Abstract
Endosperm transfer cells (ETC) mainly occur in the endosperm epithelial layer near the pedicel. They transport the nutrient unloaded by the maternal vascular tissue to filial tissues. Wall ingrowths of ETC can facilitate solute transportation. Sugar, especially glucose, is found to modulate the promoter activity of ZmMRP-1, a determinant of transfer cell-specific expression. The ZmMRP-1-encoded protein can transactivate the promoters of transfer cell-specific genes. Signalling and early events leading to wall ingrowth formation depend upon gene expression. Sucrose synthase and the cytoskeleton probably play a primary role in the wall ingrowth formation. The major solutes transferred by ETC are amino acids, sucrose, and monosaccharides, which is consistent with the expression of their transporters and transport-associated genes. In this paper, we review current opinions on the differentiation, wall ingrowth formation, and function of ETC in maize. According to the experimental materials provided by predecessors, we also give some speculations about the differentiation mechanisms of ETC and process of wall ingrowth formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endosperm develops from the central cell of the megagametophyte after introduction of the second male gamete into the diploid central cell. Of the three forms of endosperm in angiosperms, the nuclear type is prevalent in economically important species, including the cereals. Landmarks in nuclear endosperm development are the coenocytic, cellularization, differentiation, and maturation stages. The differentiated endosperm contains four major cell types: starchy endosperm, aleurone, transfer cells, and the cells of the embryo surrounding region. Specification of cell fates in the cereal endosperm appears to occur via positional signalling; cells in peripheral positions, except over the main vascular tissues, assume aleurone cell fate. Cells over the main vascular tissue become transfer cells and all interior cells become starchy endosperm cells (Olsen 2001). Endosperm transfer cells (ETC) are characterized by the presence of cell wall ingrowths, which increase the surface of the cellular membrane up to 22-fold and make ETC very efficient in the uptake of nutrients from adjacent maternal vascular tissue to the endosperm (Wang et al. 1994).
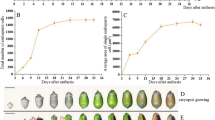
In maize, the endosperm epithelial cells facing the placentochalazal area differentiate into ETC (Thompson et al. 2001). The most basal transfer cells have more wall ingrowths than those that are successively deeper in the endosperm (Fig. 1C). When viewed in cross section, the wall ingrowths of contiguous cells tend to originate from common loci (Davis et al. 1990). The simplest morphological form of wall ingrowths is thin and rib-shaped projections deposited in the basal ETC of maize (Talbot et al. 2002). During the development of ETC, wall ingrowths go through the process of occurrence, growth, and decreasing (Fig. 1 A–D). Within the last few years, the application of molecular biological techniques has provided new evidence concerning the differentiation mechanisms of ETC in maize. New opinions on wall ingrowth formation and function of ETC have also been brought forward.
Maize endosperm transfer cells (ETC) at 6, 9, 21, and 30 days after pollination (×1,000, bar 10 μm). a There are only a few wall ingrowths in the first-layer ETC; b wall ingrowths of the first-layer ETC increase a little and wall ingrowths in cells of other inner layers also appear; c wall ingrowths of ETC increase a lot, especially the first layer; d wall ingrowths of ETC distort and decrease. a the first-layer ETC; WI wall ingrowths
Differentiation mechanisms of ETC in maize
Studies on transfer cells in the cotyledons of Vicia faba suggest the involvement of sugar uptake in the induction of transfer cell differentiation (Offler et al. 1997). Wardini et al. (2007) have concluded that transfer cells are induced by sucrose rapidly and that signalling and early events leading to wall ingrowth formation depend upon gene expression. Endosperm phenotypes resulting from mutation of the mn1 locus, which codes for INCW2, attend degeneration of the maternal placental tissues (Miller and Chourey 1992; Cheng et al. 1996). Since hydrolysis of sucrose to hexose is a key step in sugar import to the endosperm, the placental defects might result from reduced flux. The reduced grain filling1 (rgf1) mutant has a similar phenotype to mn1 but has apparently normal sugar levels, suggesting the lesion may be impaired sugar sensing. Both mn1 and rgf1 showed morphologically normal basal transfer cells but in the rgf1 mutant the levels of BETL-1 and BETL-2 proteins were decreased (Maitz et al. 2000). This did not occur in mn1 or several starch biosynthetic mutants. The basal transfer cell-specific INCW1 transcript shows sugar-dependent post-transcriptional regulation (Cheng et al. 1999). Thus, sugars influence gene expression in the basal transfer cells.
Molecular evidence suggests that positional cues demarcate the presumptive transfer cell region even prior to cellularization. The expression activity of the barley END1 gene and its ortholog from wheat were studied using in situ hybridization (Doan et al. 1996; Drea et al. 2005). In both barley and wheat END1 is expressed in the coenocyte above the nucellar projection during the free-nuclear division stage. After cellularization, END1 transcripts accumulate mainly in the ventral endosperm over the nucellar projection, but from 8 DAP a low level of expression can also be detected in the modified aleurone and the neighboring starchy endosperm (Doan et al. 1996). It was hypothesized that a signal from the placenta induces the expression of END1 and that this transcript may be anchored to the cytoskeleton in this region of the syncitial endosperm.
Rose et al. (1999) identified a family of genes that exhibit a highly conserved amino acid motif, SHAQK(Y/F)F, within the single MYB-related DNA binding domain. Members of this family of MYB-related proteins are known to be DNA-binding proteins (MybSt1; Baranowskij et al. 1994), and transcriptional regulators, such as LeMYB1 (Rose et al. 1999). Gómez et al. (2009) have shown that in epidermal cells committed to develop into aleurone cells, the ectopic expression of the transfer cell-specific transcriptional activator Myb-Related Protein-1 (MRP-1) is sufficient to temporarily transform them into transfer cells. These transformed cells acquire distinct transfer cell features, such as cell wall ingrowths and an elongated shape. In addition, they express a number of MRP-1 target genes presumably involved in defense. Gómez et al. (2009) have also shown that the expression of MRP-1 is needed to maintain the transfer cell phenotype. Later in development, an observed reduction in the ectopic expression of MRP-1 was followed by the reversion of the transformed cells, which then acquire aleurone cell features.
ZmMRP-1 also belongs to the subfamily of 1MYB transcription factors with a DNA binding domain containing a single MYB repeat. The gene is expressed specifically in ETC, accumulating at the basal pole of the endosperm coenocyte soon after fertilization. The ZmMRP-1-encoded protein is localized in nuclei and can transactivate the promoters of transfer cell-specific gene BETL-1 (Gómez et al. 2002) as well as the promoters of MEG1 (Gutiérrez-Marcos et al. 2004), and ZmTCRR-1 (Muñiz et al. 2006). A role for ZmMRP-1 in regulating transfer cell differentiation is proposed. Barrero et al. (2009) have investigated the signals controlling the expression of ZmMRP-1 through the production of transgenic lines of maize, Arabidopsis, tobacco, and barley containing ZmMRP-1 promoter: GUS reporter constructs. The GUS signal predominantly appeared in regions of active transport between source and sink tissues, including nematode-induced feeding structures, and at sites of vascular connection between the developing organs, and the main plant vasculature. In those cases, promoter induction was associated with the initial developmental stages of transport structures. Significantly, transfer cells also differentiated in these regions suggesting that, independent of species, location or morphological features, transfer cells might differentiate in a similar way under the influence of conserved induction signals. In planta and yeast experiments showed that the promoter activity is modulated by carbohydrates, glucose being the most effective inducer.
BETL-1 expression has been found to commence at 9 DAP, reach a maximum between 12 and 16 DAP, and decline after 16 DAP. The initial accumulation of the BETI polypeptide reaches a plateau by 16 DAP and declines thereafter, becoming undetectable by 20 DAP. This is an undersigned coincidence with the configuration changes of wall ingrowths in ETC (Fig. 1). Therefore, the BETL-I protein may play a role in the structural specialization of the transfer cells (Hueros et al. 1995). Maternally expressed gene1 (MEG1) shows a maternal parent-of-origin expression pattern during early stages of endosperm development but biallelic expression at later stages. MEG1 is exclusively expressed in the basal transfer region of the endosperm. The putatively processed MEG1 protein is glycosylated and subsequently localized to the labyrinthine ingrowths of the transfer cell walls. Hence, the discovery of a parent-of-origin gene expressed solely in the basal transfer region opens the door to epigenetic mechanisms operating in the endosperm to regulate certain aspects of nutrient trafficking from the maternal tissue into the developing seed (Gutiérrez-Marcos et al. 2004). ZmTCRR-1 codes for a member of the type-A response regulator class of proteins. The gene was found to be expressed exclusively in the endosperm transfer-cell layer 8–14 days after pollination, when transfer-cell differentiation is most active. The ZmTCRR-1 protein was detected not only in the transfer-cell layer, but also in the conductive tissue deep inside the endosperm, where there is no transcription of the gene. This suggests that two-component systems might be involved in intercellular signal transmission (Muñiz et al. 2006).
On the basis of above, the differentiation mechanisms of ETC in maize can be speculated as follows: (1) sucrose unloaded by phloem terminals is hydrolyzed into glucose and fructose; (2) glucose gets into the endosperm epithelial cells facing the pedicel and modulates the promoter activity of ZmMRP-1; (3) the ZmMRP-1 encodes proteins that transactivate the promoters of gene BETL-1, MEG1, and ZmTCRR-1; (4) The proteins encoded by gene BETL-1 and MEG1 (or/and other genes) probably join in the formation of wall ingrowths and the proteins encoded by gene ZmTCRR-1 join in the intracellular signal transmission. The proteins encoded by these genes are probably related to the gradual changes from the first-layer ETC to the starchy endosperm cells.
Formation of wall ingrowths in maize ETC
Wall ingrowths are a distinguishing feature of transfer cells and serve to amplify the plasma membrane surface area available for solute transport. Morphologically, two categories of ingrowths are recognized: reticulate and flange. Reticulate-type wall ingrowths are characterized by the deposition of small papillae that emerge from the underlying wall at discrete but apparently random loci, then branch, and interconnect to form a complex labyrinth of variable morphology. In comparison, flange-type ingrowths are deposited as curvilinear ribs of wall material that remain in contact with the underlying wall along their length and become variously elaborate in different transfer cell types (Talbot et al. 2002). A possible alternative mechanism for controlling the morphology of wall ingrowths is that precursor materials are locally incorporated into wall ingrowths by self-assembly (DeWitt et al. 1999). Self-assembly is a process by which molecules spontaneously form ordered structures and is influenced by specific interactions between these molecules (Jarvis 1992). The intrinsic molecular properties of cellulose may act as a “template” for the deposition of other wall polysaccharides, and these components partly organize themselves within the wall by self-assembly (Jarvis 1992, Taylor and Haigler 1993). If such a mechanism operates in the construction of wall ingrowths in transfer cells, the morphology of wall ingrowths would depend on the proper timing and position of wall precursor deposition and packing arrangements of these components within the wall (DeWitt et al. 1999).
Vaughn et al. (2007) demonstrated that wall ingrowths in the Vicia faba epidermal transfer cells are rich in cellulose, hemicellulose, pectin, callose, and arabinogalactan proteins similar to the primary cell wall. The cellulose microfibrils of flange wall ingrowths are aligned parallel along the length of the ingrowth (Talbot et al. 2007). So enzymes for cellulose synthesis are important to wall ingrowth formation. A role for sucrose synthase in wall ingrowth deposition is supported by immunocytochemical evidence showing accumulation of SuSy in coat TCs of developing cotton seed (Ruan et al. 1997), and lack of normal TC development in these cells in SuSy anti-sense lines (Ruan et al. 2003). A tip-growing mechanism for building wall ingrowths presumably requires targeted delivery of cell wall vesicles either directly or to the vicinity of the ingrowth. The actin cytoskeleton might perform such roles, possibly by directed assembly of filaments targeted to ingrowth loci, or by restricting exocytosis of vesicles to discrete domains of the plasma membrane by causing localized depolymerization of a dense cortical actin meshwork (Offler et al. 2003).
The simplest morphological form of wall ingrowths deposited in the ETC in maize is thin, rib-shaped projections. In these cells, extensive deposition of wall material occurs in the basal end of the cell. Spaces between the ribs of wall material appear to become progressively cross-linked and fused towards the base of the cell. This process apparently occurs by broadening of the ribs and the establishment of small lateral protrusions connecting adjacent ribs. These lateral protrusions resemble those observed in reticulate-type wall ingrowths and, in some instances, their formation appears to be spatially coordinated. In mature cells, most of the cell lumen are filled with wall material, with anastomosed ribs of wall ingrowth in the upper portion of the cell and a dense network of wall material in the basal portion of the cell (Talbot et al. 2002).
On the basis of the above, the process of wall ingrowth formation in maize ETC can be speculated as follows: (1) Bundling of cortical microtubules defines a plasma membrane domain where exocytosis of vesicles carrying cellulose synthesizing enzymes and matrix polysaccharides occurs (McCurdy et al. 2008). (2) Bundled cortical microtubules determine localized synthesis of cellulose microfibrils and exocytosis of non-cellulosic polysaccharides within these domains (McCurdy et al. 2008). Lining the bundles of cortical microtubules, many short wall ingrowths form (Fig. 2a). The conplane wall ingrowths elongate, branch and fuse, and become more and more flange-like (Fig. 2b). (3) Bundles of cortical microtubules are positioned between ribs of wall material, developed wall ingrowths (McCurdy et al. 2008). The mature state of the wall ingrowths is ribs of wall material with a dense network of wall material between them (Fig. 2c).
a, b Wall in growths of maize ETC under transmission electron microscope (×5,800, bar 2 μm); c wall in growths of maize ETC under scanning electron microscope (×19,200, bar 2 μm). a Wall ingrowths are short; b wall in growths elongate, brunch, and fuse; c wall ingrowths are ribs of wall material with dense network of wall material between them. WI wall ingrowths
Function of ETC in maize
In secretion or absorption processes, solutes are transported across the plasmalemma between the symplastic and apoplastic compartments. For this purpose, certain plant cells have developed a specialised transfer cell morphology characterized by wall ingrowths, which amplify the associated plasmalemma surface area up to 20-fold. In maize, the vascular terminals form a cup-shaped cushion at the base of the endosperm (Fig. 3b). At least ten layers of crushed, dead maternal cells, the placentochalazal zone (Kladnick et al. 2004) extend between the vascular terminals, and the endosperm (Fig. 3a, c). The endosperm epithelial cells facing the placentochalazal area differentiate into ETC (Thompson et al. 2001). The nutrient unloaded by the vascular terminals gets through the placentochalazal cells and enters the apoplastic compartment (Felker and Shannon. 1980). Finally, the nutrient is transported into the filial tissues by the ETC (Davis et al. 1990).
a Placentochalazal cells of maize caryopsis at 14 days after pollination under transmission electron microscope (×5,800, bar 2 μm); b the base of maize endosperm under fluorescence microscope at 17 days after pollination (×100, bar 50 μm); c the base of maize endosperm under light microscope at 9 days after pollination (×200, bar 30 μm). a The placentochalazal cells are crushed; b vascular terminals at the base of maize endosperm form a cup-shaped cushion; c there is the placentochalazal zone between vascular terminals and endosperm transfer cells. En endosperm, ETC endosperm transfer cells, VT vascular terminals, Pe pericarp, PC placentochalazal cells
The major solutes transferred are amino acids, sucrose, and monosaccharides. Expression of invertase and monosaccharide transporters early in both cereal and legume seed development orchestrates the distribution of free sugars which play an important role in regulating transfer cell function and determining final endosperm or embryo cell number (Thompson et al. 2001). Transport-associated genes expressed in ETC encode transporters catalyzing proton-coupled cotransport, such as HvSUT1 (Weschke et al. 2000). Expression of these genes confirms the specificity of the developmental stage selected for analysis (8 DAF). Characteristic for this developmental stage is the strong increase of HvSUT1 activity (Weschke et al. 2000) as well as the switch from high to low hexose to sucrose ratios in the developing endosperm (Weschke et al. 2003). Furthermore, genes encoding storage proteins are strongly up-regulated at the transcriptional level (Sreenivasulu et al. 2004). High mRNA levels of putative amino permeases in ETC at the beginning of protein accumulation suggest a role for the uptake of amino acids into the endosperm (Thiel et al. 2008). Charlton et al. (1995) suggested that seed abortion resulting from crosses between diploid maize lines with 4 N male parents is attributable to a complete suppression of the basal endosperm transfer layer (BETL) development at an early stage of seed development in such crosses. In another study, Maitz et al. (2000) reported a reduced grain filling (rgf1) locus that is associated with reduced expression of BETL markers and a loss of 70% seed weight at maturity. Similarly, the globby1 (Costa et al. 2003), empty pericarp4 (emp4; Gutierrez-Marcos et al. 2007), and baseless1 (Gutierrez-Marcos et al. 2006) mutants exhibit abnormal BETL at an early stage of seed development and, ultimately, the aborted seed lethal phenotypes.
Future prospects
ETC play an important part in nutrient transport between the vascular tissue and the endosperm in cereal. Lots of research on the ETC has been done from the morphological observation to the molecular-level investigation. However, there are still many unknown or uncertain features of the ETC.
(1) The ZmMRP-1-encoded protein is localized in nuclei and can transactivate the promoters of transfer cell-specific gene BETL-1 (Gómez et al. 2002) as well as the promoters of MEG1 (Gutiérrez-Marcos et al. 2004), and ZmTCRR-1 (Muñiz et al. 2006). ZmMRP-1, a determinant of transfer cell-specific expression (Gómez et al. 2002), is induced most effectively by glucose (Barrero et al. 2009). However, how glucose induces ETC differentiation is still not clear.
(2) Several groups of genes have been found to be expressed only or mainly in ETC, such as BETL1–4 (Hueros G 1995,1999), MEG1 (Gutiérrez-Marcos et al. 2004), and ZmTCRR-1 (Muñiz et al. 2006). However, functions have only been assigned to just a few of the genes, and conclusive evidence elucidating which genes direct wall ingrowth formation is lacking.
(3) ZmMRPI-1 and ZmMRPI-2, belonging to the C2H2 zinc finger protein family, interact with ZmMRP-1 and modulate its activity on transfer cell-specific promoters (Royo et al. 2009). This shows us a new pathway of transfer cell differentiation regulation. So further work needs to be done in this field.
(4) Wall ingrowths lie at one pole of the ETC and the most basal transfer cells have more wall ingrowths than those that are successively deeper in the endosperm. The peripheral cells at the base of the endosperm differentiate into transfer cells instead of aleurone cells. The exact mechanisms for that above need more molecular-level evidences to illuminate.
(5) DeWitt et al. (1999) described a possible alternative mechanism for controlling the morphology of wall ingrowths in which precursor materials are locally incorporated into wall ingrowths by self-assembly. McCurdy et al. (2008) suggested that sucrose synthase and the cytoskeleton play a primary role in the wall ingrowth formation and have given the models. However, the exact procedure of wall ingrowth formation needs to be clarified.
(6) ETC are characterized by the presence of cell wall ingrowths, which increase the surface of the cellular membrane up to 22-fold and make ETC very efficient in the uptake of nutrients from adjacent maternal vascular tissue to the endosperm (Wang et al. 1994). The molecular-level evidence proving the function of ETC has been provided. However, the exact way that the ETC transport the amino acids, sucrose, and monosaccharides is still uncertain.
Abbreviations
- TC:
-
Transfer cells
- ETC:
-
Endosperm transfer cells
- BETL:
-
Basal endosperm transfer layer
- DAP:
-
Days after pollination
References
Baranowskij N, Frohberg C, Prat S, Willmitzer L (1994) A novel DNA binding protein with homology to MYB oncoproteins containing only one repeat can function as a transcriptional activator. EMBO J 13:5383–5392
Barrero C, Royo J, Grijota-Martinez C, Faye C, Paul W, Sanz S, Steinbiss HH, Hueros G (2009) The promoter of ZmMRP-1, a maize transfer cell-specific transcriptional activator, is induced at solute exchange surfaces and responds to transport demands. Planta 229:235–247
Charlton WL, Keen CL, Merriman C, Lynch P, Greenland AJ, Dickinson HG (1995) Endosperm development in Zea mays; implication of gametic imprinting and paternal excess in regulation of transfer layer development. Development 121:3089–3097
Cheng WH, Taliercio EW, Chourey PS (1996) The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 8:971–983
Cheng WH, Taliercio EW, Chourey PS (1999) Sugars modulate an unusual mode of control of the cell-wall invertase gene (Incw1) through its 30 untranslated region in a cell suspension culture of maize. Proc Natl Acad Sci USA 96:10512–10517
Costa LM, Gutiérrez-Marcos JF, Brutnell TP, Greenland AJ, Dickinson HG (2003) The globby1-1 (glo1-1) mutation disrupts nuclear and cell division in the developing maize seed causing alterations in endosperm cell fate and tissue differentiation. Development 130:5009–5017
Davis RW, Smith JD, Cobb BG (1990) A light and electron microscope investigation of the transfer cell region of maize caryopses. Can J Bot 68:471–479
DeWitt G, Richards J, Mohnen D, Jones AM (1999) Comparative compositional analysis of walls with two different morphologies: archetypical versus transfer-cell like. Protoplasma 209:238–245
Doan DNP, Linnestad C, Olsen OA (1996) Isolation of molecular markers from the barley endosperm coenocyte and the surrounding nucellus cell layers. Plant Mol Biol 31:877–886
Drea S, Leader DJ, Arnold BC, Shaw P, Dolan L, Doonan JH (2005) Systematic spatial analysis of gene expression during wheat caryopsis development. Plant Cell 17:2172–2185
Felker FC, Shannon JC (1980) Movement of 14C-labeled assimilates into kernels of Zea mays L III. An anatomical examination and microautoradiographic study of assimilate transfer. Plant Physiol 65:864–870
Gómez E, Royo J, Guo Y, Thompson R, Hueros G (2002) Establishment of cereal endosperm expression domains: identification and properties of a maize transfer cell-specific transcription factor, ZmMRP-1. Plant Cell 14:599–610
Gómez E, Royo J, Muñiz LM, Sellam O, Paul W, Gerentes D, Barrero C, López M, Perez P, Hueros G (2009) The maize transcription factor myb-related protein-1 is a key regulator of the differentiation of transfer cells. The Plant Cell 21:2022–2035
Gutiérrez-Marcos JF, Costa LM, Biderre-Petit C, Khbaya B, O’Sullivan DM, Wormald M, Perez P, Dickinson HG (2004) Maternally expressed gene1 is a novel maize endosperm transfer cell-specific gene with a maternal parent-of-origin pattern of expression. Plant Cell 16:1288–1301
Gutiérrez-Marcos JF, Costa LM, Evans MMS (2006) Maternal gametophytic baseless1 is required for development of the central cell and early endosperm patterning in maize (Zea mays). Genetics 174:317–329
Gutiérrez-Marcos JF, Dal Prà M, Giulini A, Costa LM, Gavazzi G, Cordelier S, Sellam O, Tatout C, Paul W, Perez P, Dickinson HG, Consonni G (2007) empty pericarp4 encodes a mitochondrion-targeted pentatricopeptide repeat protein necessary for seed development and plant growth in maize. Plant Cell 19:196–210
Hueros G, Varotto S, Salamini F, Thompson RD (1995) Molecular characterization of Bet1, a gene expressed in the endosperm transfer cells of maize. Plant Cell 7:747–757
Hueros G, Royo J, Maitz M, Salamini F, Thompson RD (1999) Evidence for factors regulating transfer cell-specific expression in maize endosperm. Plant Mol Biol 41:403–414
Jarvis M (1992) Self-assembly of plant cell walls. Plant Cell Environ 15:1–5
Kladnick A, Chamusco K, Dermastia M, Chourey P (2004) Evidence of programmed cell death in post-phloem transport cells of the maternal pedicel tissue in developing caryopsis of maize. Plant Physiol 136:3572–3581
Maitz M, Santandrea G, Zhang Z, Lal S, Hannah LC, Salamini F, Thompson RD (2000) rgf1, a mutation reducing grain filling in maize through effects on basal endosperm and pedicel development. Plant J 23:29–42
McCurdy DW, Patrick JW, Offler CE (2008) Wall ingrowth formation in transfer cells: novel examples of localized wall deposition in plant cells. Curr Opin Plant Biol 11:653–661
Miller ME, Chourey PS (1992) The maize invertase-deficient miniature-1 seed mutation is associated with aberrant pedicel and endosperm development. Plant Cell 4:297–305
Muñiz LM, Royo J, Gómez E, Barrero C, Bergareche D, Hueros G (2006) The maize transfer cell-specific type-A response regulator ZmTCRR-1 appears to be involved in intercellular signalling. Plant J 48:17–27
Offler CE, Liet E, Sutton EG (1997) Transfer cell induction in cotyledons of Vicia faba L. Protoplasma 200:51–64
Offler CE, McCurdy DW, Patrick JW, Talbot MJ (2003) Transfer cells: cells specialized for a special purpose. Annu Rev Plant Biol 54:431–454
Olsen OA (2001) Endosperm development: cellularization and cell fate specification. Annu Rev Plant Physiol Plant Mol Biol 52:233–267
Rose A, Meier I, Wienand U (1999) The tomato I-box binding factor LeMYBI is a member of a novel class of Myb-like proteins. Plant J. 20:641–652
Royo J, Gómez E, Barrero C, Muñiz LM, Sanz Y, Hueros G (2009) Transcriptional activation of the maize endosperm transfer cell-specific gene BETL1 by ZmMRP-1 is enhanced by two C2H2 zinc finger-containing proteins. Planta 230:807–818
Ruan YL, Chourey PS, Delmer DP, Perez-Grau L (1997) The differential expression of sucrose synthase in relation to diverse patterns of carbon partitioning in developing cotton seed. Plant Physiol 115:375–385
Ruan YL, Llewellyn DJ, Furbank RT (2003) Suppression of sucrose synthase gene expression represses cotton fiber cell initiation, elongation, and seed development. Plant Cell 15:952–964
Sreenivasulu N, Altschmied L, Radchuk V, Gubatz S, Wobus U, Weschke W (2004) Transcript profiles and deduced changes of metabolic pathways in maternal and filial tissues of developing barley grains. Plant J 37:539–553
Talbot MJ, Offler CE, McCurdy DW (2002) Transfer cell wall architecture: a contribution towards understanding localized wall deposition. Protoplasma 219:197–209
Talbot MJ, Wasteneys G, McCurdy DW, Offler CE (2007) Deposition patterns of cellulose microfibrils in flange wall ingrowths of transfer cells indicate clear parallels with those of secondary wall thickenings. Funct Plant Biol 34:307–313
Taylor JG, Haigler CH (1993) Patterned secondary cell-wall assembly occurs in a self-perpetuating cascade. Acta Bot Neerl 42:153–163
Thiel J, Weier D, Sreenivasulu N, Strickert M, Weichert N, Melzer M, Czauderna T, Wobus U, Weber H, Weschke W (2008) Different hormonal regulation of cellular differentiation and function in nucellar projection and endosperm transfer cells: a microdissection-based transcriptome study of young barley grains. Plant Physiol 148:1436–1452
Thompson RD, Hueros G, Becker H, Maitz M (2001) Development and functions of seed transfer cells. Plant Sci 160:775–783
Vaughn KC, Talbot MJ, Offler CE, McCurdy DW (2007) Wall ingrowths in epidermal transfer cells of Vicia faba cotyledons are modified primary walls marked by localized accumulations of arabinogalactan proteins. J Plant Cell Physiol 48:159–168
Wang HL, Offler CE, Patrick JW (1994) Nucellar projection transfer cells in the developing wheat grain. Protoplasma 182:39–52
Wardini T, Wang XD, Offler CE, Patrick JW (2007) Induction of wall ingrowths of transfer cells occurs rapidly and depends upon gene expression in cotyledons of developing Vicia faba seeds. Protoplasma 231:15–23
Weschke W, Panitz R, Sauer N, Wang Q, Neubohn B, Weber H, Wobus U (2000) Sucrose transport into developing barley seeds: molecular characterization of two transporters and implications for seed development and starch accumulation. Plant J 21:455–467
Weschke W, Panitz R, Gubatz S, Wang Q, Radchuk R, Weber H, Wobus U (2003) The role of invertases and hexose transporters in controlling sugar ratios in maternal and filial tissues of barley caryopses during early development. Plant J 33:395–411
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Reski.
Rights and permissions
About this article
Cite this article
Zheng, Y., Wang, Z. Current opinions on endosperm transfer cells in maize. Plant Cell Rep 29, 935–942 (2010). https://doi.org/10.1007/s00299-010-0891-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0891-z