Abstract
Oxidative processes involved in cryopreservation protocols may be responsible for the reduced viability of tissues after liquid nitrogen exposure. Antioxidants that counteract these reactions should improve recovery. This study focused on oxidative lipid injury and the effects of exogenous vitamin E (tocopherol, Vit E) and vitamin C (ascorbic acid, Vit C) treatments on regrowth at four critical steps of the plant vitrification solution number 2 (PVS2) vitrification cryopreservation technique; pretreatment, loading, rinsing, and regrowth. Initial experiments showed that Vit E at 11–15 mM significantly increased regrowth (P < 0.001) when added at any of the four steps. There was significantly more malondialdehyde (MDA), a lipid peroxidation product, at each of the steps than in fresh untreated shoot tips. Vit E uptake was assayed at each step and showed significantly more α- and γ-tocopherols in treated shoots than those without Vit E. Vit E added at each step significantly reduced MDA formation and improved shoot regrowth. Vit C (0.14–0.58 mM) also significantly improved regrowth of shoot tips at each step compared to the controls. Regrowth medium with high iron concentrations and Vit C decreased recovery. However, in iron-free medium, Vit C significantly improved recovery. Treatments with Vit E (11 mM) and Vit C (0.14 mM) combined were not significantly better than Vit C alone. We recommend adding Vit C (0.28 mM) to the pretreatment medium, the loading solution or the rinse solution in the PVS2 vitrification protocol. This is the first report of the application of vitamins for improving cryopreservation of plant tissues by minimizing oxidative damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryopreservation is the use of ultra-low temperature, usually liquid nitrogen (LN) (−196°C) to store biological materials such that they remain capable of regrowth upon rewarming. It provides an option for cost effective long-term preservation of vegetatively propagated plants. Cryopreservation is applicable for long-term storage of many plant species, animal cells and microbes but regrowth variation among diverse genotypes is still a problem. Techniques used for cryopreservation impose stresses on cells and tissues that can cause damage, resulting in reduced growth or death upon rewarming. The stresses associated with vitrification-based techniques arise from excision, osmotic injury, desiccation and changes in temperature. These factors lead to the production of reactive oxygen species (ROS) that cause oxidative damage. Antioxidants are compounds that arrest ROS before oxidative damage occurs (Asada 1992). When ROS production exceeds the capacity of antioxidants in cell systems, it results in physiological decline. ROS-induced stress is thought to be a fundamental cause of cell death in cryopreserved samples (Benson 1990). ROS, including superoxide radicals, hydrogen peroxide, hydroxyl radicals and singlet oxygen, are formed as a result of partial reductions of ground-state oxygen. They are the primary ROS associated with oxidative damage in plant systems (Halliwell 2006). Damage caused by ROS includes lipid peroxidation, protein denaturation, alterations in nucleic acids, membrane disruption, severe cellular disorder (Halliwell 2006; Halliwell and Whiteman 2004) and premature senescence (Thompson et al. 1987). Hydroxyl radical is the most highly reactive of all the species and can be produced as a result of the Fenton reaction involving hydrogen peroxide in the presence of metal ions (Evans et al. 2004; Halliwell and Gutteridge 1984). Damage to the cellular membrane is critical to cell survival. Damage resulting from phospholipid degradation was observed in membranes of poplar (Populus euramenricana cv. Gelrica) cortical tissues subjected to subzero temperatures during early cryobiological studies (Heber 1968; Yoshida and Sakai 1974).
Lipids are the major class of biomolecules targeted by ROS in a membrane. Lipid peroxidation is of concern because it affects the integrity of membrane structure and alters its functions, leading to cell death. The main lipids targeted by ROS are the polyunsaturated fatty acids (PUFA) (Esterbauer et al. 1991). PUFA make up 50–90% of membrane lipids (Douce et al. 1973). When PUFA are oxidized, reactive aldehydes are formed as by-products, some of which cause damage. Malondialdehyde (MDA) is a breakdown product of PUFA (Davey et al. 2005; Yamauchi et al. 2008). MDA is a highly reactive and toxic molecule. Its attachment to nucleic acids and proteins causes modifications that disrupt biological functions (Del Rio et al. 2005; Esterbauer et al. 1991; Yamauchi et al. 2008). The degree of lipid peroxidation can be estimated by the amount of MDA present in tissues (Davey et al. 2005; Esterbauer and Cheeseman 1990). High levels of intracellular antioxidants were detected in Arabidopsis thaliana tissues during studies on oxidative stress induced by low temperature (8°C) (Havaux et al. 2005). Johnston et al. (2007) found that an increased ratio of total phenolics to ROS in shoot cultures of R. nigrum correlated with significantly improved regrowth following cryopreservation.
Vitamin E (Vit E) is a common term used for tocopherols and tocotrienols. It is an important antioxidant that is directly involved in scavenging oxygen free radicals and quenching lipid peroxidation chain reactions that occur during oxidation reactions with PUFA (Sattler et al. 2004). Vit E reactions result in the formation of tocopheroxyl radicals that react with other antioxidants to regenerate the active molecule (Carelli et al. 2005; Liebler 1993). Vitamin C (ascorbic acid, Vit C) is a well-known water soluble antioxidant that works to regenerate Vit E from its radical form (Kamal-Eldin and Appelqvist 1996; Leung et al. 1981). Vit C also has direct reactivity with hydrogen peroxide, superoxide, hydroxyl radical and lipid hydroperoxides (Shao et al. 2008). Vit C can be regenerated from its radical form via the Ascorbate–GSH cycle that uses NADPH generated from the pentose phosphate pathway as a reducing agent (Sgherri and Navari-Izzo 1995).
Several laboratories have successfully cryopreserved Rubus genotypes using a variety of cryopreservation techniques (Popov et al. 2006; Reed 2008). Recovery of shoot tips cryopreserved using these techniques on diverse genotypes varied from 15 to 100% and often was genotype dependent. Cryopreservation using plant vitrification solution number 2 (PVS2) (Sakai et al. 1990) was modified for Rubus (Gupta and Reed 2006). The steps of the PVS2 vitrification technique include: excision, pretreatment, loading solution, cryoprotectant, cooling, rewarming, rinsing and regrowth. Each of these steps presents the possibility of oxidative stress because physical damage and osmotic stress are often involved. For example, oxidative burst and ROS production were observed during embryonic axis excision and reduction of cellular water content in Castanea sativa (Roach et al. 2008). The only report of antioxidant addition during cryopreservation involved glutathione. Adding glutathione at four steps; pretreatment, loading, cryoprotectant and regrowth medium during the PVS2 vitrification technique doubled shoot recovery of cryopreserved Citrus shoot tips (Wang and Deng 2004). There is no experimental evidence available, to the best of our knowledge, that directly shows the effect of antioxidant vitamins such as Vit C and Vit E on recovery from cryopreservation or the relationship between oxidative damage caused by ROS and the role of Vit C or Vit E in reducing oxidative damage and improving regrowth of shoot tips following cryopreservation. Vit E and Vit C were chosen because of their free radical scavenging capabilities.
The goals of this study were (1) to evaluate if exogenous Vit C and Vit E added at four critical steps of the PVS2 vitrification technique can significantly improve regrowth following cryopreservation; (2) to quantify MDA, a lipid peroxidation product formed during oxidative stress, at each step and (3) to determine the relationship between lipid peroxidation and shoot-tip regrowth following cryopreservation.
Materials and methods
Plant propagation
Shoots of blackberry cultivars Chehalem and Hull Thornless were selected from the in vitro collections of the USDA-ARS, NCGR, Corvallis, OR, USA. These cultivars were chosen because they had stable but moderate regrowth of 40–50% following cryopreservation by PVS2 vitrification. This allows for improvement with the antioxidant treatments. The shoots were transferred at 3-week intervals on RUB medium (Reed 1990), consisting of MS (Murashige and Skoog 1962) mineral salts, vitamins, with doubled the concentration of iron (FeSO4 · 7H2O with Na2EDTA · 2H2O), 1 mg 1−1 N 6-benzyladenine (BA), 0.1 mg l−1 gibberellic acid (GA3) (Sigma-Aldrich Co., St Louis, MO), 0.1 mg 1−1 indole-3-butyric acid (IBA) (Sigma-Aldrich Co., St Louis, MO), 3.5 g l−1 agar (Difco, Detroit, MI), 1.45 g l−1 Gelrite (Phyto Technology Lab., Shawnee Mission, KS) and 30 g l−1 sucrose at pH 5.7, dispensed into Magenta GA7 boxes (Magenta Corp., Chicago, IL). The shoots were grown at 25°C with 16 h light/8 h dark photoperiod (80 μE m−2 s−1) and a 3-week subculture interval. Regrowth medium following cryopreservation was RUB medium but without IBA (Chang and Reed 1997) and on reduced agar (3.0 g l−1) and Gelrite (1.2 g l−1), under low light (26–30 μE). For the final Vit C experiment iron was eliminated from the regrowth medium.
Cold acclimation
After 3 weeks in the growth room, shoots were transferred to a cold acclimation (CA) chamber for 2 weeks to induce cold tolerance. The CA conditions were 22°C with 8 h light (10 μE m−2 s−1) and −1°C with 16 h dark (Reed 1988).
PVS2 vitrification
The vitrification procedure for blackberry shoot tips was previously described (Gupta and Reed 2006). Shoot tips (0.8–1 mm) from 2-week CA shoots were pretreated for 48 h on MS agar plates containing 5% (v/v) dimethyl sulfoxide (Sigma-Aldrich Co., St Louis, MO), with 3.5 g agar and 1.75 g l−1 Gelrite, under CA conditions. Shoot tips were transferred into 1.0 ml vials (Nunc, Roskilde, Denmark) and treated with 1 ml loading solution [2 M glycerol and 0.4 M sucrose solution (v/v), pH 5.8] for 20 min at 25°C. The loading solution was removed and 1 ml PVS2 cryoprotectant solution [30% glycerol, 15% ethylene glycol, 15% DMSO in liquid MS medium with 0.8 M sucrose (v/v) (Sakai et al. 1990)] was added and held for 20 min at 25°C. At this point five control shoot tips of each cultivar were rinsed three times in liquid MS medium containing 1.2 M sucrose and planted on regrowth medium. Twenty shoot tips of each cultivar in 1 ml PVS2 solution were plunged into LN and held for 30–45 min, rewarmed in 45°C water for 1 min and 25°C water for 1 min, rinsed and planted on regrowth medium in 24 well plates.
Antioxidants
The Vit E used was AQUA-E™ (Yasoo Health Inc., Morrisville, NC), a water soluble mixture containing tocopherols, tocotrienols and α-tocopheryl polyethylene glycol 1000 succinate (TPGS), provided as a gift from Dr. Andreas M. Papas. AQUA-E was added at 0, 5, 10, 15, and 20 mM. Vit E analysis found these concentrations to be approximately 0, 4, 7, 11 and 15 mM in our samples. Vit C, ascorbic acid (Sigma Chemical Co., St Louis, MO), was tested at 0, 0.14, 0.28, 0.43 and 0.58 mM. Cryopreservation testing involved separate treatments with exogenous Vit E or Vit C added at one of the following steps at a time: the pretreatment medium (48 h), loading solution (20 min), rinse solution (~5 min) or regrowth medium (6 weeks).
Application
Vitamin E
The effect of the addition of Vit E, separately to each of the four critical steps, was examined. In the pretreatment medium, DMSO is added after autoclaving, so the Vit E was added to the DMSO (v/v) and this mixture was added to the culture medium. Vit E was added in the loading and rinse solutions during solution preparation and before adjusting pH (5.7), then filter sterilized (0.45 μm Supor® membrane, PAL Corporation, Cornwall, UK). Vit E was filter sterilized and added to regrowth medium after autoclaving. Vit E was added at one step only for each experiment (pretreatment, loading, rinsing or regrowth). In one additional experiment Vit E was added in both the pretreatment and rinsing steps.
Vitamin C
The effect of Vit C was examined separately at each of the four critical steps. Vit C was added into the culture medium and solutions before the pH was adjusted to 5.7. The pretreatment and regrowth media were autoclaved while the loading and rinsing solutions were filter sterilized using membrane filters (0.45 μm, 150-ml analytical filter unit, NALGENE, Rochester, NY). Vit C could not be added to media after autoclaving because it greatly altered the medium pH. Vit C (0.14–0.58 mM) was initially added in standard medium with iron at each of the four steps (at only one step per experiment). All iron was excluded from the regrowth medium in a separate experiment. In an additional experiment, Vit C was added at all four steps but with exclusion of iron from all the media and solutions. Shoot tips from experiments without iron in the media and solutions were transferred after 14 days onto medium with iron but no Vit C.
Antioxidant combination
At each step, Vit E (11 mM) and Vit C (0.14 mM) were added separately and in combination (Vit E + Vit C) to test the antioxidants’ synergistic effects on regrowth. For the combined antioxidants study, we did not exclude iron from the regrowth medium or solutions.
Relationship between lipid peroxidation, Vit E and regrowth
Shoot tips treated with and without added Vit E (15 mM) at the pretreatment, loading and rinsing steps were cryopreserved (20 shoot tips per treatment) and planted on RUB regrowth medium. Five shoot tips per treatment (treated with all the solutions but not cryopreserved) were also planted on the regrowth medium. Ten shoot tips per treatment were assayed for Vit E and MDA. Vit E was added one step at a time.
Quantification of MDA in shoot tips
Ten shoot tips (10–30 mg fresh weight) of ‘Chehalem’ were collected from fresh shoots in the growth room (control) and from those treated with and without added Vit E at each step of cryopreservation. These were surface cleaned thoroughly with distilled water and blotted dry with filter papers. The shoot tips were placed in 1 ml cryotubes separated by treatment and transported in LN. MDA was measured by the method of Davey et al. (2005). Briefly, shoot tips were added to 100 μl of 1% butylated hydroxytoluene (BHT) stabilized with 5% (w/v) meta phosphoric acid (MPA) (Sigma, St Louis, MO) in a 2 ml microcentrifuge tube. To this solution were added 50 μl each of 1% thiobarbituric acid (TBA) in 50 mM NaOH and 25% (v/v) HCl at pH 1.0. The reaction mixture was heated to 95°C for 60 min. The MDA (TBA)2 adduct was partitioned out of the mixture with 150 μl of n-butanol. Following centrifugation, 10 μl of the supernatant was injected onto a Shimadzu HPLC reverse-phase system including an autosampler and a Beckman 5 μm ODS, 4.6 × 250 mm column. The MDA (TBA)2 adduct was eluted using an isocratic mobile phase consisting of 50% methanol and 50% of 25 mM phosphate buffer at pH 6.5 and at a flow rate of 1.5 ml/min. MDA was detected by fluorescence at excitation 532 nm and emission 553 nm. Quantitation was done using an external standard of 1, 1, 3, 3-tetraethoxypropane (Sigma, St Louis, MO) prepared using the same method as the samples.
Quantification of shoot-tip tocopherol content
Ten shoot tips each (~30 mg of fresh weight) of ‘Chehalem’, from both untreated (control) and those with and without added Vit E (15 mM), at the pretreatment, loading and rinsing steps of the PVS2 vitrification technique were prepared for analysis. Also analyzed were shoot tips of field-grown plants of ‘Chehalem’. For the assay of α- and γ-tocopherols, a modification of the method by Podda et al. (1996) was used. Briefly, the shoot tips were saponified with alcoholic KOH, extracted with hexane, dried under nitrogen, resuspended in 1:1 ethanol:methanol, then injected into a Shimadzu HPLC system (Columbia, MD). The HPLC system consisted of a Shimadzu LC-10ADvp controller, and a SIL-10ADvp auto injector with a 50 μl sample loop. Tocopherols were detected using an LC-4B amperometric electrochemical detector (Bioanalytical Systems Inc., West Lafayette, IN) with a glassy carbon working electrode, and a silver chloride reference electrode. The column used was a Waters Spherosorb ODS2 C-18 column, 100 × 4.6 mm, 3 μm particle size with a Waters Spherisorb ODS precolumn, 10 × 4.6 mm, 5 μm. An isocratic mobile phase delivery system was used, with a total run time of 6 min. The mobile phase used was 99:1 (v:v) methanol:water containing 0.1% (w:v) lithium perchlorate. The electrochemical detector was in the oxidizing mode, potential 600 mV, full recorder scale at 500 nA. Peak areas were integrated using the Shimadzu EZStart 7.2 software package, and tocopherols were quantified using authentic standards.
Quantification of shoot-tip vitamin C content
To determine that Vit C was taken up by the shoots, Vit C was analyzed in ten shoot tips each (~30 mg of fresh weight) of ‘Chehalem’, from both untreated (control) and those with added Vit C (0.14 mM), at the pretreatment step. Ascorbate was analyzed by ion-paired, reverse-phase HPLC with electrochemical detection by the method of Suh et al. (2003).
Statistics
Analysis of variance (ANOVA) was performed using SAS on the raw data and the data presented as means or percentage of means. Means grouping was with Duncan’s multiple range test (α = 0.05). Experiments were run individually for each step of the protocol. Each experiment was run three times. Twenty shoot tips were cryopreserved in LN after each antioxidant treatment (n = 60). Twenty control shoot tips were also cryopreserved without any added antioxidants (n = 60). Five additional shoot tips per treatment were planted without exposure to LN (n = 15). Regrowth data were taken at 6 weeks following reculture. Recovery from cryopreservation required shoot growth and leaf emergence from the original shoot tip.
Results
Shoot tocopherol assay
In vitro grown shoot tips without added Vit E had very low α- and γ-tocopherol concentrations (<20 nmol/shoot-tip). Exogenous application of Vit E significantly increased the α- and γ-tocopherols in the shoot tips at each step (P < 0.001). The tocopherol concentrations were higher at the loading than at the pretreatment and rinsing steps for both isomers. The γ-tocopherol was lower than the α-tocopherol concentrations in all the shoot tips (Fig. 1). The amounts taken up at each step were equally effective in improving shoot-tip regrowth at these steps (Fig. 2b). The tocopherol content of in vitro shoot tips was also compared to shoot tips from field-grown plants. Field-grown plants had three to six times the amount of tocopherol as the in vitro grown shoots but amounts present were much less than shoots with added Vit E.
Electrochemical detection of tocopherol isomers in shoot tips of ‘Chehalem’ (fresh weight) from in vitro and field-grown plants and shoot tips treated with and without 15 mM Vit E during the PVS2 vitrification procedure (log scale) (mean ± SD). Mean with the same letter are not significantly different. P < 0.001 (n = 60)
a Malondialdehyde (MDA) formation in untreated ‘Chehalem’ shoot tips (control) and PVS2 treated shoot tips with and without added Vit E (15 mM). Mean ± SD. b Regrowth with and without added Vit E (14.72 mM) after exposure to liquid nitrogen. Mean with the same letter are not significantly different. P < 0.05 (n = 60)
Malondialdehyde detection
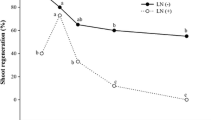
Significantly more MDA was produced in shoot tips during the three cryopreservation steps than in the control (P < 0.001). MDA formation was significantly higher after the loading treatment compared to the pretreatment and rinsing steps (Fig. 2a). We observed a significant reduction in MDA formation at each of the critical steps with added Vit E, compared to that of the controls (untreated shoot tips at the same steps) (P = 0.002). Shoot tips treated with all solutions with and without added Vit E but not exposed to LN, all grew (100%) for each treatment. Regrowth of shoot tips after LN exposure (Fig. 2b) was low (mean of 45%) when MDA (Fig. 2a) was high and high (mean of 71%) when MDA was low in presence of added Vit E. The high regrowth percentages from treatments with added Vit E were not significantly different in these steps but were significantly better than the untreated controls (P = 0.002) (Fig. 2b).
Vitamin E
Exogenous Vit E added at each step significantly improved shoot regrowth following LN exposure (P < 0.001). Shoot tips treated with solutions including Vit E but not exposed to LN, had high regrowth (96%, data not shown). Regrowth of shoot tips treated with Vit E at 11 and 15 mM and exposed to LN was significantly higher than the controls at each step (Fig. 3). Post-LN regrowth of ‘Chehalem’ shoot tips treated with Vit E (7 mM) at pretreatment and rinsing steps was also significantly better than the control (Fig. 3a).
Regrowth of a ‘Chehalem’ and b ‘Hull Thornless’ after liquid nitrogen exposure of shoot tips treated with and without vitamin E at four critical steps of the PVS2 vitrification technique: pretreatment, loading, rinsing and regrowth. Mean ± SD. Mean with the same letter are not significantly different. P < 0.05 (n = 60)
Vitamin E added at two steps
Vit E (11–15 mM) added at both the pretreatment and rinsing steps improved regrowth significantly (P < 0.001) compared to controls (data not shown). Regrowth was not significantly different from when Vit E was added one step at a time (Fig. 3). Some cryopreserved shoot tips initially formed callus before shoot emergence, thus no further testing was done with Vit E in multiple steps.
Vitamin C
Shoot tips treated with Vit C at the pretreatment step but not exposed to LN had a mean Vit C content of 37.8 μM in the tissues while the control untreated shoot tips had a mean of 0.96 μM (P < 0.05). This confirmed that Vit C was readily taken up by the shoot tips. Shoot tips with added Vit C at the four critical steps and exposed to LN had significantly higher regrowth compared to the control for both cultivars (Fig. 4). Increased regrowth always occurred at the pretreatment, loading and rinsing steps. Regrowth on RUB medium with Vit C was significantly lower (10–18%) than the controls (42–45%) for both cultivars during initial experiments. When iron was removed from the regrowth medium in later experiments, the Vit C treated shoot tips improved at the same high percentages as the other three steps. Regrowth of shoot tips on iron-free medium was followed by a transfer to RUB medium with iron (no Vit C) after 2 weeks. Shoot tips treated with Vit C and not exposed to LN had mean regrowth ≥98%. In an attempt to further improve recovery, iron was excluded from all the solutions and Vit C was applied at all four steps. Some callus formation was observed and shoots did not recover (data not shown).
Regrowth of a ‘Chehalem’ and b ‘Hull Thornless’ after liquid nitrogen exposure of shoot tips treated with and without Vit C at each of four critical steps of the PVS2 vitrification technique: pretreatment, loading, rinsing or regrowth. Mean ± SD. Mean with the same letter are not significantly different. P ≤ 0.05 (n = 60)
Combined Vit C and Vit E treatments
Vit C (0.14 mM) combined with Vit E (11 mM) at each of the critical steps resulted in regrowth that was significantly higher than controls (P < 0.001). Regrowth with Vit C alone was not significantly different from combined treatment (Vit E + Vit C). Vit E alone produced significantly less regrowth from combined treatment but the regrowth was significantly better than the control. This trend held for all four steps and both cultivars (Fig. 5). The control shoot tips that were not exposed to LN at each treatment had 100% regrowth (Fig. 6).
Effect of antioxidant treatments: Vit E (11 mM), Vit C (0.14 mM) and combined on regrowth of cryopreserved shoot tips of blackberry cultivars at four critical steps of the PVS2 vitrification technique. a Pretreatment, b loading, c rinsing and d regrowth. Mean ± SD. Mean with the same letter are not significantly different. P ≤ 0.05 (n = 60)
Six weeks regrowth of Rubus hybrid cv. Hull Thornless following antioxidant treatments in the loading solution. The top row of each plate contains shoots that had all the treatments but were not exposed to LN. The control was cryopreserved with the standard technique but without added antioxidants, Vit E = 11 mM, Vit C = 0.14 mM
Discussion
Exogenous Vit E or Vit C added at critical steps of the PVS2 vitrification technique were taken up by the shoot tips and significantly increased regrowth of both cultivars. These findings suggest that oxidative stress is an important factor in the death of plant tissues following cryopreservation. This study determined the mean regrowth at each treatment, the amount of lipid peroxidation occurring at each step of the PVS2 protocol, and then provided a solution for reducing the oxidative damage. We found a significant rise in the amount of MDA (a lipid peroxidation product) produced at each of these steps compared to fresh shoot tips or the untreated controls (Fig. 2a). Roach et al. (2008) characterized ROS formation during cryopreservation treatments (specifically ‘wounding and desiccation’) on embryonic axes of chestnut seeds (C. sativa). They found that excision led to a transient burst of superoxide in 5 min and the stress imposed by a combination of excision and dehydration from 60 to 30% water content doubled the initial rates of superoxide production.
Superoxide is known to spontaneously produce H2O2, a precursor for the formation of the highly reactive hydroxyl radicals (Halliwell and Gutteridge 1999). Our data showed that shoot tips with high MDA had reduced regrowth after cryopreservation compared to those with low MDA (Fig. 2). This agrees with a study which showed that accumulation of 4-hydroxy-2-nonenal and MDA in Daucus carota L. in vitro plant cells correlated with loss of regeneration potential (Adams et al. 1999). We observed that significantly higher lipid peroxidation occurred at the loading than at the pretreatment and rinsing steps (Fig. 3a). This result may be due to the osmotic effect of the loading solution on shoot tips. The high sucrose concentration and glycerol in the loading solution may increase stress as a result of desiccation of the cells. These results may also be due to the antioxidant effect of DMSO in the pretreatment solution and residual DMSO from the cryoprotectant in the rinsing step. DMSO is a known antioxidant and free radical scavenger (Yu and Quinn 1994) and thus it may be involved in reducing oxidative damage at these steps.
Exogenously applied Vit E significantly increased the quantities of α- and γ-tocopherols in blackberry shoot-tip cultures (Fig. 4). Adding Vit E during the steps of the cryopreservation protocol provided a simple and cost effective method of improving a plant’s tocopherol status. Alpha tocopherol was increased in cell cultures of sunflower by the addition of exogenous homogentisic acid and phytol (Caretto et al. 2004). The elevation of antioxidant status using exogenous glutathione was demonstrated with tobacco cell cultures (Schneider et al. 1992) and canola plants (Lappartient and Touraine 1997). Initial experiments to determine what concentration of Vit E could significantly increase regrowth following LN exposure showed that 11 and 15 mM significantly improved regrowth of shoot tips compared to controls at each of these steps (Fig. 1). Regrowth following exogenous Vit E addition at two steps, pretreatment and rinsing, was not significantly better than when Vit E was added one step at a time (data not shown). Intermediate callus was formed before shoots emerged suggesting that some risk might be associated with the application of Vit E at two or more steps at once. Vit E, in addition to its antioxidant effects has been shown to exert pro-oxidative effects which may be deleterious. For example, an in vitro experiment with micellar cell cultures showed that excessive doses of Vit E caused pro-oxidative effects due to partial reduction of Vit E radical (Mukai 1993). A study on rat erythrocytes showed that excessive doses of Vit E lower the activities of antioxidant enzymes in vivo (Eder et al. 2002). Vit E decreased MDA formation (Fig. 2a) and significantly improved shoot regrowth after cryopreservation (Fig. 2b), most likely due to the reduction in MDA formation. These data indicate that Vit E is effective in preventing or repairing damage caused by lipid peroxidation. Sattler et al. (2004) also showed that tocopherols were vital in preventing lipid peroxidation in seeds of A. thaliana during germination. Vit E scavenges ROS, reacts with by-products of PUFA and modulates signal transduction (Halliwell and Gutteridge 1999; Noctor 2006). Our analysis found that field-grown blackberry plants exposed to the stresses of the outdoor environment had three to six times the amount of tocopherol found in the in vitro blackberry plants grown in a controlled environment (Fig. 1). In Ribes shoot cultures, a high phenolics to ROS ratio was observed in a cold-tolerant genotype compared to one considered cold sensitive (Johnston et al. 2007). Antioxidants thus may modulate the effects of ROS.
Vitamin C is a water soluble molecule and is taken up by plants after exogenous application (Arrigoni et al. 1997; Paciolla et al. 2001). Vit C was readily taken up by Rubus shoot tips in our study. Vit C reacts with ROS including hydroxyl radicals and hydrogen peroxide and forms a stable free radical (monodehydroascorbate). This radical has a very short life and is readily converted into dehydroascorbate and Vit C, leading to the termination of the chain reactions of ROS (Halliwell 2006). Vit C (0.14–0.58 mM) significantly improved regrowth of cryopreserved blackberry shoot tips (Fig. 4). Vit C (0.1 mM) in tissue culture medium greatly improved in vitro shoot and root growth of somatic embryos of white spruce (Stasolla and Yeung 1999). The initial low regrowth of cryopreserved Rubus shoot tips on 2× MS iron regrowth medium, the standard growth medium for Rubus used in this study, is likely due to iron and Vit C participating in the Fenton reaction, leading to the production of hydroxyl radicals (Halliwell 2006; Halliwell and Gutteridge 1984). A similar growth decline caused by iron-induced reactions was observed during regeneration of Arachis hypogaea L. explants (Zheng et al. 2005). Removal of iron from the regrowth medium resulted in high regrowth of our cryopreserved Rubus shoot tips (Fig. 4).
Vitamin C was combined with Vit E to determine if a synergistic effect would be seen. Vit C recycles Vit E, keeping Vit E in its active form (Scarpa et al. 1984). Regrowth of shoot tips when Vit E and Vit C were combined was not significantly better than Vit C alone in any of the steps, suggesting that the benefit was mostly due to Vit C rather than Vit E (Fig. 5). These data suggest that antioxidant protection by exogenous Vit C is likely more efficient compared to Vit E. It is also possible that improvement would be seen if the plants tested had even lower initial recovery or lower recovery with Vit C alone. Improving Rubus regrowth from 90% with Vit C to significantly greater than 90% would be less likely than improving recovery of a cultivar with only 30% regrowth. A study of shoot organogenesis found that exogenous Vit C (0.1 mM) increased the number of shoots and the percentage of organogenesis in cultured leaf segments of Gladiolus more than α-tocopherol of equal concentration. At a higher concentration (0.5 mM) of both, Vit E was better (Gupta and Datta 2003). We did not observe decreased regrowth of shoot tips when Vit C was added in a regrowth medium with both iron and Vit E (Fig. 5d). Possibly Vit E quenched any deleterious Fenton reactions and prevented damage to the tissues. Also, we did not observe a growth decline with Vit C and the regular concentration of MS iron when both occurred in the presence of DMSO (Figs. 4, 5). This may be due to the fact that MS iron is not doubled in these solutions as it is in our regrowth medium or that DMSO may have a beneficial antioxidant protection against ROS. We did not test the antioxidant effect of Vit C on reducing lipid peroxidation of shoot tips, but exogenous Vit C is known to reduce lipid peroxidation by ROS in tomato seedlings (Shalata and Neumann 2001), and in sorghum and sunflower seedlings (Zhang and Kirkham 1996). Vit C provides antioxidant protection against the hypersensitivity of a Vit C mutant of A. thaliana to oxidative damage.
Conclusions
Vitamin E or Vit C added at critical steps during PVS2 vitrification significantly improved regrowth of shoot tips following LN exposure. MDA formation significantly increased at each step of the PVS2 vitrification protocol. Adding an exogenous water soluble form of Vit E significantly increased the α- and γ-tocopherol contents of the in vitro blackberry shoot tips and significantly decreased lipid peroxidation at each of the steps. These findings enhance our understanding of the stresses associated with cryopreservation procedures that limit regrowth and provide a solution for reducing damage to plants during these processes. This study to the best of our knowledge is the first report on the successful use of antioxidant vitamins to substantially increase regrowth of cryopreserved plants. Our studies with Vit E demonstrated that ROS are involved in lethal damage to plants via lipid peroxidation during the cryopreservation process. Although Vit E and Vit C additions greatly improve regrowth of these Rubus cultivars, further research may expand this technique to a broader range of genotypes, particularly ‘difficult to cryopreserve species’. We recommend adding Vit C to either the pretreatment, loading or rinsing step as the easiest and most economical way to improve the standard PVS2 cryopreservation protocol. It is likely that Vit C addition would be beneficial in other protocols as well.
References
Adams LK, Benson EE, Strains HJ, Bremner DH, Millam S, Deighton N (1999) Effects of the lipid peroxidation products 4-hydroxy-2-nonenal and malondialdehyde on the proliferation and morphogenetic development of in vitro plant cells. J Plant Physiol 155:376–386
Arrigoni O, Calabrese G, De Gara L, Bitonti MB, Liso R (1997) Correlations between changes in cell ascorbate and growth of Lipinus albus seedlings. J Plant Physiol 150:302–308
Asada R (1992) Ascorbate peroxidase—a hydrogen peroxide scavenging enzyme in plants. Plant Physiol 85:235–241
Benson EE (1990) Free radical damage in stored plant germplasm. International Board for Plant Genetic Resources, Italy
Carelli AA, Franco CI, Crapiste HG (2005) Effectiveness of added natural antioxidants in sunflower oil. Grasas Aceites 56:303–310
Caretto S, Speth EB, Fachechi C, Gala R, Zacheo G, Giovinazzo G (2004) Enhancement of vitamin E production in sunflower cell cultures. Plant Cell Rep 23:174–179
Chang Y, Reed BM (1997) The effects of in vitro-growth condition on the cryopreservation of Pyrus meristems. In Vitro Cell Dev Biol 33:50A
Davey MW, Stals E, Panis B, Keulemans J, Swennen RL (2005) High-throughput determination of malondialdehyde in plant tissues. Anal Biochem 347:201–207
Del Rio D, Stewart A, Pellegrini N (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovas 15:316–328
Douce R, Holtz RB, Benson AA (1973) Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem 248:7215–7222
Eder K, Flader D, Hirche F, Brandsch C (2002) Excess dietary vitamin E lowers the activities of antioxidative enzymes in erythrocytes of rats fed salmon oil. Am Soc Nutr Sci 132:3400–3404
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid-peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radical Biol Med 11:81–128
Evans MD, Dizdaroglu M, Cooke MS (2004) Oxidative DNA damage and disease: induction, repair and significance. Mutat Res 567:1–61
Gupta SD, Datta S (2003) Antioxidant enzyme activities during in vitro morphogenesis of Gladiolus and the effect of application of antioxidants on plant regeneration. Biol Plant 47:179–183
Gupta S, Reed BM (2006) Cryopreservation of shoot tips of blackberry and raspberry by encapsulation-dehydration and vitrification. Cryoletters 27:29–42
Halliwell B (2006) Reactive species and antioxidants: redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Halliwell B, Gutteridge JMC (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1–14
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford Science Publications, New York
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255
Havaux M, Eymery F, Porfirova S, Rey P, Dormann P (2005) Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17:3451–3469
Heber U (1968) Freezing injury in relation to loss of enzyme activities and protection against freezing. Cryobiology 5:188–201
Johnston JW, Harding K, Benson EE (2007) Antioxidant status and genotypic tolerance of Ribes in vitro cultures to cryopreservation. Plant Sci 172:524–534
Kamal-Eldin A, Appelqvist LA (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31:671–701
Lappartient AG, Touraine B (1997) Glutathione-mediated regulation of ATP sulfurylase activity, SO4 2− uptake, and oxidative stress response in intact canola roots. Plant Physiol 114:177–183
Leung H, Vang MJ, Mavis RD (1981) The co-operative interaction between vitamin E and vitamin C in suppression of peroxidation of membrane phospholipids. Biochim Biophys Acta 664:266–272
Liebler DC (1993) The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol 23:147–169
Mukai K (1993) Synthesis and kinetic study of antioxidant and prooxidant actions of vitamin E derivatives. In: Parker L, Fuchs J (eds) Vitamin E in health and disease. Marcel Dekker, New York, pp 97–119
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Noctor G (2006) Metabolic signaling in defense and stress: the central roles of soluble redox couples. Plant Cell Environ 29:409–425
Paciolla C, De Tullio MC, Chiappetta A, Innocenti AM, Bitonti MB, Liso R, Arrigoni O (2001) Short- and long-term effects of dehydroascorbate in Lupinus albus and Allium cepa roots. Plant Cell Physiol 42:857–863
Podda M, Weber C, Traber MG, Packer L (1996) Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res 37:893–901
Popov AS, Popova EV, Nikishina TV, Vysotskaya ON (2006) Cryobank of plant genetic resources in Russian Academy of Sciences. Int J Refrig 29:403–410
Reed BM (1988) Cold acclimation as a method to improve survival of cryopreserved Rubus meristems. Cryoletters 9:166–171
Reed BM (1990) Multiplication of Rubus germplasm in vitro: a screen of 256 accessions. Fruit Var J 44:141–148
Reed BM (2008) Cryopreservation of temperate berry crops. In: Reed B (ed) Plant cryopreservation: a practical guide. Springer, New York, pp 333–364
Roach T, Ivanova M, Beckett RP, Minibayeva FV, Green I, Pritchard HW, Kranner I (2008) An oxidative burst of superoxide in embryonic axes of recalcitrant sweet chestnut seeds as induced by excision and desiccation. Physiol Plant 133:131–139
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16:1419–1432
Scarpa MA, Rigo M, Maiorino M, Ursini F, Gregolin C (1984) Formation of α-tocopherol radical and recycling of α-tocopherol by ascorbate during peroxidation of phosphatidylcholine liposomes. Biochim Biophys Acta 801:215–219
Schneider A, Martini N, Rennenberg H (1992) Reduced glutathione (GSH) transport into cultured tobacco cells. Plant Physiol Biochem 30:29–38
Sgherri CLM, Navari-Izzo F (1995) Sunflower seedlings subjected to increasing water deficit stress: oxidative stress and defense mechanisms. Physiol Plant 93:25–30
Shalata A, Neumann PM (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52:2207–2211
Shao H-B, Chu L-Y, Lu Z-H, Kang C-M (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4:8–14
Stasolla C, Yeung EC (1999) Ascorbic acid improves conversion of white spruce somatic embryos. In Vitro Cell Dev Biol Plant 35:316–319
Suh J, Zhu B-Z, Frei B (2003) Ascorbate does not act as a pro-oxidant towards lipids and proteins in human plasma exposed to redox-active transition metal ions and hydrogen peroxide. Free Radical Biol Med 34:1306–1314
Thompson JE, Legge RL, Barber RF (1987) The role of free radicals in senescence and wounding. New Phytol 105:317–344
Wang Z, Deng X (2004) Cryopreservation of shoot-tips of citrus using vitrification: effect of reduced form of glutathione. Cryoletters 25:43–50
Yamauchi Y, Furutera A, Seki K, Toyoda Y, Tanaka K, Sugimoto Y (2008) Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol Biochem 46:786–793
Yoshida S, Sakai A (1974) Phospholipid degradation in frozen plant cells associated with freezing injury. Plant Physiol 53:509–511
Yu Z-W, Quinn PJ (1994) Dimethyl sulphoxide: a review of its applications in cell biology. Biosci Rep 14:259–281
Zhang JX, Kirkham MB (1996) Lipid peroxidation in sorghum and sunflower seedling as affected by ascorbic acid, benzoic acid and propyl gallate. J Plant Physiol 149:489–493
Zheng Q, Ju B, Liang L, Xiao X (2005) Effects of antioxidants on the plant regeneration and GUS expressive frequency of peanut (Arachis hypogaea) explants by Agrobacterium tumefaciens. Plant Cell Tissue Organ 81:83–90
Acknowledgments
Research was funded by the United States Department of Agriculture (USDA), Agricultural Research Service CRIS project 5358-21000-038-00D. Vitamin E and malondialdehyde analysis was done by the Linus Pauling Institute. Thanks are extended to Jeanine DeNoma for her technical advice and to the Ford Foundation International Fellowship Program, and the University Club of Portland Inc. for fellowships to Esther Uchendu.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Merkle.
Rights and permissions
About this article
Cite this article
Uchendu, E.E., Leonard, S.W., Traber, M.G. et al. Vitamins C and E improve regrowth and reduce lipid peroxidation of blackberry shoot tips following cryopreservation. Plant Cell Rep 29, 25–35 (2010). https://doi.org/10.1007/s00299-009-0795-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0795-y