show different transgene expression levels and patterns due to effects including integration position, copy number and silencing triggers arising from partial or complex integrations. Therefore, it is unwise to take multiple primary regenerated shoots from one selected callus as clonal ‘replicates’ without molecular confirmation that they have the same transgene integration pattern.
Organogenesis may involve cooperation of multiple callus cells in meristem formation (Bonnel et al. 1983), whereas somatic embryos arise from single cells (Ho and Vasil 1983). Therefore, it is unlikely that shoots arising by somatic embryogenesis will be chimaeric, unless the duration of callus proliferation on selection is insufficient (Bower et al. 1996). If multiple plants are desired for analysis of replicate clonal transformants in the first vegetative generation out of tissue culture, one approach to minimise the risk is to apply an additional shoot proliferation step to single excised shoots, after selection and regeneration using embryogenic callus as described above. We tested such shoots from Q117 and Q208 on MSC0 medium supplemented with various combinations of BA and Kinetin. MP4 medium with 0.1 mg l−1 BA and 0.0225 mg l−1 kinetin proved most effective, yielding 4–6 clonal shoots per transgenic line, at the same size as the starting shoot within 6 weeks.
Time required in culture and effect on vigour of regenerated plants
All cultivars except Q204 produced embryogenic calli suitable for bombardment within 3–4 weeks after initiation, regenerable calli within 5–6 weeks on selection, organised shoots within 2 weeks on MSC0 and shoots with roots suitable for potting within 10 weeks on MSC0. Emergence of transgenic calli on selection is asynchronous, and more independent transformants can often be obtained by extending the selection period up to 8–10 weeks. As the transformation efficiency is high, the events that emerge and regenerate earlier on selection can be used to minimise time in callus culture. If the supply of material to initiate cultures is limiting, it is common to extend the proliferation steps somewhat. In practice, the time in callus culture is typically about 11–15 weeks, or 19–24 weeks from callus initiation to transgenic plantlets in soil.
In our hands, bombardment of cultured explants before the formation of callus visible to the naked eye resulted in much lower transformation efficiencies per explant and a requirement for a longer period on selection after bombardment to yield regenerable callus pieces, which led to about the same total time in culture. However, amenable cultivars could be transformed at intermediate frequencies (2–8 independent transgenic calli per bombardment) by gene transfer into pre-embryogenic callus stages (typically 2–3 weeks after initiation) for a slight reduction in total culture time.
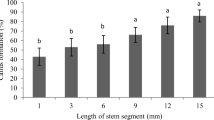
Cultivars including Q117, Q127, Q135, Q174 and Tellus were readily maintained as regenerable embryogenic callus cultures for many months, but when the objective is precise addition of a transgenic trait we prefer to minimise time in culture because of the risk of accrued genetic changes. We have not found reliable genetic tests for such change. However, we incidentally generated transgenic lines with the same genetic constructs in recipient genotype Q127 after various periods in culture. In the first field planting, there was an apparent reduction in plant vigour assessed by stalk diameter with time in culture beyond 23 weeks (Fig. 3a). Further work is required to evaluate the physiological or genetic basis for such effects and the extent of recovery in vigour through initial rounds of field propagation.
Effect of time in culture on stalk diameter (a) and height (b) of transgenic lines in their first field planting. Filled box (0 week) results are for field-sourced recipient genotype Q127. Plots show the median and range from 25–75th percentile in the box, and whiskers show the spread of measurements
Discussion
The practical use of sugarcane genetic manipulation to introduce commercially useful traits depends on the amenability of elite genotypes to the required tissue culture and selection regimens. Previous reports have been encouraging, with regenerable embryogenic callus produced at various efficiencies from multiple-tested sugarcane genotypes (Fitch and Moore 1993; Gandonou et al. 2005). But there have been no published comparisons across multiple genotypes through to selection and regeneration of transgenic plants. Therefore, we tested 16 Australian commercial sugarcane genotypes for response to callus initiation, proliferation, selection and regeneration steps used in microprojectile-mediated transformation.
Embryogenic callus has been preferred for most efficient gene transfer, selection and plant regeneration in sugarcane (Bower et al. 1996; Joyce et al. 2010). Cultivars varied substantially in rate of callus initiation and the proportion of embryogenic callus (Table 1). Nevertheless, all but one (Q204) yielded sufficient embryogenic callus for production of transgenic plant lines, through variations to the routine culture conditions.
The key factors that allowed success with initially recalcitrant genotypes were:
-
1.
Arrangement for good (non water-stressed) growth conditions before collecting cane tops as explants for callus initiation;
-
2.
Early subculture in the event of excess phenolic production by explants;
-
3.
Selective subculture to enrich for nodular embryogenic callus suitable for bombardment within 1 month after initiation (Q138, Q170, Q174, Q183, Tellus);
-
4.
Minimal light during subcultures to avoid premature plant regeneration (Q135, Q209);
-
5.
Lower light intensity (300 Lux) during initial regeneration steps (KQ228, Q208);
-
6.
Lowered concentration of 2,4-D (1.5 mg l−1) during callus initiation and maintenance in case of genotypes that produced many albino plantlets (Q208, KQ228) or other growth abnormalities (Q138) in regenerated plants;
-
7.
Lower geneticin concentration (25 mg l−1) during regeneration (KQ228).
We were surprised by the extent to which embryogenic callus production was affected by (seasonal) changes in source plant physiology, particularly in ‘recalcitrant’ genotypes such as KQ228. Water stress can trigger large changes in levels of endogenous phytohormones such as abscisic acid (ABA) (Sugiharto et al. 2002), which can reduce the capacity for embryogenic callus initiation in some plants (Rudus et al. 2009). This observation requires further testing in sugarcane.
Subculture away from excessive phenolics and preferential subculture of nodular embryogenic callus are straightforward, except that they can increase workload and time needed in culture for those genotypes that initially produce only a small proportion of the preferred callus type. Genotype-dependent effects of light on growth and differentiation in vitro have been observed in other monocot culture systems (Garcia et al. 2007; Rikiishi et al. 2008) and are easily managed once they are found to affect particular genotypes. Regeneration and rooting in the presence of antibiotic seem useful to reduce the possibility of chimaeric material, especially when the time on selection as callus is minimised. However, some genotypes (such as KQ228) appear more antibiotic-sensitive during regeneration and may require reduced antibiotic concentration during this stage. This may be recognised by a low proportion of selected calli forming shoots, and an alternative solution might be to extend selection on callus by one or two subcultures to avoid chimaeras, then regenerate without selection and confirm the presence of the transgene by PCR.
The formation of albino plants and abnormal multiple buds are indicators of culture-induced somaclonal variation. Concentration of 2,4-D in the culture medium is known to influence the frequency of variants in several plant cell culture systems (Burner and Grisham 1995; Garcia et al. 2007). We found that a decrease in 2,4-D concentration from 3 to 1.5 mg l−1 in the callus proliferation medium reduced the incidence of albinos and other multiple-bud phenotypes among regenerated plants for several genotypes. This warrants further exploration, because genetic variations may occur at a much higher frequency than visible morphological variations.
There is some evidence that mutations including gross chromosome variations accumulate with time in callus culture (Burner and Grisham 1995; Fukui 1983; Kaeppler et al. 2000). Most random changes are likely to have adverse effects on commercial performance in highly selected lines, and we saw evidence of reduced stalk diameter in first-generation, field-grown plants regenerated after more than 23 weeks in culture (Fig. 3). More work is needed on reliable and sensitive molecular markers of genomic change, and to understand the extent of recovery during subsequent field vegetative generations from adverse phenotypes that may be influenced by transient epigenetic effects in culture. Our approach is to minimise total time in callus culture, which we found to require several weeks for callus initiation because earlier bombardment required longer on selection to produce regenerable-sized callus.
Although substantially more work was required to process more explants and/or subcultures for sufficient embryogenic callus yields in less amenable cultivars, all except Q204 were used to generate at least 50 independent transgenic sugarcane lines of normal morphology, suitable for planting and evaluation under field conditions.
We have previously cautioned that this highly efficient transformation system can yield multiple independent transformed cell lines in a single piece of selected callus (Bower et al. 1996). The qRT-PCR analyses on multiple lines documented in the present report indicate that this can occur in a substantial proportion of selected calli, even when the number of transformants per bombardment is low. Some callus areas are apparently highly competent for transformation. Therefore, it is unwise to take multiple shoots from a single callus as clonal replicates without molecular analysis. However, multiplication from a single excised shoot (carefully excluding any underlying callus) can be used to obtain 4–6 clonal replicates within 6 weeks.
Q204 is exceptional among sugarcane genotypes tested to date, in showing a severe necrotic response that precluded callus formation within the range of conditions tested here. If it becomes important to apply gene transfer directly into such genotypes, our initial approach would be to test physical pre-treatments such as heat or cold to suppress the initial necrotic reaction (Gurel et al. 2009), or chemical agents such as anti-oxidants in the excision step and the initial culture medium (Enriquez-Obregon et al. 1998). It may also be useful to test media that circumvented the tissue-culture recalcitrance of the related genus Sorghum (Raghuwanshi and Birch 2010).
Conclusion
With attention to explant quality and minor adjustments to tissue culture conditions, 15 out of 16 tested Australian sugarcane cultivars were sufficiently amenable to embryogenic callus culture, selection and plant regeneration to produce multiple independent transgenic lines per bombardment, with a total tissue-culture duration below 23 weeks. Identification here of the adjustments needed to achieve this success with initially recalcitrant genotypes should simplify the optimisation process for future cultivars.
References
Arencibia AD, Carmona ER (2006) Sugarcane (Saccharum spp.). In: Wang K (ed) Agrobacterium protocols. Humana Press, New York, pp 227–235
Arencibia A, Vazquez RI, Prieto D, Tellez P, Carmona ER, Coego A, Hernandez L, De la Riva GA, Selman-Housein G (1997) Transgenic sugarcane plants resistant to stem borer attack. Mol Breed 3:247–255
Bonnel E, Demarly Y, Essad S (1983) Evolution anatomique des tissus foliares de canne a sucre (Saccharum sp.) cultives in vitro. Can J Bot 61:830–836
Bower R, Elliott AR, Potier BAM, Birch RG (1996) High-efficiency, microprojectile-mediated cotransformation of sugarcane, using visible or selectable markers. Mol Breed 2:239–249
Burner DM, Grisham MP (1995) Induction and stability of phenotypic variation in sugarcane as affected by propagation procedure. Crop Sci 35:875–880
Butterfield MK, Irvine JE, Garza MV, Mirkov TE (2002) Inheritance and segregation of virus and herbicide resistance transgenes in sugarcane. Theor Appl Genet 104:797–803
Chagvardieff P, Bonnel E, Demarly Y (1981) La culture in vitro de tissus somatiques de canne a sucre (Saccharum sp.). Agronomie Tropicale 36:266–278
Chen WH, Davey MR, Power JB, Cocking EC (1988) Control and maintenance of plant regeneration in sugarcane callus cultures. J Exp Bot 39:251–261
Croft BJ, Magarey RC, Allsopp PG, Cox MC, Willcox TG, Milford BJ, Wallis ES (2008) Sugarcane smut in Queensland: arrival and emergency response. Aust Plant Path 37:26–34
Enriquez-Obregon GA, Vazquez-Padron RI, Prieto-Samsonov DL, De la Riva GA, Selman-Housein G (1998) Herbicide-resistant sugarcane (Saccharum officinarum L.) plants by Agrobacterium-mediated transformation. Planta 206:20–27
Fitch MMM, Moore PH (1993) Long-term culture of embryogenic sugarcane callus. Plant Cell Tiss Org Cult 32:335–343
Fukui K (1983) Sequential occurrence of mutations in a growing rice callus. Theor Appl Genet 65:225–230
GalloMeagher M, Irvine JE (1996) Herbicide resistant transgenic sugarcane plants containing the bar gene. Crop Sci 36:1367–1374
Gambley RL, Bryant JD, Masel NP, Smith GR (1994) Cytokinin-enhanced regeneration of plants from microprojectile bombarded sugarcane meristematic tissue. Aust J Plant Physiol 21:603–612
Gandonou C, Errabii T, Abrini J, Idaomar M, Chibi F, Senhaji NS (2005) Effect of genotype on callus induction and plant regeneration from leaf explants of sugarcane (Saccharum sp.). Afr J Biotechnol 4:1250–1255
Garcia R, Cidade D, Castellar A, Lips A, Magioli C, Callado C, Mansur E (2007) In vitro morphogenesis patterns from shoot apices of sugar cane are determined by light and type of growth regulator. Plant Cell Tiss Org Cult 90:181–190
Gurel S, Gurel E, Kaur R, Wong J, Meng L, Tan HQ, Lemaux PG (2009) Efficient, reproducible Agrobacterium-mediated transformation of sorghum using heat treatment of immature embryos. Plant Cell Rep 28:429–444
Hamerli D, Birch RG (2010) Transgenic expression of trehalulose synthase results in high concentrations of the sucrose isomer trehalulose in mature stems of field-grown sugarcane. Plant Biotechnol J (In press)
Heinz DJ, Mee GWP (1969) Plant differentiation from callus tissue of Saccharum species. Crop Sci 9:346–348
Ho WJ, Vasil IK (1983) Somatic embryogenesis in sugarcane (Saccharum officinarum L.) I. The morphology and physiology of callus formation and the ontogeny of somatic embryos. Protoplasma 118:169–180
Iskandar HM, Simpson RS, Casu RE, Bonnett GD, Maclean DJ, Manners JM (2004) Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression. Plant Mol Biol Rep 22:325–337
James G (2004) Sugarcane. Blackwell Science Ltd, Oxford
Joyce P, Kuwahata M, Turner N, Lakshmanan P (2010) Selection system and co-cultivation medium are important determinants of Agrobacterium-mediated transformation of sugarcane. Plant Cell Rep 29:173–183
Kaeppler SM, Kaeppler HF, Rhee Y (2000) Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol 43:179–188
Kresovich S, McGee RE, Wadsworth SJ (1985) Comparisons of in vitro responsiveness of callus cultures derived from immature inflorescence and leaf base tissues of two interspecific hybrids of sugar cane (Saccharum spp.). Sugar Cane 6:8–10
Liu M-C (1983) Sugarcane. In: Sharp WR, Evans DA, Ammirato PV, Yamada Y (eds) Handbook of plant cell culture, vol 2, crop species. Macmillan, New York, pp 572–605
Luehrsen KR, Walbot V (1993) Firefly luciferase as a reporter for plant gene expression studies. Promega Notes 44:24–29
Mudge SR, Birch RG (1998) T-DNA tagging and characterisation of a novel meristem-specific promoter from tobacco. Aust J Plant Physiol 25:637–643
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Petrasovits LA, Purnell MP, Nielsen LK, Brumbley SM (2007) Production of polyhydroxybutyrate in sugarcane. Plant Biotechnol J 5:162–172
Raghuwanshi A, Birch RG (2010) Genetic transformation of sweet sorghum. Plant Cell Rep 29:997–1005
Ramgareeb S, Snyman SJ, van Antwerpen T, Rutherford RS (2010) Elimination of virus and rapid propagation of disease-free sugarcane (Saccharum spp. cultivar NCo376) using apical meristem culture. Plant Cell Tiss Org Cult 100:175–181
Rikiishi K, Matsuura T, Maekawa M, Takeda K (2008) Light control of shoot regeneration in callus cultures derived from barley (Hordeum vulgare L.) immature embryos. Breed Sci 58:129–135
Rossouw D, Kossmann J, Botha FC, Groenewald JH (2010) Reduced neutral invertase activity in the culm tissues of transgenic sugarcane plants results in a decrease in respiration and sucrose cycling and an increase in the sucrose to hexose ratio. Funct Plant Biol 37:22–31
Rudus I, Weiler EW, Kepczynska E (2009) Do stress-related phytohormones, abscisic acid and jasmonic acid play a role in the regulation of Medicago sativa L. somatic embryogenesis? Plant Growth Reg 59:159–169
Snyman SJ, Meyer GM, Richards JM, Haricharan N, Ramgareeb S, Huckett BI (2006) Refining the application of direct embryogenesis in sugarcane: effect of the developmental phase of leaf disc explants and the timing of DNA transfer on transformation efficiency. Plant Cell Rep 25:1016–1023
Sugiharto B, Ermawati N, Mori H, Aoki K, Yonekura-Sakakibara K, Yamaya T, Sugiyama T, Sakakibara H (2002) Identification and characterization of a gene encoding drought-inducible protein localizing in the bundle sheath cell of sugarcane. Plant Cell Physiol 43:350–354
Vickers JE, Grof CPL, Bonnett GD, Jackson PA, Morgan TE (2005) Effects of tissue culture, biolistic transformation, and introduction of PPO and SPS gene constructs on performance of sugarcane clones in the field. Aust J Agric Res 56:57–68
Wang ML, Goldstein C, Su W, Moore PH, Albert HH (2005) Production of biologically active GM-CSF in sugarcane: a secure biofactory. Transgenic Res 14:167–178
Wu L, Birch RG (2007) Doubled sugar content in sugarcane plants modified to produce a sucrose isomer. Plant Biotechnol J 5:109–117
Zhang L, Xu J, Birch RG (1999) Engineered detoxification confers resistance against a pathogenic bacterium. Nat Biotechnol 17:1021–1024
Zhang SZ, Yang BP, Feng CL, Chen RK, Luo JP, Cai WW, Liu FH (2006) Expression of the Grifola frondosa trehalose synthase gene and improvement of drought-tolerance in sugarcane (Saccharum officinarum L.). J Integr Plant Biol 48:453–459
Acknowledgments
We thank Kylie O’Neill, Sophie O’Neill, Kerrin Henderson, Alam Cheng, Lilian Chou, Isagani Arao, Majid Danesh and James Lee for outstanding technical assistance. We thank Terry Morgan and the CSR Technical Field Department team for generous assistance in provision of plant material. This research was supported through a collaboration between CSR Sugar Limited and The University of Queensland with funding also from the Sugar Research and Development Corporation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Lakshmanan.
A contribution to the Special Issue: Plant Biotechnology in Support of the Millennium Development Goals.
Rights and permissions
About this article
Cite this article
Basnayake, S.W.V., Moyle, R. & Birch, R.G. Embryogenic callus proliferation and regeneration conditions for genetic transformation of diverse sugarcane cultivars. Plant Cell Rep 30, 439–448 (2011). https://doi.org/10.1007/s00299-010-0927-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0927-4