Abstract
β-1,3 glucanase (E.C.3.2.1.39) is the key enzyme involved in the hydrolytic cleavage of 1,3 β-D glucosidic linkages in β-1,3 glucans. This work describes a comparative analysis of expression patterns of β-1,3 glucanase gene in relation to changes in fruit pulp softening rates in three banana cultivars, Rasthali (AAB), Kanthali (AB), and Monthan (ABB). Analysis of transcript and protein levels of β-1,3 glucanase gene during ripening revealed differential timing in expression of the gene which correlated well with the variation in enzymatic activity of glucanase and fruit pulp softening rates in the three cultivars. Exogenously applied ethylene strongly induced β-1,3 glucanase expression during the early ripening days in Rasthali, while the expression of the gene was marginally stimulated following ethylene treatment in preclimacteric Kanthali fruit. Conversely, in Monthan, β-1,3 glucanase expression was very low throughout the ripening stages, and ethylene treatment did not induce the expression of the gene in this cultivar. Analysis of glucanase activity using protein extracts from unripe and ripe fruit of Monthan with crude cell wall polysaccharide fractions (used as substrate) indicated that the natural substrate for glucanase remained almost unutilized in this cultivar due to low in vivo glucanase activity. Furthermore, the recombinant β-1,3 glucanase protein, overexpressed in E. coli, showed requirement for substrates with contiguous β-1,3 linkages for optimal activity. Overall, our results provide new information on the expression profile of β-1,3 glucanase gene in connection with the pattern of changes in fruit firmness at the physiological and molecular levels during ripening in three banana cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Softening of fruit is one of the major factors controlling fruit quality and postharvest life. Several theories have been offered to explain the mechanisms by which softening occurs during fruit ripening; this aspect of ripening remains poorly understood (Ali et al. 1998; Cosgrove 2001; Rose and Bennett 1999). Loss of turgor pressure and subsequent degradation and decrease in starch content during ripening have been shown to play an important role in fruit softening. However, changes in the wall structures and compositions catalyzed by the action of expansins and hydrolases are considered as the key factors for softening of fruit flesh (Brady 1987; Brummell et al. 2004; Fischer and Bennett 1991; Trivedi and Nath 2004).
Tropical fruits like banana (Musa paradiciaca, L.), mango (Mangifera indica, L.), papaya (Carica papaya, L.), and guava (Psidium guajava, L.) contain distinct levels of structural and non-structural carbohydrates, despite of the fact that these fruits displayed considerable difference in their softening rates during ripening and postharvest storage (Chin et al. 1999; Kojima et al. 1994). Regarding non-structural carbohydrates, mature fruits of banana and mango contain about 20–25% of fresh weight of starch, while papaya and guava contain only negligible amounts of starch. However, these differences in starch content have not always been related with the firmness level or softening rates of fruit during ripening (Hall 1987). It has been suggested that difference in the architecture of the primary walls of fruit may also contribute to the differences in their softening rates (Cosgrove 2001). However, several lines of evidences have indicated that the enzyme-catalyzed modifications of cell wall structures and components like hemicelluloses, xyloglucans etc. contribute significantly to the softening of fruits (Ali et al. 1998; Giovannoni et al. 1992; Micheli 2001).
In plants, β-1,3 glucanases (β-1,3 glucs) are widely distributed family of evolutionary related protein with the ability to hydrolyze β-1,3 glucosidic linkages in β-1,3 glucans. While the major biological functions of β-1,3 glucanases have been shown to be associated with plant reproductive biology and defense in response to pathogenic infection. Significant evidences have accumulated in recent years to indicate the functional importance of this enzyme in non-defense pathways like cell-division, seed germination, microsporogenesis, fertilization and embryogenesis, fruit ripening, wound, and abiotic stress responses (Bewley and Black 1994; Dong and Dunstan 1997; Hird et al. 1993; Leubner-Metzger and Meins 1999).
Earlier studies have reported β-1,3 gluc as a prominent ripening upregulated gene in Cavendish banana (Clendennen and May 1997; Kesari et al. 2007). Catalytically active form of β-1,3 glucanase had been purified and characterized at biochemical level from Cavendish banana (Peumans et al. 2000). However, corresponding knowledge about the expression profile of β-1,3 gluc gene during fruit ripening is not yet available in various other cultivars of banana which display distinct ripening behaviur (Roy Choudhury et al. 2008a). In addition, previous studies did not report the precise physiological function of the gene during banana fruit ripening. Therefore, the major objectives of the present study were to investigate the expression profile of β-1,3 gluc gene and changes in the enzymatic activity of β-1,3 glucanase in association with the pattern of changes in fruit firmness during ethylene-mediated post harvest ripening in the three banana cultivars Rasthali, Kanthali, and Monthan. To further understand the relative abundance of the catalytically active form of β-1,3 glucanase in the fruit pulp of the banana cultivars, we have studied the changes in glucanase activity using both recombinant β-1,3 glucanase protein and fruit protein extracts with the crude polysaccharide fractions of fruit (used as substrate for β-1,3 glucanase) from three banana cultivars and correlated these observations with the fruit softening pattern in the selected cultivars.
Materials and methods
Plant materials and ethylene treatment
The banana cultivars Rasthali (Musa sp., AAB group), Kanthali (AB group) and Monthan (ABB group) were obtained from West Bengal State Council of Science and Technology, India). Plants were grown in soil from August to April at the Bose Institute Experimental field. The average temperature was ~32 and ~18°C during the light and dark periods, respectively. The relative humidity ranged between 70–97%.
To study the β-1,3 gluc expression pattern and fruit pulp softening rates during ex-planta ripening, unripe green bananas (pre climacteric stage) were harvested from three different cultivars. The hands were cut from a bunch of 80 days post anthesis (DAP) for each cultivar to avoid heterogeneity due to differences during development. Banana hand from each cultivar was separated into individual fruits and kept at room temperature (25°C) until the fruit were fully ripe (15 days after harvest). Different ripening stages of the cultivars were previously studied by day-wise measurement of ethylene biosynthesis and ACC oxidase activity to characterize the climacteric ethylene production time of the selected cultivars (9, 10, and 12 days after harvest for Rasthali, Kanthali and Monthan, respectively) as well as to identify the preclimacteric, climacteric, and postclimacteric ripening stages (Roy Choudhury et al. 2008a). To avoid differences in ripening behavior of fruit among different hands of the same cultivar, fruit from the same hand were used as a sample group in each experiment for each cultivar. Again, for each cultivar, pulp tissues were collected from the various stages of ripening, tissues frozen in liquid N2, and stored at −80°C for isolation of RNA, protein extracts and polysaccharide fractions. All experiments were repeated at least three times with five replicates in each case.
Ethylene treatment to the preclimacteric bananas (day 0 after harvest, 80 days post anthesis) from each cultivar was given by following the protocol described by Roy Choudhury et al. (2008a). Fruit were treated with 100 μl/l ethylene for 24 h at 25°C in the dark and then allowed to ripen in air until fully ripe.
Potassium permanganate (KMnO4) was used as an ethylene absorbent for an ethylene inhibition treatment (Scott et al. 1970). Ethylene-treated banana fruit were placed in a sealed airtight container along with 15 g of potassium permanganate enclosed in a paper bag for 16 h at 25°C in the dark. Fruit were then ripened in air at 25°C until fully ripe. Samples were harvested every 24 h after ethylene treatment or both ethylene and potassium permanganate treatments. Representative samples from the respective preclimacteric, climacteric, and postclimacteric stages of the cultivars were stored at −80°C for the extraction of total RNA and proteins.
Detection of glucanase activity
Glucanase activity was determined by using laminarin (Sigma) as a substrate (Abeles et al. 1970). One gram of pulp tissue was extracted with 3 ml of 50 mM K-acetate buffer (pH 4.8) in a mortar and pestle and then homogenate was centrifuged at 10,000×g at 4°C for 10 min. Supernatant fraction (~25 μg of proteins) was added to 0.1 ml of 2% laminarin and incubated for 1 h at 50°C. The reaction was terminated by addition of 3 ml of DNS reagent (Dinitro salicylic acid) and then heating the tubes for 5 min in boiling water bath. Glucanase activity was measured spectrophotometrically at 510 nm.
Measurement of fruit firmness
Five fruits from control and each treatment were used to measure fruit firmness. To estimate fruit firmness, peel from one side of the banana finger was removed and measurement was carried out at three different places using Penetrometer and recorded as force in Newton (N). Average of five readings was taken as measure of firmness of individual fruit.
RNA isolation, RT-PCR and molecular cloning of banana β-1,3 gluc
Total RNA was isolated from pulp tissues of banana fruits by the modification of SDS–Phenol method as described earlier (Roy Choudhury et al. 2008a). First strand cDNA was synthesized from 5 μg of total RNA using Thermoscript reverse transcriptase (Life Technologies Inc.) following the manufacturer’s instructions. The cDNA clones for β-1,3 glucanase were isolated from ripe pulp tissue of the three banana cultivars by RT-PCR with the gene-specific oligos, Glu5: 5′-GTACGAGCTCATGGCAACAAAAGCTTC-3′ and Glu3: 5′-GTACGAGCTCCTAAAAGCTTATTTGGTA-3′ made from the banana β-1,3 glucanase gene sequence (EU014210). The reaction for RT-PCR comprised 30 cycles of 94°C for 1 min, 43°C for 1 min and 72°C for 2 min. The amplified cDNAs were cloned in pBluescript cloning vector (Stratagene) for sequence verification. For semi quantitative RT-PCR analysis, a first cycle of 5 min at 94°C, 45 s at 43°C, and 1 min at 72°C was followed by 45 s at 94°C, 45 s at 43°C, and 1 min at 72°C for 24 cycles. The conditions were chosen so that none of the RNAs analyzed reached a plateau at the end of the amplification protocol. We also performed a negative control containing RNA instead of cDNA to rule out genomic DNA contamination in each set of reactions. Equal amounts of PCR products were loaded on 1% agarose gel for transcript profile analysis.
Preparation of protein extracts and Western blotting
Protein extracts were prepared by homogenizing 5 g of pulp tissues by following the protocol described previously (Roy Choudhury et al. 2008b). ~25 μg of protein samples were electroblotted on to PVDF membrane and immunoprobed with anti-tobacco class I β-1,3 glucanase (1:1,000 dilution) polyclonal antibody by following the method described previously (Roy Choudhury et al. 2008b).
Expression and purification of recombinant β-1,3 glucanase
The full-length cDNA of banana β-1,3 gluc was subcloned into pQE30:UA (Qiagen) bacterial expression vector. pQE30:UA-β-1,3 gluc recombinant plasmid encoding the N-terminal 6×His-tagged β-1,3 gluc was expressed in E. coli M15 host strain carrying the pREP4 repressor (Qiagen). Cells were cultured at 37°C with a rotary shaker until OD600 reached 0.6. His-tagged protein was induced with the addition of 1 mM IPTG followed by further incubation for 5 h at 37°C. After centrifugation, the harvested bacterial cell pellet was resuspended in lysis buffer containing 50 mM NaH2PO4, 300 mM NaCl, 10 μg/ml leupeptin, 1 mM PMSF, and 10 mM imidazole. Lysozyme was added at final concentration of 1 mg/ml and incubated on ice for 4 h. Cells were then sonicated on ice for 10 s, six times with 20 s interval. After centrifugation soluble fraction was purified by Ni-NTA resin (Qiagen) by following the manufacturer’s instructions. 10 μg of affinity resin purified recombinant protein was used for glucanase activity assay against laminarin, AZCL-Pachyman, AZCL-He Cellulose, and AZCL-barley glucan.
Preparation of crude polysaccharide fractions
The banana pulp tissues from unripe (0-day after harvest or 80 days after post anthesis) and fully ripe (15 day after harvest or 95 days after post anthesis) fruit were homogenized in 0.1% solution (w/v) of ascorbic acid. The starch granules were allowed to settle and the supernatant collected for dialysis against distilled H2O to remove low molecular mass compounds. The dialyzed fraction was incubated for 30 min at room temperature with 50 mM NaOH to inactivate the enzymes present in the crude extract.
Results
Differential pattern of glucanase activity during ripening in banana cultivars
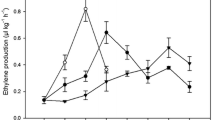
Earlier studies have predicted the possible function of β-1,3 glucanase in fruit pulp softening during banana ripening (Peumans et al. 2000). Therefore, to study the fruit softening pattern during ripening in the three banana cultivars during ripening, we first analyzed the changes in the enzymatic activity of β-1,3 glucanase in the pulp tissues of banana on a daily basis starting from green unripe stage (preclimacteric) to fully ripe stage (postclimacteric). Low glucanase activity was detected during the early stages of ripening (days 1–5 after harvest) in the banana cultivars ripened naturally (Fig. 1a–c). Glucanase activity was gradually increased as ripening progressed towards the climacteric for the respective cultivars like Rasthali (~9.6 nmol glucose equivalent min−1 mg protein−1) and Kanthali (~6.21 nmol glucose equivalent min−1 mg protein−1). In Monthan fruit, glucanase activity was very low during ripening days and increased only marginally at the later stages of ripening. In Rasthali, Kanthali, and Monthan glucanase activity levels were found to be ~15.4, ~11.1, and ~5.2 nmol glucose equivalent min−1 mg protein−1, respectively, in the ripe postclimacteric fruit (day 15 after harvest). These values were ~2.4, ~1.9 and ~1.2-fold higher as compared to those obtained in the preclimacteric fruit of the corresponding cultivars (day 1 after harvest).
Analysis of glucanase activity in banana pulps of three cultivars Rasthali (R), Kanthali (K), and Monthan (M) ripened naturally (a). Changes in glucanase activity in banana fruit after exogenous application of ethylene (b) or ethylene followed by KMnO4 treatment (c). Data points are mean of five replications, ±1 SE
Glucanase activity was prominently enhanced during the early stages (days 3–5 after harvest) of ripening following ethylene treatment, particularly in Rasthali (Fig. 1a). In this cultivar, glucanase activity increased steadily during the subsequent stages of ripening and then gradually declined towards the full-ripe stage (days 11–15 after harvest). The effect of ethylene on glucanase activity was less pronounced in Kanthali during the early days of ripening (Fig. 1b). In this cultivar, glucanase activity increased only marginally in the later stages (days 7–9) and then remained almost unchanged in the fully ripe fruit (days 11–15 after harvest). Conversely, no appreciable effect of ethylene was noticed on glucanase activity in Monthan (Fig. 1c).
To further substantiate the above results, ethylene-treated banana fruit were subjected to treatment with KMnO4 to reduce the immediate effect of ripening ethylene produced after exogenous ethylene treatment. In Rasthali, KMnO4 treatment sharply inhibited the ethylene-induced glucanase activity (~1.8-fold) in preclimacteric fruit (days 1–7 post ethylene treatment) (Fig. 1a). In Kanthali, where ethylene treatment only marginally stimulated glucanase activity during early ripening, KMnO4 treatment caused ~1.3-fold inhibition of glucanase activity in preclimacteric fruit (days 1–7 post ethylene treatment) Fig. 1b). Interestingly, in Monthan, potassium permanganate treatment of ethylene-treated fruit had a negligible inhibitory effect on glucanase activity (~1.02-fold) (Fig. 1c) during similar ripening stages.
Fruit pulp softening rates correlate well with the glucanase activity levels
To further evaluate the possible physiological functional of β-1,3 glucanase in ripening and fruit softening process, we next carried out a daily basis measurement of the changes in fruit firmness during ripening days in three banana cultivars. In Rasthali, fruit firmness decreased sharply with the onset of climacteric period (day 9 after harvest) (Fig. 2a). On the other hand, in Kanthali, fruit firmness decreased slowly with the ripening and then decreased further as the fruit ripened towards the postclimacteric period (days 11–15 after harvest) (Fig. 2b). Conversely, in Monthan, the fruit remained considerably hard throughout the ripening stages and only marginal pulp softening was detected in the postclimacteric fruit (Fig. 2c). Whereas approximately 80 and 50% decrease in fruit firmness were observed during days 9–15 after harvest in Rasthali and Kanthali, respectively, ~13% loss in fruit firmness was detected in Monthan during similar ripening days.
In Rasthali, which showed distinct stimulation of glucanase activity in the early days of ripening after ethylene treatment, fruit firmness declined sharply within 3–4 days post ethylene treatment and then decreased further in the later stages of ripening as compared to fruit ripened naturally (Fig. 2a). In Kanthali, the response was not as pronounced as in Rasthali. In this cultivar, fruit softening was initiated on day 7 after ethylene treatment after which fruit firmness decreased marginally in the later stages of ripening (day 10 onwards, Fig. 2b). In Monthan fruit, only negligible decrease in fruit firmness was detected in the later stages of ripening (day 11 onwards) after ethylene treatment (Fig. 2c).
To further verify the differential responsiveness of the cultivars towards ethylene in relation to fruit softening, ethylene-treated banana fruits were subjected to treatment with KMnO4. In Rasthali, this treatment sharply inhibited the ethylene-mediated decline in fruit firmness (~1.9-fold) during early ripening days (days 1–7 post ethylene treatment) (Fig. 2a). In Kanthali, where exogenous ethylene treatment only moderately induced fruit softening during early ripening stages, fruit softening rate was ~1.3-fold inhibited after KMnO4 treatment during days 1–7 post ethylene treatment (Fig. 2b). In Monthan, KMnO4 treatment of ethylene-treated fruit had negligible inhibitory effect on fruit-softening rates (~1.1-fold inhibition in contrast to ethylene treated fruit) during similar ripening stages (Fig. 2c). Overall, the differential fruit softening rates observed during the ripening stages (both natural and after ethylene treatment) were consistent with the changes in glucanase activity levels in the banana cultivars.
Isolation and identification of cDNAs for β-1,3 gluc from three banana cultivars
To investigate the fruit softening patterns in the three cultivars at the molecular level and to study the expression profile of β-1,3 gluc during ripening, we cloned the cDNA for β-1,3 gluc from three banana cultivars by using gene-specific primers. The cDNA sequence of β-1,3 gluc from Rasthali showed high degree of sequence similarity (~97–98%) with the β-1,3 gluc cDNAs isolated from Kanthali and Monthan fruit both at the nucleotide and amino acid level, respectively. Existence of marginal dissimilarity in the sequence may indicate to cultivar difference. The coding sequence of β-1,3 gluc isolated from Rasthali appeared to be a full-length cDNA of 1026 bp encoding a protein of 341 amino acid sequences with the predicted molecular mass of 37.5-kDa. However, existence of a putative cleavage site for a signal peptidase was found to be located between Ser-28 and Ile-29 (SignalP-NN program-http://www.us.expasy.org) (Von Heijne 1986) residues resulting in mature protein of ~34-kDa instead of 37-kDa.
Phylogenetic analysis based on the deduced amino acid sequences of Musa balbisiana β-1,3 glucanase (Accession No. AAS48700) and other fruit-specific β-1,3 glucanase sequences from Citrus sinensis (Accession No. CAA03908), Prunus persica (Accession No. AAL30426), Vitis vinifera (Accession No. AAF44667), Mangifera indica (Accession No. ABD16200), Ziziphus jujuba (Accession No. AAY25165) and Capsicum annuum (Accession No. AAF34761) have revealed that Musa acuminata β-1,3 glucanase (cultivar Rasthali) was closely related to the β-1,3 glucanase of Vitis vinifera and also indicated the existence of common ancestor of fruit-specific β-1,3 glucanases (Supplementary Fig. 1). Phylogenetic analysis of the β-1,3 glucanase amino acid sequences was performed using MEGA version 4.1 (Tamura et al. 2007). Neighbor-joining method was used for phylogenetic tree searching and inference. The phylogenetic tree was tested by bootstrap analysis with 1,000 replications.
Changes in β-1,3 gluc gene expression during ripening in three banana cultivars
We next analyzed the expression of β-1,3 gluc gene in the fruit pulp of three banana cultivars during the ripening days by RT-PCR (Fig. 3). As shown in Fig. 3a, in Rasthali, β-1,3 gluc mRNA level was relatively low at the preclimacteric stages (1–3 days after harvest). It was gradually increased at the onset of climacteric period (day 9) and reached a maximum level at the postclimacteric stage (days 10–15 after harvest). In Kanthali, β-1,3 gluc transcript level was fairly low during the early stages of ripening (days 1–7 after harvest) and at the onset of climacteric (day 10). However, in this cultivar, the abundance of β-1,3 gluc mRNA was marginally increased during the postclimacteric stages (days 11–15) (Fig. 3b). Conversely, in Monthan, β-1,3 gluc mRNA level was almost undetectable during ripening days and very low expression level of the gene was detected at the post climacteric stage (days 13–15 after harvest (Fig. 3c). β-1,3 gluc transcript accumulation patterns in the pulp tissues of the banana cultivars were consistent with the differential glucanase activity levels in the three cultivars. The changes in β-1,3 glucanase protein levels in the fruit pulp tissues, as observed by Western blotting using anti-tobacco β-1,3 glucanase antibody correlated well with the β-1,3 gluc mRNA levels during the ripening days in the three cultivars (Fig. 3d–f).
Changes in β-1,3 glucanase mRNA abundance level during ripening in three different cultivars [Rasthali (R), Kanthali (K), and Monthan (M)] of banana ripened naturally at 25°C (upper panel; a, c, e). Transcript accumulation level of β-1,3 glucanase was analyzed by RT-PCR with gene-specific primers. ~5 μg total RNA was taken from each cultivar and from each stage for RT-PCR analysis and then equal amount of amplified product was used for analysis. Representative gel images from at least three independent experiments are shown in a–c. Transcript profile of MA-actin was used as internal control (lower panel; a, b, c). Protein gel blot analysis with total protein extracts prepared from the respective preclimacteric, climacteric, and postclimacteric stages of ripening from three different cultivars of banana and immunodetection of β-1,3 glucanase protein by using β-1,3 glucanase antibody (upper panel; d, e, f). Molecular weight of the immunoreactive band is indicated. Steady state protein level of Actin at the indicated conditions was measured by immunoblot as internal control (lower panel; d, e, f). RT-PCR analysis of the β-1,3 glucanase expression pattern during the various stages of ripening in three cultivars [Rasthali (C), Kanthali (K), and Monthan (M)] following ethylene treatment (upper panel; g, i, k). Steady state mRNA level of MA-actin at the indicated conditions was measured by RT-PCR as internal control (lower panel, g, i and k). Immunoblot analysis with total protein extracts prepared from different stages of ripening following ethylene treatment from three different cultivars of banana and immunodetection of β-1,3 glucanase protein by using β-1,3 glucanase antibody (upper panel, h, j and l). Steady state protein level of Actin at the indicated conditions was measured by immunoblot as internal control (lower panel, h, j and l)
Ethylene treatment differentially stimulates β-1,3 glucanase expression in the three banana cultivars
To study the effect of exogenously applied ethylene on the expression of β-1,3 gluc gene, β-1,3 gluc transcript accumulation pattern was studied from the preclimacteric, climacteric, and postclimacteric stages (as characterized from the naturally ripening fruit) of the cultivars following exogenous application of ethylene. In contrast to the low β-1,3 gluc mRNA levels in the early natural ripening, ethylene treatment caused a considerable increase in β-1,3 gluc transcript level at the preclimacteric stage in Rasthali (Fig. 3g). In this cultivar, the abundance of β-1,3 gluc mRNA was further increased in the later stages of ripening but declined thereafter almost to the preclimacteric level in the fully ripe fruit. In Kanthali, β-1,3 gluc transcript level also increased in preclimacteric fruit after ethylene treatment in contrast to fruit ripened naturally but did not increase further as observed in Rasthali (Fig. 3i). In Monthan, ethylene treatment did not induce β-1,3 gluc expression during the early ripening stages and negligible increase was detected at the climacteric and postclimacteric stages (days 13–15 after harvest) (Fig. 3k). The changes in protein levels of β-1,3 glucanase in the ethylene-treated fruit pulp tissues were mostly in agreement with the transcript levels of β-1,3 gluc during ripening (Fig. 3h, j and l).
Ethylene-mediated induction of β-1,3 gluc gene expression both at transcript and protein levels at the preclimacteric stages in Rasthali and Kanthali was distinctly inhibited by potassium permanganate treatment of the ethylene-treated fruits (Fig. 4a–d). However, in contrast to Kanthali, in ethylene-treated Rasthali fruit, ethylene-induced expression of β-1,3 gluc was considerably increased both at transcript and protein levels at postclimacteric stage after KMnO4 treatment (Fig. 4a–d). In ethylene-treated Monthan fruit, no noticeable effect of potassium permanganate treatment on the expression of β-1,3 gluc was observed during ripening (Fig. 4e, f).
Detection of β-1,3 glucanase mRNA abundance during the various stages of ripening in three cultivars, namely Rasthali (R), Kanthali (K), and Monthan (M) following ethylene and KMnO4 treatment as revealed by RT-PCR analysis (upper panel, a, c and e). Steady state mRNA level of MA-actin at the indicated conditions was measured by RT-PCR as internal control (lower panel, a, c and e). Immunoblot analysis with total protein extracts prepared from different stages of ripening following ethylene and KMnO4 treatment from three different cultivars of banana and immunodetection of β-1,3 glucanase protein by using β-1,3 glucanase antibody (upper panel, b, d and f). Steady state protein level of Actin at the indicated conditions was measured by immunoblot as internal control (lower panel, b, d and f)
Activity and substrate specificity of banana recombinant β-1,3 glucanase
To further analyze the biochemical properties of banana fruit-specific β-1,3 glucanase, the full length cDNA for β-1,3 gluc (cv Rasthali) was cloned into pQ30:UA expression vector and the His-tagged recombinant protein was overexpressed in E. coli M15 (pREP-4) host strain. The overexpression of β-1,3 gluc cDNA in E. coli produced considerable amount of the recombinant protein in soluble fraction and showed glucanase activity on laminarin (with contiguous β-1,3 linkages) as substrate. The purified recombinant β-1,3 gluc protein was clearly recognized by anti-tobacco β-1,3 glucanase antibody in immunoblotting experiment (Fig. 5b), while no cross-reacting band could be detected with rabbit pre-immune serum, indicating the specificity of recognition of the antibody (data not shown). Using laminarin as a substrate, the recombinant enzyme showed a pH optimum of around 4.8 and was consistent with the native enzyme reported from Cavendish banana (Peumans et al. 2000) (Fig. 5c). The thermal stability of recombinant β-1,3 glucanase was analyzed by measuring glucanase activity after incubating the E. coli lysate (overexpressing β-1,3 gluc) supernatant or Ni-NTA column purified enzyme at temperature ranging from 50–70°C for 10–30 min. β-1,3 glucanase activity was almost unaffected by incubation at 50 or 60°C for 20 to 30 min, respectively. The recombinant enzyme was found to be heat stable up to 65°C for 30 min. However, longer incubation period beyond 65°C distinctly reduced the enzyme activity even after 10 min of incubation (Table 1).
a Overexpression and purification of recombinant banana β-1,3 glucanase in E.coli. Separation of noninduced control (lane 2) and IPTG induced (lane 3) total protein extracts, obtained from M15 (Prep4) E.coli cells (harboring pQE30: banana β-1,3 glucanase recombinant plasmid) by 10% SDS-PAGE and stained with coomassie blue. After cell lysis considerable amount of recombinant protein remained in soluble fraction (lane 4), while small amount of recombinant protein remained insoluble fraction (lane 5). The soluble fraction was further purified by Ni-NTA chromatography (lane 6). Arrow indicating the position of recombinant β-1,3 glucanase on gel. b Immunodetection of recombinant β-1,3 glucanase with anti-tobacco class I β-1,3 glucanase antibody (1:1,000 dilution) using noninduced E.coli lysate (lane 1), IPTG induced E.coli lysate (Overexpressing the recombinant his-tagged β-1,3 glucanase) (lane 2) and Ni-NTA resin purified protein fraction (lane 3). Molecular mass markers are shown on the left of figures. c pH dependence of the banana recombinant β-1,3 glucanase. d Detection of glucanase activity by using 10 μg of purified recombinant protein and equal amounts (~15 μg) of polysaccharide fractions from unripe (UR, day 1 after harvest) and ripe pulp (R, day 15 after harvest) of three banana cultivars where fruit were ripened naturally. e Glucanase activity was studied using 10 μg of recombinant protein and equal amounts of polysaccharide fractions (~15 μg) from ethylene-treated unripe (UR, day 1 after harvest) and ripe pulp (R, day 15 after harvest) of the three cultivars. f Equal amounts of protein extracts (~25 μg) from ripe fruit of Rasthali, Kanthali, and Monthan were incubated with the polysaccharide fractions from unripe (UR, day 1 after harvest) and ripe fruits (R, day 15 after harvest) of the respective cultivars for detection of glucanase activity, where fruit ripened naturally. g Analysis of glucanase activity by using ~25 μg of ripe fruit (R, day 15 after harvest) protein extracts from Rasthali with the cell wall polysaccharide fractions (utilized as substrate) from unripe (UR, day 1 after harvest) and ripe (R, day 15 after harvest) fruit of Monthan and vice versa. Equal amount of polysaccharide fractions (~15 μg) were used from unripe (UR) and ripe (R) fruit of Monthan and Rasthali ripened naturally
To understand the nature of in vivo substrate for β-1,3 glucanase, the substrate specificity of the recombinant enzyme was also analyzed by using other substrates besides laminarin. The catalytic activity of the enzyme was compared by using AZCL-Pachyman (with contiguous β-1,3 linkages), AZCL-HE-Cellulose (a β-1,4 glucan substrate) and AZCL-barley β-glucan (a β-1,3: β-1,4-glucan substrate). As shown in Table 2, negligible activity could be detected with AZCL-HE-Cellulose, while the activity of the recombinant enzyme was ~10 times higher with AZCL-Pachyman as compared to AZCL-barley β-glucan (Table 2). Together, these results may suggest that the recombinant enzyme cannot utilize β-1,4-glucan as substrate and may not efficiently utilize with β-1,3: β-1,4 linkages. The optimum activity of the enzyme could be detected only with substrate having consecutive β-1,3 linkages (as observed with laminarin and AZCL-Pachyman).
Low glucanase activity in Monthan results in inefficient utilization substrate for β-1,3 glucanase in the fruit pulp
To further investigate the differential glucanase activity and softening rates in the banana cultivars, the activity of purified recombinant banana β-1,3 glucanase enzyme was assayed against the crude polysaccharide fractions (utilized as substrate) prepared from unripe and ripe fruit (day 1 and day 15 after harvest) of Rasthali, Kanthali, and Monthan, respectively. The recombinant enzyme typically showed considerably higher activity with polysaccharide fractions from unripe fruit as compared to ripe fruit pulp in Rasthali and Kanthali (Fig. 5d). These results indicate that the substrate for β-1,3 glucanase mostly exists in the unripe banana and along with the climacteric ethylene production and induction of catalytic activity of β-1,3 glucanase, the substrate disappears in the ripe fruit. In contrast to this, in Monthan, recombinant enzyme showed high glucanase activity against both unripe and ripe polysaccharide fractions (Fig. 5d). These results may indicate that in Monthan the in vivo substrate for β-1,3 glucanase undergo least degradation along with the ripening. In addition, the recombinant enzyme showed low activity with polysaccharide fractions isolated from ethylene-treated unripe fruit of Rasthali and Kanthali (Fig. 5e) as compared to fruit ripened naturally (Fig. 5d). Glucanase activity was further decreased when ethylene-treated ripe fruit polysaccharide fraction from Rasthali and Kanthali were used as substrate. On the other hand, the recombinant enzyme showed considerable glucanase activity against the unripe and ripe polysaccharide fractions isolated from ethylene-treated Monthan fruit (Fig. 5e). Together, these results may suggest ethylene-induced glucanase activity and degradation of its substrate during early ripening periods in Rasthali and Kanthali, while in Monthan, ethylene treatment did not induce glucanase activity. Thus, in this cultivar, the substrate for β-1,3 glucanase remained mostly unaffected even after ethylene treatment in unripe and ripe fruit, allowing the recombinant enzyme to access considerable amount of substrate.
In another set of experiments, equal amounts of protein extracts (~25 μg, as used for glucanase activity assay) from ripe fruit of Rasthali, Kanthali, and Monthan were added to the polysaccharide fraction from unripe and ripe fruit of the respective cultivars. In Rasthali and Kanthali, glucanase activity was high with unripe polysaccharide fractions and reduced when ripe polysaccharide fractions was used as substrate. Conversely, in Monthan fruit protein extract, very low glucanase activity was detected against both unripe and ripe fruit polysaccharide fractions (Fig. 5f). Furthermore, when ripe fruit protein extract from Rasthali was incubated with unripe and ripe polysaccharide fractions from Monthan fruit, higher glucanase activity was detected in unripe polysaccharide fraction and a slightly low activity with ripe polysaccharide fractions (Fig. 5g). On the other hand, similar experiments with ripe fruit protein extract from Monthan and polysaccharide fraction from unripe and ripe fruit of Rasthali yielded low glucanase activity with both unripe and ripe polysaccharide fractions (Fig. 5g). Taken together, these results may indicate that wall polysaccharide fractions (may include the natural substrate of banana β-1,3 glucanase) may not vary considerably among the three cultivars. However, in Monthan the natural substrate for β-1,3 glucanase may not be fully utilized due to low glucanase activity level in vivo and ethylene did not stimulate the endogenous glucanase activity in this cultivar, resulting in considerably high glucanase activity against polysaccharide preparations from both unripe and ripe fruits after ethylene treatment when either recombinant enzyme or protein extract from Rasthali were used as enzyme source. In control experiment, crude polysaccharide fraction was incubated on the absence of recombinant β-1,3 glucanase or fruit protein extracts for the detection of glucanase activity and generation of ethanol-soluble sugar fractions.
Discussion
The β-1,3 glucanases appear to have a broad range of functions in plant tissues including both defense and non-defense related activities. Antimicrobial activity has been reported in a number of specific plant tissues such as seed (Bewley and Black 1994), bud (Krabel et al. 1993), style of flower (Ori et al. 1990), anther (Hird et al. 1993), and fruit (Dong and Dunstan 1997). Differential screening of cDNA library in banana has revealed increased transcript abundance of gene encoding β-1,3 gluc during ripening period in banana fruit pulp (Clendennen and May 1997). Peumans et al. (2000) have reported the purification and biochemical characterization of an abundant class of enzymatically active form of fruit-specific β-1,3 glucanase from Cavendish banana and also predicted the possible physiological function of this protein in softening during banana fruit ripening. Kesari et al. (2007) have recently shown that a number of stress/defense related genes like osmotin, PR1 and β-1,3 gluc are induced in response to ethylene in banana. Therefore, it can be argued that in addition to the usual function, these defense, and/or stress-related genes like β-1,3 gluc or osmotin or PR1 encoding enzymes, which may have alternative functional task during ethylene-mediated ripening process in banana. In non-climacteric fruit such as strawberry, a higher β-xylosidase activity (responsible for degradation of cell wall xylan) together with higher mRNA and protein levels of the enzyme has been reported in softest strawberry cultivar (Bustamante et al. 2006), indicating the function of the enzyme in fruit softening. Previous research has also reported considerable expression level of two β-1,3 glucanase genes in strawberry fruit during ripening (Shi et al. 2006). On the other hand, since the anti-fungal property of banana β-1,3 gluc is still not well established and the fruit pulp tissue remains well protected from invading fungal pathogen, the function of this enzyme in defense is questionable. Based on these observations, we readdressed the issue of potential role of this enzyme in fruit pulp softening during ripening in banana fruit.
Although it is possible that various fruit contain similar enzymes for the modification of cell walls, the complexity of this mechanism and its regulation to control the overall process of cell wall modification and fruit softening is not yet fully understood (Ali et al. 1998; Hadfield et al. 1998; Smith and Gross 2000). However the timing, rate and magnitude of enzyme catalyzed modifications vary remarkably with the fruit type (McCollum et al. 1989; Mitcham and McDonald 1992; Muda et al. 1995; Redgwell et al. 1992). In this work, we have studied the fruit pulp softening rates in the three banana cultivars along with the changes in expression profile and enzymatic activity of β-1,3 gluc gene during ripening to understand the possible involvement of the enzyme in softening in the banana cultivars.
Daily estimation of glucanase activity and changes in fruit firmness during ripening has revealed an interesting and distinct pattern of fruit pulp softening in the three cultivars. A notable difference in the expression profile of β-1,3 gluc gene during the ripening days was also evident in the three cultivars. The expression profiles of β-1,3 gluc showed appreciable correlation with the differential pattern of glucanase activity and fruit pulp softening rates in Rasthali, Kanthali, and Monthan. Maximum expression of β-1,3 gluc and glucanase activity was detected in Rasthali, the softest cultivar, while Monthan, the hardest cultivar, showed highly reduced expression of the gene as well as glucanase activity.
In Rasthali, fruit firmness decreased noticeably at the onset of climacteric stages. Conversely, in Kanthali, fruit pulp softening was initiated slowly at postclimacteric stages. Postclimacteric Monthan (day 15 after harvest) fruit showed ~3.4, and 2.2-times less glucanase activity, while ~10 times firmer fruit at the similar stage of ripening as compared to Rasthali (AAB) and Kanthali (AB), respectively. On the other hand, in Monthan (ABB), consistent with the highly reduced β-1,3 gluc transcript level, glucanase activity remained very low throughout the ripening stages and only negligible pulp softening was observed in mature fruit in Monthan even after ethylene treatment. It was interesting to observe that the expression level of β-1,3 gluc, glucanase activity and fruit softening rates changed considerably as a consequence of incorporation of B complement in the triploid banana genome. β-1,3 gluc expression and glucanase activity was fairly high in the most popular cultivar Cavendish with autotriploid genome (AAA) (data not shown). The expression and activity of the gene decreased slightly in Rasthali (AAB), while in Monthan (ABB) β-1,3 gluc expression and softening rate decreased appreciably. Kanthali (AB) displayed an intermediate trait in between Rasthali and Monthan in relation to β-1,3 gluc expression, glucanase activity, and fruit softening rates. As like Rasthali, we have also found somewhat similar kind of expression of β-1,3 gluc, glucanase activity and fruit softening pattern in other banana cultivars bearing AAB genome. On the other hand, similar to Monthan, cultivars with ABB genome showed highly reduced expression of β-1,3 gluc and softening rates during ripening days (data not shown). Together, these comparative observations may suggest the possible physiological function of β-1,3 glucanase in fruit pulp softening during ripening in banana fruits and differential stimulation of glucanase activity during ripening in these cultivars.
It was also interesting to note that although the mRNA level of β-1,3 gluc reached the maximum level during the early ripening following exogenous application of ethylene (as clearly observed in Rasthali) and then gradually declined at the later stages, the glucanase activity was still high at the later stages of ripening. Protein gel blot analysis also revealed considerable accumulation of β-1,3 glucanase protein even in the mature ethylene-treated fruits. One of the possible explanations for this discrepancy between the activity and mRNA level may be due to the faster degradation of β-1,3 gluc mRNA than the protein and/or due to the activity of other proteins which might have protected the rapid loss of activity of glucanase at the post phase of ripening.
The study with recombinant protein and polysaccharide fractions provided interesting clue for the differential utilization of the natural substrate for β-1,3 glucanase during ripening in the three cultivars. Study of glucanase activity using purified recombinant enzyme and fruit protein extracts from unripe and ripe fruit with wall polysaccharide fractions as substrate have demonstrated that under natural conditions there was clear difference in the activity level of glucanase in the banana cultivars. In Monthan, higher glucanase activity of the recombinant protein against both unripe and ripe fruit wall polysaccharide fractions (naturally ripened or ethylene treated fruit) have indicated that in this cultivar, the substrate for glucanase remain mostly unutilized possibly due to low glucanase activity in vivo and even ethylene treatment did not induce the endogenous glucanase activity during ripening days in this cultivar. These observations also reflect the difference in the expression level of the gene and abundance of catalytically active β-1,3 glucanase protein, resulting in distinct glucanase activity and pulp softening rates in the cultivars.
Our studies in relation to β-1,3 gluc expression have demonstrated that the gene is induced at the onset of climacteric ethylene production during fruit ripening. However, analysis of β-1,3 gluc mRNA accumulation pattern during ripening days in the three cultivars has indicated differential expression profile of the gene and correlated well with the pattern of ripening ethylene production rates in the cultivars as observed previously (Roy Choudhury et al. 2008a). Induction of β-1,3 gluc expression shortly after ethylene treatment (as in Rasthali) and inhibition of expression after KMnO4 treatment clearly suggested that β-1,3 gluc gene is regulated by ethylene. However, differential transcriptional stimulation of β-1,3 gluc during ripening in Rasthali and Kanthali may indicate distinct regulation pattern of the gene in the two cultivars. In contrast, very low expression of the gene during natural or ethylene-mediated ripening in Monthan together with remarkably reduced glucanase activity and fruit softening rate may indicate ethylene insensitive nature of this cultivar. Taken together, it may be suggested that β-1,3 gluc is ripening-regulated while differentially regulated in the three banana cultivars, leading to distinct fruit softening pattern in these cultivars. Although we were unable to specifically identify the natural substrate for banana β-1,3 glucanase in the wall polysaccharide fractions, our results have provided interesting information about the difference on the availability of catalytically active form of β-1,3 glucanase particularly in Rasthali and Monthan fruit.
We have shown contrasting pattern of transcriptional stimulation of β-1,3 gluc gene in the cultivars, especially in Rasthali and Monthan in response to ethylene. This has raised a more important question whether there are differences in the promoters of β-1,3 gluc gene in the cultivars, particularly in relation to ethylene-mediated transcriptional induction of the respective promoters. Therefore, further research is needed on the transcriptional apparatus of β-1,3 gluc gene to gain insight into the mechanism of ethylene-mediated regulation of this gene in different banana cultivars.
Change history
11 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00299-023-03122-6
References
Abeles FB, Bosshart RP, Forrence LE, Habig WH (1970) Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol 47:129–134
Ali ZM, Ng SY, Othman R, Goh LY, Lazan H (1998) Isolation, characterization and significance of papaya β-galactanases to cell wall modifications and fruit softening during ripening. Physiol Plant 104:105–115
Bewley JD, Black M (1994) Seeds: physiology of development and germination, 2nd edn. Plenum Press, New York
Brady CJ (1987) Fruit ripening. Annu Rev Plant Physiol 38:155–173
Brummell DA, Cin VD, Crisosto CH, Labavitch JM (2004) Cell wall metabolism during maturation, ripening and senescence of peach fruit. J Exp Bot 55:2029–2039
Bustamante CA, Rosli HG, Anon MC, Civello PM, Martinez GA (2006) β-Xylosidase in strawberry fruit: isolation of a full-length gene and analysis of its expression and enzymatic activity in cultivars with contrasting firmness. Plant Sci 171:497–504
Chin LH, Ali ZM, Lazan H (1999) Cell wall modifications, degrading enzymes and softening of Carambola fruit during ripening. J Exp Bot 50:767–775
Clendennen KS, May DG (1997) Differential gene expression in ripening banana fruit. Plant Physiol 115:463–469
Cosgrove DJ (2001) Wall structure and wall loosening. A look backwards and forwards. Physiol Plant 125:131–134
Dong J-Z, Dunstan DI (1997) Endochitinase and β-1,3-glucanase genes are developmentally regulated during somatic embryogenesis in Picea glauca. Planta 201:189–204
Fischer RL, Bennett AB (1991) Role of cell wall hydrolases in fruit ripening. Annu Rev Plant Physiol Plant Mol Biol 42:675–703
Giovannoni JJ, DellaPenna D, Bennett AB, Fischer RL (1992) Polygalacturonase and tomato fruit ripening. Hortic Rev 13:67–109
Hadfield KA, Rose JKC, Yaver DS, Berka RM, Bennett AB (1998) Polygalacturonase gene expression in ripe melon fruit supports a role for polygalacturonase in ripening associated pectin disassembly. Physiol Plant 117:363–373
Hall CB (1987) Firmness of tomato tissues according to cultivars and ripeness. J Am Soc Hortic Sci 112:663–665
Hird DL, Worrall D, Hodge R, Smartt S, Paul W, Scott R (1993) The anther-specific protein encoded by the Brassica napus and Arabidopsis thaliana A6 gene displays similarity to β-1,3-glucanases. Plant J 4:1023–1033
Kesari R, Trivedi PK, Nath P (2007) Ethylene-induced ripening in banana evokes expression of defense and stress related genes in fruit tissue. Postharvest Biol Technol 46:136–143
Kojima K, Sakurai N, Kuraishi S (1994) Fruit softening in banana: correlation among stress-relaxation parameters, cell wall components and starch during ripening. Physiol Plant 90:772–778
Krabel D, Eschrich W, Wirth S, Wolf G (1993) Callase-(1,3-β-D-glucanase) activity during spring reactivation in deciduous trees. Plant Sci 93:19–23
Leubner-Metzger G, Meins F Jr (1999) Functions and regulation of plant β-1,3-glucanases (PR-2), Review. In: Datta SK, Muthukrishnan S (eds) Pathogenesis-related proteins in plants. CRC Press LLC, Boca Raton, pp 49–76
McCollum TG, Huber DJ, Cantliffe DJ (1989) Modification of polyuronides and hemicelluloses during muskmelon fruit softening. Physiol Plant 76:303–308
Micheli F (2001) Pectin methylesterase: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6:414–419
Mitcham EJ, McDonald RE (1992) Cell wall modification during ripening of ‘Keitt’ and ‘Tommy Atkins’ mango fruit. J Am Soc Hortic Sci 117:919–924
Muda P, Seymour GB, Errington N, Tucker GA (1995) Compositional changes in cell wall polymers during mango fruit ripening. Carbohydr Polym 26:255–260
Ori N, Sessa G, Lotan T, Himmelhoch S, Fluhr R (1990) A major stylar matrix polypeptide (sp41) is a member of the pathogenesis-related proteins superclass. EMBO J 9:3429–3436
Peumans WJ, Barre A, Derycke V, Rougé P, Zhang W, May GD, Delcour JA, Leuven FV, Van Damme EJM (2000) Purification, characterization and structural analysis of an abundant -1,3-glucanase from banana fruit. Eur J Biochem 267:1188–1195
Redgwell RJ, Melton LD, Brasch DJ (1992) Cell wall dissolution in ripening kiwifruit (Actinidia deliciosa). Solubilization of the pectic polymers. Physiol Plant 98:71–81
Rose JKC, Bennett AB (1999) Cooperative disassembly of the cellulose-xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends Plant Sci 4:176–183
Roy Choudhury S, Roy S, Saha PP, Singh SK, Sengupta DN (2008a) Characterization of differential ripening pattern in association with ethylene biosynthesis in the fruits of five naturally occurring banana cultivars and detection of a GCC-box specific DNA binding protein. Plant Cell Rep 27:1235–1249
Roy Choudhury S, Roy S, Sengupta DN (2008b) Characterization of transcriptional profiles of MA-ACS1 and MA-ACO1 genes in response to ethylene, auxin, wounding, cold and different photoperiods during ripening in banana fruit. J Plant Physiol 165:1865–1878
Scott KJ, McGlasson WB, Roberts EA (1970) Potassium permanganate as an ethylene absorbent in polyethylene bags to delay ripening of bananas during storage. Aust J Exp Agric 10:237–240
Shi Y, Zhang Y, Shih DS (2006) Cloning and expression analysis of two β-1,3-glucanase genes from Strawberry. J Plant Physiol 163:956–967
Smith DL, Gross KC (2000) A family of at least seven β-galactosidase genes is expressed during tomato fruit development. Plant Physiol 123:1173–1183
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol Biol Evol 24:1596–1599
Trivedi P, Nath P (2004) MaExp1, an ethylene-induced expansion from ripening banana fruit. Plant Sci 6:1351–1358
Von Heijne G (1986) A new method for predicting signal sequence cleavage sites. Nucleic Acids Res 14:4683–4690
Acknowledgments
We thank Prof. Frederick Meins, FMIBR, Basel, Switzerland for providing the antibody of β-1,3 glucanase. We gratefully acknowledge the financial assistance from Council for Scientific and Industrial Research (CSIR). We thank Mr. Jadav Ghosh, Department of Botany, Bose Institute, for the necessary technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Lakshmanan.
S. Roy Choudhury and S. Roy contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2009_764_MOESM1_ESM.tif
Supplementary Fig 1. The phylogenetic tree was constructed based on the deduced amino acid sequences of different fruit-specific β 1,3 glucanases. The tree was constructed using neighbor joining method with the best tree mode by Mega4.1 version. A value of 0.1 corresponds to a difference of 10% between the two sequences (TIFF 723 kb)
Rights and permissions
About this article
Cite this article
Roy Choudhury, S., Roy, S. & Sengupta, D.N. Characterization of cultivar differences in β-1,3 glucanase gene expression, glucanase activity and fruit pulp softening rates during fruit ripening in three naturally occurring banana cultivars. Plant Cell Rep 28, 1641–1653 (2009). https://doi.org/10.1007/s00299-009-0764-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0764-5