Abstract
MicroRNAs (miRNAs) are a class of small non-coding RNAs which play a critical role in plant growth and development. To detect strawberry miRNAs and discover the expression difference between conventional and micropropagated strawberry plants, we carried out the detection and quantification of strawberry miRNAs by microarray. The main findings were that 74 miRNAs were checked in strawberry plants and four miRNA genes displayed clear expression difference between conventional and micropropagated strawberry plants, including two up-regulated genes (miR535 and miR390) and two down-regulated genes (miR169a and miR169d). The ratios of conventionally propagated strawberry plant/micropropagated strawberry plant for miR535, miR390, miR169a and miR169d were 2.6884, 2.2673, 0.2496 and 0.3814, respectively. Quantitative reverse transcription polymerase chain reaction applied to the two up-regulated genes (miR535 and miR390) validated the microarray result. This is the first report on differential expression of miRNAs in conventional and micropropagated plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Strawberry is one of the most widely consumed fruits throughout the world. Micropropagation to multiply elite cultivars by tissue culture technology has been used on a commercial basis in Europe since the 1970s (Boxus 1974), and its use is now growing in China. Despite the widespread cultivation of micropropagated strawberry plants, several problems are encountered in the use of this means of multiplication (Litwińczuk 2004). One of the most serious of these concerns the appearance of unwanted variation among micropropagated plantlets. This variability reflects a combination of sequence mutation (which occurs at a relatively low frequency) and the much more common epigenetic events (Kaeppler et al. 2000). The phenomenon of de novo epigenetic variation among micropropagated plants has been well established (Cassells and Curry 2001), but little emphasis has been given to exploring the mechanism.
Epigenetics is used in reference to the alteration of phenotype, morphological or molecular, without change in either the coding sequence of a gene or the upstream promoter region (Rapp and Wendel 2005). Epigenetic variation is the changes in gene expression that are mitotically or meiotically heritable (Schubert et al. 2005). The alteration of gene activity can be accomplished by several self-reinforcing and inter-related covalent modifications on DNA and/or chromosomal proteins, such as DNA methylation and histone modifications, by chromatin remodeling, such as repositioning of nucleosomes, and by RNA silencing mediated by small RNAs (Liu and Wendel 2003; Baulcombe 2004).
miRNAs are small, non-coding regulatory RNAs which regulate gene expression by guiding targeted mRNA cleavage or translational inhibition (Bartel 2004; Sunkar and Zhu 2004; Park et al. 2005; Brodersen et al. 2008). A growing body of evidence suggests that they are important controllers in many aspects of plant growth and development (Chen 2004; Lauter et al. 2005; Kim et al. 2005; Wang et al. 2005). Dysfunction of individual miRNAs or their ability to properly regulate their targets has been shown to cause normal plants to produce a series of changes, such as loss of organ polarity and altered vascular development (miR165/166; Juarez et al. 2004; McHale and Koning 2004; Kim et al. 2005), floral and leaf-patterning defects (miR159; Palatnik et al. 2003; Achard et al. 2004; Millar and Gubler 2005), defective organ separations and aberrant numbers of floral organs (miR164; Laufs et al. 2004; Baker et al. 2005), floral development and timing defects (miR172; Aukerman and Sakai 2003; Chen 2004), aberrant phyllotaxis and abortion of the shoot apical meristem (miR168; Vaucheret et al. 2004), and symmetry defects of cotyledon and leaf shape (miR160; Mallory et al. 2005).
Till now, miRNAs have been identified from the model species Arabidopsis thaliana (Sunkar and Zhu 2004; Adai et al. 2005), Oryza sativa (Sunkar et al. 2005) and Populus trichocarpa (Lu et al. 2005; Tuskan et al. 2006). They are also identified from other plant species, such as Nicotiana tabacum (Billoud et al. 2005), Medicago truncatula, Glycine max, Saccharum officinarum and Zea mays (Dezulian et al. 2005), Triticum aestivum (Yao et al. 2007), Lycopersicon esculentum (Yin et al. 2008), Gossypium hirsutum (Qiu et al. 2007), Brassica napus (Xie et al. 2007), the moss Physcomitrella patens (Arazi et al. 2005; Talmor-Neiman et al. 2006a), and the algae Chlamydomonas reinhardtii (Molnár et al. 2007; Zhao et al. 2007b). Although the monocot and eudicot clades are thought to have diverged from one another over 125 Mya, it has been suggested that the sequence of many plant miRNAs has remain conserved between these two ancient plant groups (Bonnet et al. 2004; Jones-Rhoades and Bartel 2004; Wang et al. 2004; Adai et al. 2005). For example, a majority of 42 miRNAs families including 117 miRNAs in Arabidopsis have also been found in other species, such as rice, maize, and sorghum (Griffiths-Jones 2004). Axtell and Bartel (2005) have found that the miR159/319 family exists in ten land plant species. This allows an opportunity to apply experimental strategies in a plant whose genome is not sequenced, based on conversed miRNA sequence information taken from well researched plant species.
The genome sequences of cultivated strawberry (Fragaria × ananassa Duch.) are still largely unknown. To date, there is no report on detection and identification of miRNAs in strawberry. Thus we set out to use microarray and quantitative-reverse transcription-polymerase chain reaction (Q-RT-PCR) to compare the miRNA content of conventional and micropropagated strawberry plants, in order to gain insight into the expressional difference of miRNAs in epigenetic variation of tissue-cultured plants.
Materials and methods
Plant material
The strawberry cultivar ‘Toyonoka’ was maintained in a greenhouse at Shenyang Agricultural University and conventionally propagated by runner plants. The micropropagation procedure was as follows: runners were collected from actively growing plants and disinfected with 0.1% w/v HgCl2 as suggested by Dai et al. (2007). Shoot tips of ~0.3 mm in length were excised from each runner and placed on MS medium (Murashige and Skoog 1962) supplemented with 0.2 mg/l 6-benzylaminopurine, 0.1 mg/l gibberellic acid and 0.01 mg/l indole-3-butyric acid (IBA) to establish in vitro shoot proliferation in May 2007. The explants were held at 23 ± 2°C under white fluorescent light (60 μmol s−1 m−2) with a 14 h photoperiod. Plantlets were sub-cultured onto the same medium at 5-week intervals. Unrooted plantlets were transferred to half-strength MS medium supplemented with 0.01 mg/l IBA. Rooted plants were transferred to plug trays containing peat moss, vermiculite and soil (2:1:1 v/v) in a solar greenhouse in the end of January 2008. The survived micropropagated plants were replanted to 12 cm plastic pots in the mid April, at the same time, the conventionally propagated runner plants of ‘Toyonoka’ strawberry were dug from open field and replanted to 12 cm plastic pots in the solar greenhouse. On the sixth of May, ten strawberry plants with same the plant size and vigor were selected from the micropropagated and conventionally propagated groups separately. The young leaves were collected from both conventional and micropropagated plants, treated with liquid nitrogen and stored at −70°C.
RNA extraction and microarray analysis
Total RNA was isolated from strawberry leaves essentially as described by Chang et al. (2007). Before precipitation, the RNA was treated with 15 U RNase-free DNase I (TaKaRa Biotechnology Co., Dalian, China) at 37°C for 4 h. RNA integrity was assessed by 1.0% agarose gel electrophoresis, and its concentration was estimated in a DU800 spectrophotometer (Beckman Coulter, Fullerton, CA, USA). High molecular weight RNA was precipitated and removed by the addition of 12.5% (v/v) PEG8000 and 1.25 M NaCl. The labeling of the low molecular weight RNA fraction used a modified version of the protocol described by Thomson et al. (2004). Briefly, the labeling reaction contained RNA (typically 25 μg before PEG precipitation), 0.1 mM ATP, 50 mM HEPES, pH 7.8, 3.5 mM DTT, 20 mM MgCl2, 10 mg/ml BSA, 10% DMSO, and 500 ng 5′-phosphate-cytidyl-uridyl-Cy3-3′ or 5′-phosphate-cytidyl-uridyl-Cy5-3′ (Dharmacon) with 20 units T4 RNA ligase (New England Biolabs, Inc, Ipswich, MA, USA). The labeling reaction was allowed to proceed at 4°C for 4 h. Labeled RNA was precipitated with 0.3 M sodium acetate, pH 5.2, 2.5 volumes ethanol, and resuspended in 16 μl hybridization solution containing 3 × SSC, 0.2% SDS, 15% formamide and 50 × Denhardt’s.
A miRNA microarray (CapitalBio, Beijing, China) containing 426 well-characterized miRNAs from Arabidopsis, rice, maize, sorghum, etc and various controls was used (see supplementary material table 1). The procedure for microarray hybridization and data evaluation were as previously described in detail (Yang et al. 2002). In brief, the hybridization was performed at 42°C for 16 h, and then washed in a solution containing 2 × SSC and 0.2% SDS at 42°C for 4 min. After the last washing with 0.2 × SSC solution at room temperature for 4 min and spin-drying, slides were scanned with LuxScan 10 K-A scanner (CapitalBio, Beijing, China) and raw pixel intensities were extracted with LuxScan 3.0 software. Each miRNA probe had three replicate spots on a microarray, and the levels of significance of differentially expressed miRNAs were analyzed using SAM (Significance Analysis of Microarrays) software.
A space and intensity-dependent normalization based on the LOWESS program (Yang et al. 2002) was employed. For each test and control sample, two hybridizations were performed by using a reversal fluorescent strategy. Cy3 and Cy5 intensities were normalized and corrected by a coefficient based on the ratio of control genes. Only genes whose intensity value of fluorescent signals maintained beyond 400 in both microarrays were selected as checked genes. And the alteration tendency kept consistency (both above twofold) in both microarrays were selected as differentially expressed genes.
Reverse transcription PCR (RT-PCR)
RT-PCR employed a stem-loop primer (Fig. 3a) to detect miRNAs. The primers were based on those described by Chen et al. (2005). Their 3′ end was complementary to both the ~8 nucleotides at the miRNA 3′ end and the ~7 nucleotides of the primer’s 5′ end, which ensured the formation of a stem-loop structure. Subsequent PCRs used a 5′ primer matching the ~10 nucleotides at the 5′ end of the target miRNA. The 3′ primer was a universal reverse transcription (RT) primer. This primer design left three ‘unconstrained’ nucleotides to recognize specific miRNAs, and these were used to confirm the identity of the amplified miRNA following cloning and sequencing of the PCR amplicon.
The 20 μl RT reactions contained 2 μg total RNA, 1 μl 5 μM stem-loop primer (Table 1), 1 μl 10 mM dNTP, 1 μl 5 U/μl AMV reverse transcriptase (TaKaRa Biotechnology Co, Dalian, China), 4 μl 5 × AMV buffer and 1 μl 40 U/μl RNase inhibitor. The template, primer and dNTP mixture was heated to 65°C for 5 min, and quenched on ice for at least 5 min. Then the remaining reagents were added, and the complete reaction incubated at 16°C for 30 min, followed by 60 cycles of 20°C/30 s, 42°C/30 s and 50°C/1 s. The AMV reverse transcriptase was inactivated by heating the reactions to 85°C for 10 min, and then cooling and holding at 4°C. All reactions (including no template and no RT controls) were performed in duplicate.
PCRs (20 μl) contained 1 μl cDNA, 0.2 μl 5 U/μl Taq DNA polymerase (TianGen, Beijing, China), 2 μl 10 × PCR buffer, 1.6 μl 25 mM MgCl2, 1 μl 2.5 mM dNTP, 1.6 μl 5 μM forward primer and 1.6 μl 5 μM reverse PCR primer (Table 1). The PCR regime consisted of an incubation at 95°C for 10 min and 55°C for 2 min, followed by 35 cycles of 95°C/1 s and 65°C/1 min, ending with an incubation of 72°C for 5 min. Amplified PCR products were separated by electrophoresis through 2.5% agarose gels and visualized by ethidium bromide staining.
PCR fragments were recovered from the agarose gel using a DNA purification kit (TaKaRa Biotechnology Co., Dalian, China) and ligated into a pMD19-T cloning vector (TaKaRa Biotechnology Co., Dalian, China) for transformation into Escherichia coli JM109 competent cells. Plasmids were isolated from randomly selected colonies, and clone identity was established by PCR. Plasmid inserts were commercially sequenced by Sangon Biological Engineering & Technology and Service Co. Ltd. (Shanghai, China).
Q-RT-PCR
In order to verify the data obtained from microarray, Q-RT-PCR (Fig. 3b) experiments using a stem-loop primer were performed using a standard TaqMan PCR protocol on an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The 20 μl reaction included 1 μl RT product, 8 μl 2.5 × realMaster Mix (TianGen, Beijing, China), 1 μl 20 × probe enhancer solution, 0.5 μl 10 μM TaqMan probe, 1 μl 10 μM forward primer and 1 μl 10 μM reverse primer. The reactions were incubated in a 96-well plate (Applied Biosystems) at 95°C for 10 min, followed by 40 cycles of 95°C/15 s and 60°C/60 s. Relative fold changes in miRNA expression were calculated using the comparative Ct (2−∆∆Ct) method with 18S rRNA as the endogenous control. Four biological replicates of two kinds of strawberry plants were performed, and in each biological replicate the genes and no template control were carried out in triplicate. The threshold cycle (Ct) was defined as the cycle number at which the fluorescence signal exceeded the fixed threshold.
Results
Analysis of miRNAs in strawberry plants by microarray
In order to simultaneously test whether the expression of any of the mainly known miRNAs was different between conventional and micropropagated strawberry plants, the miRNA microarray containing 426 probes that were complementary to mainly known plant miRNAs was used. On the miRNA microarray, eight short oligos possessing no homology with any existing miRNA sequences were designed as negative controls, and a transcriptional repressor Hex as positive control was also included (see supplementary material table 1). Total RNA was extracted from two kinds of strawberry plants and low molecular weight RNA was obtained after removing high molecular weight RNA with PEG8000. One miRNA chip was hybridized with Cy5 probe pair of conventionally propagated strawberry plants and Cy3 probe pair of micropropagated strawberry plants, while the other miRNA chip was hybridized with Cy5 probe pair of micropropagated strawberry plants and Cy3 probe pair of conventionally propagated strawberry plants. The hybridized microarray was scanned by laser channels for Cy3 and Cy5 emissions. The fluorescent signal intensity of a spot was decided by Cy5 and Cy3 which allowed internal normalization of the experiments. The ratio of the conventionally propagated strawberries and micropropagated strawberries’ fluorescent signal intensities was calculated as a relative measure to determine changes in the differential expression of the oligomer sequence represented by miRNA spots on the microarrays.
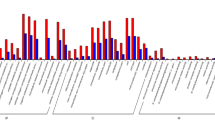
A double-color fluorescent comparison profile (Cy3 and Cy5 labeled) of the microarray is shown in Fig. 1. The standard quality-control measures indicated that the micorarray used in this analysis showed a similar distribution of intensities, and none of the negative control probes gave a detectable signal in any of the samples. On the miRNA microarray, 1,278 spots were used to provide three replicates of 426 known miRNA genes because of each miRNA probe with three replicate spots on a microarray (including 348 documented in the Sanger Centre miRBase database (http://microrna.sanger.ac.uk/sequences/, version 9.1) and the rest was predicted by Sunkar et al (2005) and Chinese Academy of Sciences). The six replicated experiments based on two chips revealed that 74 miRNAs whose intensity value of fluorescent signals maintained beyond 400 in both microarrays were checked in strawberry leaves. Of these, the 46 present in the public access (Sanger) database have been listed in Table 2, and these represent 21 miRNA gene families. Their conservation was analyzed between distantly related land plants, for example the dicotyledonous Arabidopsis, the monocotyledonous rice and maize and the moss P. patens according to Sanger database. In the 21 miRNA gene families (Fig. 2), 15 (miR157, 159, 162, 165, 166, 167, 169, 171, 172, 390, 391, 393, 394, 396 and 399) of these miRNAs were also detected in A. thaliana, and the sequences of 12 of them were completely conserved in rice (the exceptions were miR157, 165 and 391). Three rice miRNA families (miR156, 170 and 535) were also present in strawberry. Five miRNA families (miR156, 159, 166, 167 and 169) were conserved across maize, sorghum and soybean. Among them, four miRNA families (miR159, 166, 167 and 169) deeply conserved in many plants from eudicots to monocots, even to gymnosperms (Table 2). This is consistent with the result of Axtell and Bartel (2005). miR169 represents the largest miRNA family in Arabidopsis, which are encoded by 14 different genomic loci (http://asrp.cgrb.oregonstate.edu). It is represented by at least 11 loci in maize, 16 loci in rice, 31 loci in P. trichocarpa and 9 loci in Sorghum bicolor (http://microrna.sanger.ac.uk/sequences/). Our result showed that there were five members for miR169 family in F. ananassa, which was the second largest miRNA family in the checked 21 miRNA families in strawberry.
Detection of miRNAs in strawberry plants by RT-PCR
RT-PCR based on stem-loop primers was demonstrated to be a method with high sensitivity and specificity for detecting miRNAs in animals and plants (Chen et al. 2005; Tang et al. 2006; Varkonyi-Gasic et al. 2007). In order to verify the assay effect of miRNA microarray, RT-PCR using stem-loop primer (Fig. 3a) was carried out to detect the presence of miRNA in strawberry leaves. RT-PCR primers were targeted to the 14 specific miRNAs (miR156, 157, 159, 162, 164, 165, 167, 168, 169, 170, 171, 172, 390 and 535) identified by the microarray analysis. The resulting amplicons were of the predicted length of ~75 bp (Fig. 3c). All the amplicons were sequenced and analyzed. The results showed that their sequence included both that of the primers and the ‘unconstrained’ nucleotides. All 14 miRNAs were detectable in both the conventional and micropropagated plants.
Differentially expressed miRNAs between conventional and micropropagated strawberry plants
A criterion of fold change >2 and P value < 5% was used to examine the differentially expressed miRNAs by microarray. The SAM software generated a plot of miRNAs which were expressed differentially between conventional and micropropagated strawberry plants (Fig. 4). We found that four miRNAs produced such a difference, including two up-regulated genes (marked in red) and two down-regulated genes (green). The former were miR535 (ratio = 2.6884) (a rice sequence) and miR390 (ratio = 2.2673) (an A. thaliana sequence), while the latter were miR169a (ratio = 0.2496, from A. thaliana) and miR169d (ratio = 0.3814, from rice).
In many cases of study global gene expression patterns by microarray, attempts were made to confirm the microarray results using Q-RT-PCR (Thomson et al. 2004; Poole et al. 2007). Q-RT-PCR is generally more sensitive than Northern blot analysis, and allows absolute quantification of transcript abundance. So Q-RT-PCR analysis was performed to determine which locus was responsive to two kinds of propagated methods in this paper. When Q-RT-PCR (Fig. 3b) was applied to the two up-regulated genes to validate the microarray result, the ratio of miR535 expression between the conventional and micropropagated plants was 4.262, while that for miR390 was 3.820 (Fig. 5). The same result that the expression of miR535 and miR390 was both higher in conventionally propagated strawberry plants than that in micropropagated strawberry plants was found by using two methods.
Discussion
Currently, a variety of biochemical, molecular, and bioinformatic approaches and technologies have been developed for miRNA analysis and detection (Schena et al. 1995; Eisen and Brown 1999). Using microarray technology, it is now possible to perform high-throughput profiling of the expression of all the known miRNAs to examine their expression profiles under different conditions (Garzon et al. 2006; Zhao et al. 2007a; Liu et al. 2008). This technology has proven to be semiquantitative, sensitive, and highly reproducible in experiments with vertebrate miRNAs (Baskerville and Bartel 2005). Many plant miRNAs are members of closely related families that differ by a few nucleotides or only one nucleotide in sequences. In order to distinguish between different members of a miRNA family, several normalization methods, such as LOWESS normalization, basing on ‘housekeeping’ genes and replicate hybridizations for each miRNA, arraying separate spots for closely related family members, have been developed for microarray data (Thomson et al. 2004; Axtell and Bartel 2005). These methods had also been adopted in this study. Luo et al. (2007) showed that the difference by only one nucleotide in the center portion of the sequence of miRNAs could be differentiated in microarray analysis because the fluorescent signal intensities of perfect match were more above 2.1 times than that of mismatch with only one nucleotide difference about Let-7.
Some plant miRNA families have remained conserved not only in sequence but also in function. For example, miR166 is deeply conserved across eudicots, monocots, and gymnosperms, whose conservation in function between eudicots and monocots has been experimentally demonstrated (McConnell et al. 2001; Emery et al. 2003; Tang et al. 2003; Juarez et al. 2004; McHale and Koning 2004; Zhong and Ye 2004), and the conservation of target mRNA cleavage at the canonical site has also been shown to occur in the lycopod Selaginella kraussiana (Floyd and Bowman 2004). The sequence of miR169 is also deeply conserved in many plants, whose target genes in A. thaliana and M. truncatula encode the transcription factors HAP2s (Jones-Rhoades and Bartel 2004; Combier et al. 2006). HAP (also known as NF-Y or CBF) is a ubiquitous transcription factor with high affinity and sequence specificity for the CCAAT box, a cis-element present in 25% of eukaryotic gene promoters (Li et al. 2008). HAP protein complexes appear to play central roles during development by regulating cell division and/or differentiation in eukaryotes (Bhattacharya et al. 2003; Agostino et al. 2006). In plants, HAP genes have been shown to be key regulators of embryogenesis, chloroplast biogenesis, and abscisic acid response, etc (Lotan et al. 1998; Kwong et al. 2003; Lee et al. 2003; Miyoshi et al. 2003; Warpeha et al. 2007). In M. truncatula, MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development (Combier et al. 2006). The complementary expression pattern of MtHAP2-1 and miR169 indicates that the miR169-mediated restriction of MtHAP2-1 expression to the nodule meristematic zone is essential for the differentiation of nodule cells. NFYA5, a member of the Arabidopsis NF-YA (HAP2) family, is important in controlling stomatal aperture and drought resistance. NFYA5 contains a target site for miR169, and is mainly regulated by miR169a (Jones-Rhoades and Bartel 2004; Li et al. 2008). NFYA5 knockout plants and plants overexpressing miR169a have showed greater stomatal apertures and are more sensitive to drought stress than wild-type plants (Li et al. 2008). In vitro plants have larger stomatal apertures (Brainerd and Fuchigami 1982; Short et al. 1987). In this study, miR169a and miR169d expressed at very high levels in micropropagated strawberry plants. So we deduce that the expression of miR169 could be up-regulated by tissue culture condition.
Trans-acting small interfering RNAs (ta-siRNAs) are a class of plant endogenous siRNAs that have been discovered as regulators of gene expression in plants (Allen et al. 2005; Williams et al. 2005; Talmor-Neiman et al. 2006b). In contrast to other classes of endogenous siRNAs, they typically direct the cleavage of perfectly complementary mRNA targets (Vazquez et al. 2004). Ta-siRNA primary transcript processing represents a new function for miRNAs and a departure from the canonical function of miRNAs as direct negative regulators (Allen et al. 2005). Three families of TAS genes, TAS1, TAS2 and TAS3, have been identified in Arabidopsis (Allen et al. 2005; Yoshikawa et al. 2005). Among these, the TAS3 was identified as target of miR390 in A. thaliana and P. patens (Allen et al. 2005; Talmor-Neiman et al. 2006b). Homologous transcripts of TAS3, with miR390 target sites, have been found in various dicotyledonous and monocotyledonous species, which suggests that TAS3 is conserved in higher plants (Allen et al. 2005). TAS3 ta-siRNAs target the mRNAs associated with several auxin response factors (ARFs), including ARF3 and ARF4 (Allen et al. 2005; Adenot et al. 2006; Fahlgren et al. 2006; Garcia et al. 2006). ARFs are transcriptional activators and repressors that bind with specificity to TGTCTC auxin response element in promoters of primary/early auxin response genes (Tiwari et al. 2003). The juvenile-to-adult phase transition in A. thaliana is normally suppressed by TAS3 ta-siRNAs via the negative regulation of ARF3 mRNA (Fahlgren et al. 2006). ‘Toyonoka’ is a short-day strawberry cultivar, which initiate flower buds either under short-day conditions (less than 10 h of day length) or when temperature is less than 15°C. But in a previous study, we found the characteristics of flower–bud differentiation and flowering of micropropagated strawberry plants changed, i.e., after 60 days of acclimatization in a greenhouse, the micropropagated plants began to initiate flower buds when temperature was more than 20°C and the day length was 13 h (Mi et al. 2007). Here, we have shown, using both a microarray and a Q-RT-PCR approach, that miR390 expression was suppressed in micropropagated strawberry plants. Further experiments are needed to find out whether the low expression of miR390 conduces to the change of flower–bud differentiation characteristics of micropropagated strawberry plants.
Epigenetically induced morphological variation has become a topic of interest in the plant world, such as two classic examples of the concerning the genus Brassica (Pires et al. 2004) and Linaria vulgaris (Cubas et al. 2001). Epigenetic variation is also common among micropropagated plants, such as mantled variation in the oil palm (Jaligot et al. 2000) and hyperflowering response in micropropagated strawberry plants (Jemmali et al. 1994). There were reports on DNA methylation differences exist between the conventional and micropropagated plants (Rival et al. 2008), but there has been no attempt to correlate miRNA expression with epigenetic variation in micropropagated plants. The relationship between miRNAs and epigenetics is complicated. DNA methylation and histone modifications can control the expression of miRNAs (Saito et al. 2006), on the other hand, miRNAs can be involved in establishing DNA methylation (Bao et al. 2004) and may regulate chromatin structure by regulating key histone modifiers (Tuddenham et al. 2006). Here we have been able to demonstrate the existence of differences in miRNA expression between conventional and micropropagated strawberry plants. Among these, it proved that miR535 showed the largest expression difference between the conventional and micropropagated strawberry plants. Until now, miR535 has been detected in several plants, including rice, P. patens and California poppy Eschscholzia californica (Sunkar et al. 2008; Talmor-Neiman et al. 2006a; Barakat et al. 2007). Its target gene has been predicted in rice (Guo et al. 2008), but it need to be supported by further experimental data. And the role of miR535 in epigenetic variation of micropropagated plants needs to be tested by its over-expression or silencing in transgenic plants.
Abbreviations
- IBA:
-
Indole-3-butyric acid
- RT:
-
Reverse transcription
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- Q-RT-PCR:
-
Quantitative-reverse transcription-polymerase chain reaction
- cDNA:
-
Complementary deoxyribonucleic acid
References
Achard P, Herr A, Baulcombe DC, Harberd NP (2004) Modulation of floral development by a gibberellin-regulated microRNA. Development 131(14):3357–3365
Adai A, Johnson C, Mlotshwa S, Archer-Evans S, Manocha V, Vance V, Sundaresan V (2005) Computational prediction of miRNAs in Arabidopsis thaliana. Genome Res 15:78–91
Adenot X, Elmayan T, Lauressergues D, Boutet S, Bouche N, Gasciolli V, Vaucheret H (2006) DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr Biol 16:927–932
Agostino SD, Strano S, Emiliozzi V, Zerbini V, Mottolese M, Sacchi A, Blandino G, Piaggio G (2006) Gain of function of mutant p53: the mutant p53/NF-Y protein complex reveals an aberrant transcriptional mechanism of cell cycle regulation. Cancer Cell 10:191–202
Allen E, Xie Z, Gustafson AM, Carrington JC (2005) MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121:207–221
Arazi T, Talmor-Neiman M, Stav R, Riese M, Huijser P, Baulcombe DC (2005) Cloning and characterization of micro-RNAs from moss. Plant J 43:837–848
Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15:2730–2741
Axtell MJ, Bartel DP (2005) Antiquity of microRNAs and their targets in land plants. Plant Cell 17:1658–1673
Baker CC, Sieber P, Wellmer F, Meyerowitz EM (2005) The early extra petals1 mutant uncovers a role for microRNA miR164c in regulating petal number in Arabidopsis. Curr Biol 15:303–315
Bao N, Lye KW, Barton MK (2004) MicroRNA binding sites in Arabidopsis class Ш HD-ZIP mRNAs are required for methylation of the template chromosome. Dev Cell 7:653–662
Barakat A, Wall K, Leebens-Mack J, Wang YJ, Carlson JE, dePamphilis CW (2007) Large-scale identification of microRNAs from a basal eudicot (Eschscholzia californica) and conservation in flowering plants. Plant J 51:991–1003
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Baskerville S, Bartel DP (2005) Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 11:241–247
Baulcombe D (2004) RNA silencing in plants. Nature 431:356–363
Bhattacharya A, Deng JM, Zhang Z, Behringer R, de Crombrugghe B, Maity SN (2003) The B subunit of the CCAAT box binding transcription factor complex (CBF/NF-Y) is essential for early mouse development and cell proliferation. Cancer Res 63:8167–8172
Billoud B, de Paepe R, Baulcombe D, Boccara M (2005) Identification of new small non-coding RNAs from tobacco and Arabidopsis. Biochimie 87:905–910
Bonnet E, Wuyts J, Rouzé P, Van de Peer Y (2004) Detection of 91 potential conserved plant microRNAs in Arabidopsis thaliana and Oryza sativa identifies important target genes. Proc Natl Acad Sci USA 101:11511–11516
Boxus P (1974) The production of strawberry plants by in vitro micropropagation. J Hort Sci 49:209–210
Brainerd KE, Fuchigami LH (1982) Stomatal functioning of in vitro and greenhouse apple leaves in darkness, mannitol, ABA and CO2. J Exp Bot 33:388–392
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O (2008) Widespread translational inhibition by plant miRNAs and siRNAs. Science 320:1185–1190
Cassells AC, Curry RF (2001) Oxidative stress and physiological, epigenetic and genetic variability in plant tissue culture: implications for micropropagators and genetic engineers. Plant Cell Tissue Organ Cult 64(2–3):145–157
Chang L, Zhang Z, Yang H, Li H, Dai H (2007) Detection of strawberry RNA and DNA viruses by RT-PCR using total nucleic acid as a template. J Phytopathol 155:431–436
Chen X (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303:2022–2025
Chen CF, Ridzon DA, Broomer AJ, Zhou ZH, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ (2005) Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33:179–187
Combier JP, Frugier F, de Billy F, Boualem A, EI-Yahyaoui F, Moreau S, Vernié T, Ott T, Gamas P, Crespi M, Niebel A (2006) MtHAP2–1 is a key transcriptional regulator of symbiotic nodule development regulated by microRNA169 in Medicago truncatula. Genes Dev 20(22):3084–3088
Cubas P, Coen E, Zapater JMM (2001) Ancient asymmetries in the evolution of flowers. Curr Biol 11:1050–1052
Dai H, Zhang Z, Guo X (2007) Adventitious bud regeneration from leaf and cotyledon explants of Chinese hawthorn (Crataegus pinnatifida Bge. var. major N.E.Br.). In Vitro Cell Dev Biol Plant 43:2–8
Dezulian T, Palatnik JF, Huson D, Weigel D (2005) Conservation and divergence of microRNA families in plants. Genome Biol 6:13
Eisen MB, Brown PO (1999) DNA arrays for analysis of gene expression. Methods Enzymol 303:179–205
Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL (2003) Radial patterning of Arabidopsis shoots by class Ш HD-ZIP and KANADI genes. Curr Biol 13:1768–1774
Fahlgren N, Montgomery T, Howell MD, Allen E, Dvorak SK, Alexander AL, Carrington JC (2006) Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr Biol 16(9):939–944
Floyd SK, Bowman JL (2004) Gene regulation: ancient microRNA target sequences in plants. Nature 428:485–486
Garcia D, Collier SA, Byrne ME, Martienssen RA (2006) Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr Biol 16:933–938
Garzon R, Pichiorri F, Palumbo T, Iuliano R, Cimmino A, Aqeilan R, Volinia S, Bhatt D, Alder H, Marcucci G, Calin GA, Liu CG, Bloomfield CD, Andreeff M, Croce CM (2006) MicroRNA fingerprints during human megakaryocytopoiesis. Proc Natl Acad Sci USA 103(13):5078–5083
Griffiths-Jones S (2004) The microRNA registry. Nucleic Acids Res 32:D109–D111
Guo X, Gui Y, Wang Y, Zhu QH, Helliwell C, Fan L (2008) Selection and mutation on microRNA target sequences during rice evolution. BMC Genomics 9:454
Jaligot E, Rival A, Beulé T, Dussert S, Verdeil JL (2000) Somaclonal variation in oil palm (Elaeis guineensis Jacq.): the DNA methylation hypothesis. Plant Cell Rep 19(7):684–690
Jemmali A, Boxus P, Dekegel D, Heule GV (1994) Occurrence of spontaneous shoot regeneration on leaf stipules in relation to hyperflowering response in micropropagated strawberry plantlets. In Vitro Cell Dev Biol 30p:192–195
Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14:787–799
Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MCP (2004) MicroRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428:84–88
Kaeppler SM, Kaeppler HF, Rhee Y (2000) Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol 43:179–188
Kim J, Jung JH, Reyes JL, Kim YS, Kim SY, Chung KS, Kim JA, Lee M, Lee Y, Kim VN, Chua NH, Park CM (2005) MicorRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J 42(1):84–94
Kwong RW, Bui AQ, Lee H, Kwong LW, Fischer RL, Goldberg RB, Harada JJ (2003) LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development. Plant Cell 15:5–18
Laufs P, Peaucelle A, Morin H, Traas J (2004) MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristems. Development 131:4311–4322
Lauter N, Kampani A, Carlson S, Goebel M, Moose SP (2005) MicroRNA 172 down-regulates glossy15 to promote vegetative phase change in maize. Proc Natl Acad Sci USA 102:9412–9417
Lee H, Fischer RL, Goldberg RB, Harada JJ (2003) Arabidopsis LEAFY COTYLEDON1 represents a functionally specialized subunit of the CCAAT binding transcription factor. Proc Natl Acad Sci USA 100:2152–2156
Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, Lu XY, Cui X, Jin H, Zhu JK (2008) The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20:2238–2251
Litwińczuk W (2004) Field performance of ‘Senga Sengana’ strawberry plants (Fragaria × ananassa Duch.) obtained by runners and in vitro through axillary and adventitious shoots. Electronic Journal of Polish Agricultural Universities, Horticulture 7(1): art–03
Liu B, Wendel JF (2003) Epigenetic phenomena and the evolution of plant allopolyploids. Mol Phylogenet Evol 29:365–379
Liu HH, Tian X, Li YJ, Wu CA, Zheng CC (2008) Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 14:836–843
Lotan T, Ohto M, Yee KM, West MA, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93:1195–1205
Lu S, Sun YH, Shi R, Clark C, Li L, Chiang VL (2005) Novel and mechanical stress-responsive microRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell 17:2186–2203
Luo MY, Tian ZG, Xu Z, Zhang L, Wang YX, Cheng J (2007) Construction and application of a microarray for profiling microRNA expression. Prog Biochem Biophys 34(1):31–41
Mallory AC, Bartel DP, Bartel B (2005) MciroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 17:1360–1375
McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK (2001) Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411:709–713
McHale NA, Koning RE (2004) MicroRNA-directed cleavage of Nicotiana sylvestris PHAVLOUTA mRNA regulates the vascular cambium and structure of apical meristems. Plant Cell 16:1730–1740
Mi JM, Zhang ZH, Li H, Gao XY, Du GD (2007) Blossoming and fruiting of micropropagated strawberry plants after transplantation. J Fruit Sci 24(4):472–476
Millar AA, Gubler F (2005) The Arabidopsis GAMYB-Like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 17:705–721
Miyoshi K, Ito Y, Serizawa A, Kurata N (2003) OsHAP3 genes regulate chloroplast biogenesis in rice. Plant J 36:532–540
Molnár A, Schwach F, Studholme DJ, Thuenemann EC, Baulcombe DC (2007) miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature 447:1126–1129
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D (2003) Control of leaf morphogenesis by microRNAs. Nature 425:257–263
Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poething RS (2005) Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci USA 102(10):3691–3696
Pires JC, Zhao J, Schranz EM, Leon EJ, Quijada PA, Lukens LN, Osborn TC (2004) Flowering time divergence and genomic rearrangements in resynthesized Brassica polyploids (Brassicaceae). Biol J Linn Soc Lond 82:675–688
Poole R, Barker G, Wilson ID, Coghill JA, Edwards KJ (2007) Measuring global gene expression in polyploidy; a cautionary note from allohexaploid wheat. Funct Integr Genomics 7:207–219
Qiu CX, Xie FL, Zhu YY, Guo K, Huang SQ, Nie L, Yang ZM (2007) Computational identification of microRNAs and their targets in Gossypium hirsutum expressed sequence tags. Gene 395:49–61
Rapp RA, Wendel JF (2005) Epigenetics and plant evolution. New Phytol 168:81–91
Rival A, Jaligot E, Beule T, Finnegan EJ (2008) Isolation and expression analysis of genes encoding MET, CMT and DRM methyltransferases in oil palm (Elaeis guineensis Jacq.) in relation to the ‘mantled’ somaclonal variation. J Exp Bot 1–11
Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, Jones PA (2006) Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell 9:435–443
Schena M, Shalon D, Davis RW, Brown PO (1995) Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270:467–470
Schubert D, Clarenz O, Goodrich J (2005) Epigenetic control of plant development by Polycomb-group proteins. Curr Opin Plant Biol 8:553–561
Short KC, Warburton J, Roberts AV (1987) In vitro hardening of cultured cauliflower and chrysanthemum plantlets to humidity. Acta Hortic 212:329–334
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16:2001–2019
Sunkar R, Girke T, Jain PK, Zhu JK (2005) Cloning and characterization of microRNAs from Rice. Plant Cell 17:1397–1411
Sunkar R, Zhou XF, Zheng Y, Zhang WX, Zhu JK (2008) Identification of novel and candidate miRNAs in rice by high throughput sequencing. BMC Plant Biol 8:25
Talmor-Neiman M, Stav R, Frank W, Voss B, Arazi T (2006a) Novel microRNAs and intermediates of microRNA biogenesis from moss. Plant J 47:25–37
Talmor-Neiman M, Stav R, Klipcan L, Buxdorf K, Baulcombe DC, Arazi T (2006b) Identification of trans-acting siRNAs in moss and an RNA-dependent RNA polymerase required for their biogenesis. Plant J 48:511–521
Tang G, Reinhart BJ, Bartel DP, Zamore PD (2003) A biochemical framework for RNA silencing in plants. Gens Dev 17:49–63
Tang F, Hajkova P, Barton SC, Lao K, Surani MA (2006) MicroRNA expression profiling of single whole embryonic stem cells. Nucleic Acids Res 34(2):e9
Thomson JM, Parker J, Perou CM, Hammond SM (2004) A custom microarray platform for analysis of microRNA gene expression. Nat Methods 1:47–53
Tiwari SB, Hagen G, Guilfoyle T (2003) The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15:533–543
Tuddenham L, Wheeler G, Ntounia-Fousara S, Waters J, Hajihosseini MK, Clark I, Dalmay T (2006) The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett 580:4214–4217
Tuskan GA, DiFazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A et al (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313:1596–1604
Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3:12
Vaucheret H, Vazquez F, Crété P, Bartel DP (2004) The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev 18:1187–1197
Vazquez F, Vaucheret H, Rajagopalan R, Lepers C, Gasciolli V, Mallory AC, Hilbert JL, Bartel DP, Crété P (2004) Endogenous trans-acting siRNAs regulate the accumulation of Arabidopsis mRNAs. Mol Cell 16(1):69–79
Wang XJ, Reyes JL, Chua NH, Gaasterland T (2004) Prediction and identification of Arabidopsis thaliana microRNAs and their mRNA targets. Genome Biol 5(9):R65
Wang JW, Wang LJ, Mao YB, Cai WJ, Xue HW, Chen XY (2005) Control of root cap formation by microRNA-targeted auxin response factors in Arabidopsis. Plant Cell 17:2204–2216
Warpeha KM, Upadhyay S, Yeh J, Adamiak J, Hawkins SI, Lapik YR, Anderson MB, Kaufman LS (2007) The GCR1, GPA1, PRN1, NF-Y signal chain mediates both blue light and abscisic acid responses in Arabidopsis. Plant Physiol 143:1590–1600
Williams L, Carles CC, Osmont KS, Fletcher JC (2005) A database analysis method identifies an endogenous trans-acting short-interfering RNA that targets the Arabidopsis ARF2, ARF3, and ARF4 genes. Proc Natl Acad Sci USA 102:9703–9708
Xie FL, Huang SQ, Guo K, Xiang AL, Zhu YY, Nie L, Yang ZM (2007) Computational identification of novel microRNAs and targets in Brassica napus. FEBS Lett 581:1464–1474
Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP (2002) Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30:e15
Yao Y, Guo G, Ni Z, Sunkar R, Du J, Zhu JK, Sun Q (2007) Cloning and characterization of microRNAs from wheat (Triticum aestivum L.). Genome Biol 8:R96
Yin Z, Li C, Han X, Shen F (2008) Identification of conserved microRNAs and their target genes in tomato (Lycopersicon esculentum). Gene 414:60–66
Yoshikawa M, Peragine A, Park MY, Poethig RS (2005) A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev 19:2164–2175
Zhao B, Liang R, Ge L, Li W, Xiao H, Lin H, Ruan K, Jin K (2007a) Identification of drought-induced microRNAs in rice. Biochem Biophys Res Commun 354:585–590
Zhao T, Li GL, Mi SJ, Li S, Hannon GJ, Wang XJ, Qi YJ (2007b) A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev 21:1190–1203
Zhong R, Ye ZH (2004) Amphivasal vascular bundle 1, a gain of function mutation of the IFL1/REV gene, is associated with alterations in the polarity of leaves, stem and carpels. Plant Cell Physiol 45(4):369–385
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (30671432) and Program for New Century Excellent Talents in University (NCET-07-0565).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Reski.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, H., Zhang, Z., Huang, F. et al. MicroRNA expression profiles in conventional and micropropagated strawberry (Fragaria × ananassa Duch.) plants. Plant Cell Rep 28, 891–902 (2009). https://doi.org/10.1007/s00299-009-0693-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0693-3