Abstract
Cotton (Gossypium hirsutum), the most important textile crop worldwide, often encounters water stress such as drought or waterlog during its growth season (Summer). To investigate molecular mechanism of water regulation in cotton plants, three cDNAs encoding the plasma membrane intrinsic protein (PIP) were isolated from cotton root cDNA library, and designated GhPIP1;1, GhPIP2;1 and GhPIP2;2, respectively. All of the three PIP proteins displayed water channel activity in Xenopus laevis oocytes. GhPIP2;1 and GhPIP2;2 proteins, however, showed much higher water transport activity than that of the GhPIP1;1 protein. Northern blot analysis revealed that all of the three genes were preferentially expressed in young roots. Further analysis by Real-time quantitative RT-PCR revealed that the transcripts of all the three genes were accumulated at high levels in 3-day-old young roots, but dramatically declined to much lower levels in 6–14 days old roots during seedling development, suggesting that expressions of the isolated GhPIP genes are developmentally regulated in roots. Additionally, expressions of the three genes were remarkably up-regulated or down-regulated under different stresses such as NaCl, cold, PEG (polyethylene glycol) treatments. Collectively, the results suggest that these genes may be involved in root development and in response to stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water movement in cells is a precisely regulated process during plant development. Besides diffusion, water pass through cellular membrane rapidly by water channels consisting of aquaporins (AQPs), which belong to the major intrinsic protein (MIP) family. AQPs are small (23–34 kDa) transmembrane proteins, consisting of six transmembrane α-helices (H1–H6) connected by five short loops (loop A–loop E) with both N and C termini facing to the cytosol. Two highly conserved asparagine-proline-alanine (NPA) motifs locate at loop B and loop E which both form hemi-helix (HB and HE) dipping into the membrane from opposite sides. Through the interaction of six transmembrane α-helices and the two hemi-helices, AQPs form a membrane water-selective channel (Chaumont et al. 2001). AQPs generally exist as tetramers which are important for the protein folding and stability in the plasma membrane (Murata et al. 2000; Sui et al. 2001; Chaumont et al. 2005).
In plants, AQP proteins encoded by multigenes form a large family. There are 36 AQP members in maize (Chaumont et al. 2001), and 35 AQPs in Arabidopsis (Johanson et al. 2001). Based on the amino-acid sequence similarity, plant AQPs can be classified into four subfamilies, plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), NOD26-like intrinsic proteins (NIPs) and small basic intrinsic proteins (SIPs) (Johanson and Gustavsson 2002; Johanson et al. 2001; Chaumont et al. 2001). The PIPs are mainly targeted to the plasma membrane and TIPs are generally localized to the vacuolar membrane (Chaumont et al. 2005). The NIPs which are close homologues of GmNOD26 are abundantly expressed in the peribacteriod membrane of symbiotic nitrogen-fixing nodules of soybean roots (Wallace et al. 2006), while in non-leguminous plants, they are often localized in plasma and intracellular membranes (Mizutani et al. 2006; Takano et al. 2006; Maurel 2007). SIPs have been mostly found to be present in the endoplasmic reticulum (ER) membrane (Ishikawa et al. 2005). The PIP subfamily can be further divided into PIP1 and PIP2 isoforms based on sequence homology (Fetter et al. 2004). Previous studies indicated that most plant PIP2 proteins show high water channel activity, but PIP1 members are often inactive or have low activity (Chaumont et al. 2000; Marin-Olivier et al. 2000; Moshelion et al. 2002; Fetter et al. 2004). However, coexpression of some plant PIP1s and PIP2s in Xenopus oocytes resulted in a greater increase in permeability coefficient (P f ) than did the expression of PIP2s alone (Fetter et al. 2004; Temmei et al. 2005). A number of water-channel activities of AQPs are involved in regulation of water flux and homeostasis at the subcellular, transcellular, tissue and whole-plant levels (Chaumont et al. 2001). The activities of plant AQPs may be regulated by gating mechanisms, and the factors related to the gating action likely contain phosphorylation, heteromerization, pH, Ca2+, pressure, solute gradients, temperature and so on (Chaumont et al. 2005). For instance, the water transport activity of PM28A is regulated by phosphorylation (Johansson et al. 1998). Although most plant AQPs have been found to be water channels (Johansson et al. 2000; Tyerman et al. 2002), a few plant AQPs which do not exhibit a strict specificity for water channel can also transport some small neutral molecules such as glycerol, urea, CO2 (Gerbeau et al. 1999; Guenther and Roberts 2000; Weig and Jakob 2000; Ciavatta et al. 2001), NH3 and NH4 + (Niemietz and Tyerman 2000; Jahn et al. 2004), boron (Dordas et al. 2000; Takano et al. 2006) and H2O2 (Henzler and Steudle 2000; Bienert et al. 2007).
The large numbers of PIP and TIP genes in the plants are expressed in the tissue-specific and developmental-regulated manner. Both PIP and TIP gene products have been localized in the vascular tissue and its surrounding cells, and also in the zones of the elongated cells that are undergoing vacuolization, but seldom in meristematic tissues (Schäffner 1998). A study revealed that many Arabidopsis AQPs are predominantly expressed in either root or flower organs, but no AQP members seem to be leaf-specific (Alexandersson et al. 2005). Both NtPIP2;1 and NtPIP1;1 genes in tobacco were detected in most tissues of anther, but only NtPIP1;1 RNA accumulated in the stigma (Bots et al. 2005). Previous study indicated that the water permeability of the cells was declined by repressing NtAQP1 expression in tobacco (Siefritz et al. 2002). Overexpression of Arabidopsis AtPIP1b in tobacco remarkably increased plant growth, transpiration and photosynthesis rate, whereas transgenic tobacco plants wilted more quickly under drought stress (Aharon et al. 2003). In Arabidopsis, the expression of all 13 PIP genes was up- or down-regulated under abiotic stresses (Jang et al. 2004).
Roots usually provide the input of water in higher plants and establish a critical link in the soil–plant–air continuum which has to be maintained in the most adverse environmental or physiological conditions. Roots, in particular, show a remarkable capacity to alter their water permeability in response to day/night cycles, nutrient deficiency and other stresses. Rapid and significant (>30%) increases or decreases in water permeability (hydraulic conductivity, L p ) of roots may be observed upon short-term exposure to stresses. These rapid changes are mostly accounted for by changes in cell membrane permeability, and are mediated by aquaporins (reviewed in Javot and Maurel 2002). Hence, aquaporins may play significant roles in cotton roots which are usually exposed to irregular cycles of wetting and drying during summer. However, none of cotton aquaporin genes was well characterized in roots, and particularly, the activities and functions of these genes in root development remain to be elucidated so far. Here, we report identification of three new PIP genes which were predominantly expressed in developmentally-regulated manner in cotton roots. Overexpression of the cotton PIP genes in X. laevis oocytes dramatically increased water permeability of the cells, indicating that these PIP proteins possess water transport activities.
Materials and methods
Plant materials and growth
Cotton (Gossypium hirsutum cvs. Xuzhou 142, Emian No. 9 and No. 10) seeds were surface-sterilized with 70% (v/v) ethanol for 1 min and 10% (v/v) H2O2 for 2 h, then washed with sterile water. The sterilized seeds were germinated on 1/2MS medium under a 16 h light/8 h dark cycle at 25°C for 5–6 days. In the stress experiments, the sterilized seeds were germinated on 1/2MS medium under a 16 h light/8 h dark cycle at 25°C for 4–5 days, and then seedlings were transferred in 4°C for cold treatment, or transferred onto 1/2MS medium containing NaCl or PEG (polyethylene glycol 4,000) for treatment.
Roots, cotyledons and hypocotyls were cut from sterile seedlings, and other tissues for RNA extraction were derived from cotton plants grown in the field. All tissues were collected 2 h after light onset since some plant aquaporins display a diurnal expression pattern, with a peak of expression at 2–4 h after the beginning of the light period (Hachez et al. 2006).
Construction of cotton root cDNA library and isolation of GhPIP1;1, GhPIP2;1 and GhPIP2;2 cDNAs
Total RNA was extracted from young roots (3–10 days old) as described previously (Li et al. 2002). Poly(A)+ mRNA was prepared from a pool of root total RNA by using an mRNA purification kit (Qiagen). Complementary DNA was synthesized and cloned into the EcoR I–Xho I sites of the ZAP express vector and packaged using a ZAP-cDNA Gigapack Gold III cloning kit (Stratagene) according to the manufacturer’s instruction.
More than 4,000 cDNA clones were randomly selected from the cotton root cDNA library for sequencing. Some GhPIP clones with partial sequences were identified. Then a 500 bp fragment of GhPIP including partial coding region and 3′-untranslated region was labeled with α-32P-dCTP and used as probe to screen cotton root cDNA libraries. 2 × 105 cDNA clones were screened and over 100 clones were identified. Among them, the three unique cDNA clones (GhPIP1;1, GhPIP2;1 and GhPIP2;2) were obtained.
Northern blot analysis
Total RNA was extracted from young fibers, ovules, anthers, petals, leaves, cotyledons, hypocotyls and roots of cotton. The isolated total RNA was purified by RNA purification kit (Qiagen USA, Valencia, CA). RNA samples (16 μg per lane) from different cotton tissues were separated on 1.2% agarose–formaldehyde gel and transferred onto Hybond-N nylon membranes by capillary blotting. 0.2 kb 3′-UTR (3′-untranslated region) sequences of GhPIP1, GhPIP2;1 and GhPIP2;2 cDNAs were amplified as gene-specific probes by PCR, respectively. The α-32P-dCTP labeled gene-specific probes were prepared through random primer method (Random Primer DNA Labeling Kit Ver.2, TAKARA). Northern blot hybridization was performed as described previously (Li et al. 2002). The membrane was exposed to X-film (Eastman Kodak, Rochester, NY) with two intensifying screens at −70°C for 1–3 days.
Quantitative RT-PCR analysis
The expression of GhPIP genes in cotton roots was analyzed by real-time quantitative reverse transcriptase (RT)-PCR using the fluorescent intercalating dye SYBR-Green in a detection system (MJ Research, Opticon 2). A cotton polyubiquitin gene (GhUBI1, access number in GenBank: EU604080) was used as a standard control in the RT-PCR reactions. Two-step RT-PCR procedure was performed in all experiments using a method described earlier (Li et al. 2005). In brief, total RNA was reversely transcribed into cDNAs which were used as templates in real-time PCR reactions with gene-specific primers. The primer sequences were as follows: GhPIP1;1: up-chain primer, 5′>TGGGCGCCATATGCGGGGCG<3′, down-chain primer, 5′>AAGCTCATAATCATGGAATCATG<3′; GhPIP2;1: up-chain primer, 5′>GCTGATGGATACAGCACTGGC<3′, down-chain primer, 5′>AGCAAAGTAAAAGGCGTAGATTC<3′; GhPIP2;2: up-chain primer, 5′>GTCTCTAACTTTAACCTTATCAC<3′, down-chain primer, 5′>AAGGAAAAGGAACTGAACTCCAT<3′. Real-time PCR reaction was performed using Real-time PCR Master Mix (TOYOBO, Japan) according to the manufacturer’s instruction. The Ct (cycle threshold), defined as the PCR cycle at which a statistically significant increase of reporter fluorescence is first detected, is used as a measure for the starting copy numbers of the target gene. Relative quantity of the target GhPIP expression level was performed using the comparative Ct method. The relative value for the expression level of each GhPIP gene was calculated by the equation Y = 10ΔCt/3.5 × 100% (ΔCt is the differences of Ct between the control GhUBI1 products and the target GhPIP products, i.e. ΔCt = Ct GhUBI1 − Ct GhPIP). For the accurate amplification of each specific target gene among the highly homologous PIP family, we carefully designed the primer sets for each PIP gene based on the sequences corresponding to 3′-untranslational region. To achieve optimal amplification, PCR conditions for every primer combination were optimized for annealing temperature, and PCR products were verified by melting curve analysis and confirmed on an agarose gel. The efficiency of each primer pair was detected using GhPIP cDNAs as standard templates, and the RT-PCR data were normalized with the relative efficiency of each primer pair. The data of the real-time RT-PCR are mean values and standard errors (bar) of three independent experiments with three biological replicates of root materials.
DNA and protein sequence analysis
Nucleotide and amino acid sequences were analyzed using DNAstar (DNAstar Inc). The GhPIPs peptide sequences were aligned with the ClustalW program (http://www.ebi.ac.uk), and phylogenetic analysis was employed to investigate the evolutionary relationships among the aquaporins. A minimum evolution tree was generated in MEG3.1. A bootstrap analysis with 1,000 replicates was performed to assess the statistical reliability of the tree topology.
In vitro complementary RNA synthesis
The cDNA of GhPIPs were subcloned into pGEM-7Z vector using the flanking restriction sites EcoRI and XbaI of the ZAP vector. Capped cRNA transcripts were synthesized in vitro with mMAHINE SP6 Kit (Ambion) with XbaI linearized vector.
Oocyte preparation, cRNA injection, and osmotic water permeability assay
Xenopus laevis oocytes of stages V and VI were isolated and defolliculated by digestion at room temperature for 1 h with 2 mg/ml collagenase A (Sigma) in ND96 solution (96 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2 and 5 mM Hepes-NaOH, pH 7.4, 220 mOsm/kg). 50 nl volume of in vitro transcripts (50 ng) of the target genes, using the same volume of distilled water as negative control, were injected into the oocytes, and then the oocytes were incubated at 19°C for 48 h in ND96 solution supplemented with 10 μg/ml penicillin and 10 μg/ml streptomycin. To measure the osmotic water P f , a single oocyte was transferred to five-fold diluted ND96 solution. Changes in oocyte volume were monitored at room temperature with a microscope video system by taking digital images at 30 s intervals. Oocytes volumes (V) were calculated from the measured area of each oocyte. The osmotic P f was calculated for the first 5 min using the formula P f = V 0[d(V 0/V)/dt]/[S 0 × V W (Osmin − Osmout)], with an initial volume (V 0) of 9 × 10−4 cm3, an initial oocytes surface area (S 0) of 0.045 cm2, and a molar volume of water (V W) of 18 cm3/mol (Zhang and Verkman 1991).
Results
Isolation and characterization of three GhPIP genes
By screening cotton root cDNA libraries with a 500 bp GhPIP probe, we isolated three unique PIP cDNAs. Sequence analysis predicted that one gene (designated GhPIP1;1, accession number in GenBank: EF079900) encodes a protein with 289 amino acids belonging to PIP1 isoform, and the other two (designated GhPIP2;1, GhPIP2;2, accession number in GenBank: EF079901 and EF079902) encode polypeptides consisting of 285 and 286 amino acids, respectively, belonging to PIP2 members.
The GhPIP1;1 cDNA consists of 935 bp, including 870 bp open reading frame (ORF). It shares high sequence homology at nucleotide level (approximately 77% identities) in the coding region and at amino acid level (approximately 87% identities) with GhAQP1 (ABD63904) and GhPIP1-2 (ABR68794), the other two cotton PIP1 genes reported previously. There is 13% substitution rate at amino acid level compared with each other (Fig. 1). In addition, the deduced amino acid sequence of GhP1P1;1 shows 91% identity with Medicago truncatula MtPIP1;1 (AAK66766) and 87%–88% identity with Arabidopsis AtPIP1s. The GhPIP2;1 cDNA consisting of 1,116 bp, shares 86% homology at nucleotide level, and 91% identity at amino acid level with GhPIP2;2 which is 1,105 bp in length. They have 74–83% identity with Arabidopsis PIP2 proteins, and shows 81% identity with maize ZmPIP2;1 (AAK26758). Additionally, there is approximately 67% identity between GhPIP1;1 and GhPIP2s.
Comparison of the three predicted amino acid sequences of cotton PIPs with the other known cotton PIP proteins. Amino acid sequences are aligned by ClusterW software. Amino acid substitutions are highlighted in black and six transmembrane-helix (H1–H6) are shown in the box. Overstriking letters refer to the most highly conserved amino acid sequences of MIP. The accession numbers of these known proteins in GenBank are as follows: GhAQP1(ABD63904) and GhPIP1-2 (ABR68794)
Although the predicted proteins encoding by these three genes belong to different PIP subgroups, all of them contain six putative transmembrane α-helices, highly conserved amino acid sequence ‘HINPAVTFG’ and two ‘NPA’ motifs (Fig. 1). By contrary, the terminal domains of GhPIPs display relatively high variability. GhPIP1s contain large N-terminal domain, while GhPIP2s contain large C-terminal domain (Fig. 1).
Phylogenetic relationship of GhPIP proteins
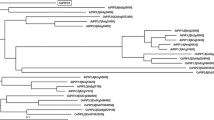
To investigate the evolutionary relationships of three GhPIP proteins, all 13 PIP protein sequences of Arabidopsis as well as cotton AQPs reported previously were selected from GenBank for phylogenetic analysis. GlpF, a member of the MIP family from Escherichia coli, was employed as an outgroup. As shown in the phylogenetic tree (Fig. 2), 18 PIP proteins split into two groups obviously. GhPIP1-2 and GhAQP1 form a distinct clade and locate at the clade based to AtPIP1;4 and AtPIP1;5. Although GhPIP1;1 shares high sequence homology with GhAQP1 and GhPIP1-2, it inhabits a single clade which is basal to the branch containing all other PIP1 proteins. The result suggest that GhPIP1;1 may diverge relatively earlier from other GhPIP1s during evolution. In the branch of PIP2s, GhPIP2;1 is a sister group to the AtPIP2;2/AtPIP2;3 clade, while GhPIP2;2 is basal to the clade containing AtPIP2;2, AtPIP2;3 and GhPIP2;1.
Phylogenetic relationships of cotton PIP proteins with Arabidopsis PIP proteins. The Minimum Evolution tree was constructed in MEGA3.1 from 1,000 bootstrap replicates. The accession numbers of the known proteins of Arabidopsis in GenBank are as follows: AtPIP1;1 (AAM19914.1), AtPIP1;2 (AAM14193), AtPIP1;3 (AAK15545.1), AtPIP1;4 (AAN15649.1), AtPIP1;5 (AAL24430.1), AtPIP2;1 (AAK73268.1), AtPIP2;2 (AAD18142), AtPIP2;3 (AAM20335.1), AtPIP2;4 (AAM64801.1), AtPIP2;5 (AAM61408.1), AtPIP2;6 (AAK74048.1), AtPIP2;7 (AAM10142.1), AtPIP2;8 (AAM15086.1). The accession numbers of the known cotton aquaporins are as follows: GhAQP1 (ABD63904), GhPIP1-2 (ABR68794). GlpF is a glycerol facilitator from Escherichia coli (Genbank accession number CAH19327) which belongs to MIP family and was used as an outgroup
Overexpression of GhPIPs in Xenopus laevis oocytes enhance water permeability of the cells
To test the water transport activity of GhPIPs, their capped sense cRNA were injected into Xenopus laevis oocytes. After a 2-days incubation for cRNA translation and the target proteins to the oocyte membrane, the oocytes were transferred to a hypoosmotic solution and the increase in area was measured in time and used to calculate the increase in volume. This change in volume was used to calculate the relative water permeability (P f ) of the oocytes. The results of the experiments are presented in Fig. 3. The oocytes expressed the GhPIP1;1 proteins showed a P f of 18.79 ± 4.02 μm/s (n = 30) which was about two-fold higher than that of the controls injected with water (P f = 9.47 ± 5.30 μm/s, n = 30). Notably, the P f values of the oocytes expressed GhPIP2;1 and GhPIP2;2, respectively, were 30.58 ± 7.34 μm/s and 37.26 ± 10.01 μm/s (n = 30), which were much higher than that of the oocytes injected with GhPIP1;1 cRNA. In addition, We also coexpressed GhPIP1;1 and GhPIP2s in the oocytes. However, statistical analysis revealed that the P f values of the cells coexpressed GhPIP1;1 and GhPIP2s did not show significant differences from those of the oocytes expressed GhPIP2s alone (data not shown). The above data demonstrated that all of the three GhPIP proteins possess water-transport capacity, and the GhPIP2 activity was higher than that of GhPIP1.
Functional expression assay of GhPIP proteins in Xenopus oocytes. a Increase in relative volume of oocytes injected with GhPIPs cRNA after transfer to hypoosmotic medium (see “Methods”). b P f -values of oocytes injected with GhPIPs cRNA, using water as control. *Independent t tests demonstrated that there was significant difference between the P f -values of oocytes injected with water and GhPIP1;1 cRNA (t test for equality of means, P value < 0.05). **Very significant difference between the P f -values of oocytes injected with water and GhPIP2s cRNA (P value < 0.01)
Expressions of the three GhPIP genes are organ-specific/preferential and are root-developmentally regulated
Northern blot results showed that the expressions of all the three genes are organ-preferential (Fig. 4). Strong expression of GhPIP1;1 gene was found in roots, and its moderate expression was detected in petals, but only weak or no signals were observed in other tissues (Fig. 4a). The expression of GhPIP1;1 in cotton organs and tissues was also analyzed by real-time quantitative RT-PCR. The results revealed that the transcripts of GhPIP1;1 were predominantly accumulated in 3-day-old roots which is four-fold higher than in petals (data not shown). Similarly, the GhPIP2;2 was also mainly expressed in young roots. However, its transcripts were relatively largely accumulated in hypocotyls, but not in petals, unlike GhPIP1;1 (Fig. 4b). On the other hand, the GhPIP2;1 products were predominantly accumulated in young hypocotyls, and relative high levels of its transcripts were detected in young roots and petals, but no or weak expression was observed in other tissues (Fig. 4b).
RNA gel blot analysis of GhPIP1;1, GhPIP2;1 and GhPIP2;2 genes expression in cotton tissues. Upper panel of a and the upper, middle panels of b: autoradiograph of RNA hybridization; bottom panel: RNA gel before being transfered to membrane showing equal loading of RNAs. a GhPIP1;1 expression patterns: R roots; C cotyledons; L leaves; H hypocotyls; P petals; A anthers; O 10 DPA (days post anthesis) ovules; F6–F12 fiber of 6, 9, 10, 12 DPA, respectively; b GhPIP2;1 and GhPIP2;2 expression patterns: R1–R4 Roots of 3, 6, 9, 14-day-old, respectively; C Cotyledons; L Leaves; H Hypocotyls; P Petals; A Anthers; O 10 DPA ovules; F 10 DPA fiber
Furthermore, as shown in Figs. 4b and 5, both GhPIP2;1 and GhPIP2;2 expression are developmentally-regulated in cotton roots. At early stage of development (3-day-old), high levels of the gene products were detected in roots. As roots further developed, the expression activities of both genes were dramatically declined to much lower level (6-day-old roots), and till undetectable level (9–14 days old roots). Similarly, the GhPIP1;1 gene was also expressed at the highest level in 3-day-old roots, while dramatically declined with further growth of the roots (Fig. 5). Interestingly, the expression levels of all the three genes in the tip regions (20 mm) were much higher than the upper regions of 6-day-old roots (Fig. 5). The results suggest that all the GhPIP1;1, GhPIP2;1 and GhPIP2;2 genes are mainly expressed in young cells which are actively undergoing cell division or elongation during early root development.
Expression of GhPIP genes during root development. Relative values of the GhPIP gene expressions in the development of cotton roots are shown as percentage of GhUBI1 expression activity (see “Methods”). Error bars represent standard deviation. *Independent t tests demonstrated that there was significant difference between 6-day-old roots and the tip regions of 6-day-old roots (P value < 0.05). **Very significant difference between 6-day-old roots and the tip regions of 6-day-old roots (P value < 0.01). 3, 6, 9 and 14d refer to 3, 6, 9, 14-day-old roots respectively. And 6d-t refers to tip regions (20 mm from root tip) of 6-day-old roots
Expression levels of the three GhPIP genes are regulated in roots upon different stresses
To investigate whether the expression of the GhPIP genes are stress-induced, total RNA were exacted from roots under different stresses for 12 h. The expression profiles of the three genes are shown in Fig. 6a. After salt (1% NaCl) treatment, the expressions of all three genes were remarkably up-regulated in roots. A significant increase was also observed in the transcription levels of GhPIP2;1 after 16% PEG treatment. In contrast, the expression levels of both GhPIP2;1 and GhPIP2;2 genes decreased after cold treatment. On the other hand, the expression of GhPIP1;1 were up-regulated slightly as cold and PEG treatments, and so did GhPIP2;2 expression with PEG treatment, compared with controls.
Expression of the GhPIP1;1, GhPIP2;1 and GhPIP2;2 genes in roots under salt, cold and PEG stresses. Relative value of GhPIP gene expression in cotton roots was shown as percentage of GhUBI1 expression activity (see “Methods”). Error bars represent standard deviation. *Independent t tests demonstrated that there was significant difference between (P value < 0.05). **Very significant difference between treated and un-treated roots (P value < 0.01). a Different treatments: cotton roots of 5-day-old were dealt with 1% NaCl, 4°C or 16% PEG for 12 h. CK un-treated roots (control); NaCl roots dealt with 1% NaCl; Cold roots dealt with 4°C; PEG roots dealt with 16% PEG. b Concentration gradient treatments: 5-day-old roots dealt with different concentrations of NaCl or PEG for 12 h. The concentration of NaCl is 0.25, 0.5, 0.75, 1.0% in turn and that of PEG is 4, 8 and 16% in turn. c Time gradient treatments: 5-day-old roots dealt with 1% NaCl or 16% PEG for 3, 6, 12, 24, 48 h, respectively
More details were obtained by investigating the expression patterns of GhPIP1;1 and GhPIP2;1 in 5-day-old roots dealt with different concentrations of NaCl or PEG for 12 h as well as in the roots dealt with 1% NaCl or 16% PEG for different time. The same experiments had not been done to GhPIP2;2 because of its low abundance in the root and the similar expression profiles like GhPIP2;1 under different stresses.
After a low concentration of NaCl treatment, the expression level of GhPIP1;1 was slightly up-regulated, while GhPIP2;1 was down-regulated obviously in roots. Transcription levels of both genes achieved the peak value in roots dealt with 0.75% NaCl, and then decreased remarkably after a higher concentration of NaCl (1.0%) treatment (Fig. 6b). Unlike the salt stress, the transcripts of GhPIP1;1 in roots dealt with a low concentration of PEG (4%) were much higher than with the higher concentration of PEG treatment. With different concentrations of PEG treatments, the expression levels of GhPIP2;1 were all up-regulated significantly in roots, compared to control (Fig. 6b).
Time-course analysis of the expression patterns of GhPIP1;1 and GhPIP2;1 under stresses were shown in Fig. 6c. Within 6 h treatment with 1% NaCl, the transcripts of GhPIP1;1 increased obviously and reached its maximum at 12 h, and then decreased slightly after 48 h treatment. When seedlings were treated by 16% PEG, the expression levels of GhPIP1;1 in roots were up-regulated slightly from 6 to 24 h and restored to normal level after 48 h treatment. The expression patterns of GhPIP2;1 response to 16% PEG were similar to that of GhPIP1;1 response to 1% NaCl. GhPIP2;1 seems to be less sensitive to 1% NaCl and its expression levels were slightly up-regulated till 12 h treatment. Collectively, the data revealed that the expression of all three GhPIP genes in cotton roots was up- or down-regulated in response to different stresses.
Discussion
The data presented in this study revealed that the isolated cotton PIP1;1, PIP2;1 and PIP2;2 genes encode the proteins which are homologues of the known plant PIP-type aquaporins. Comparing with the PIP genes from different species, they share high sequence homology not only at nucleotide level (58–80%) but also at amino acid level (60–91%). In the deduced amino acid sequences, they all display the most conserved amino acid sequence ‘HINPATFVG’ of MIP family and two ‘NPA’ motifs (Fig. 1). Furthermore, all of the three PIP proteins also contain the conserved sequence (R/K)DYX(E/D)PP(P/R)X3–4(E/D)XXELXXWSF(Y/W)R which is found in all PIP members (Schäffner 1998), although GhPIP1;1 possesses a longer N-termini than GhPIP2;1 and GhPIP2;2. GhPIP1;1 has the typical C-termini sequence [YHX(I/V)(V/I)IRA(M/I)XF(H/K)XXX] of PIP1, while both GhPIP2;1 and GhPIP2;2 contain the PIP2-conserved sequence [YHQ(F/Y)XLRA(G/S)(A/G)XK(A/X)LGSFRSX(PXX/A)] at the C-termini. Phylogenetic analysis (Fig. 2) showed that GhPIP1;1 had a more close evolutional relationship with AtPIP1s (87–88% identity) than AtPIP2s (63–68% identity), while the situations of GhPIP2;1 and GhPIP2;2 were reverse. Given the data together, our results strongly indicated that the three genes can be classified into two PIP subfamilies, i.e. GhPIP1;1 belongs to plant PIP1 subfamily and the other two belong to plant PIP2 subfamily.
It is widely accepted that the plant plasma membrane PIP1 and PIP2 subgroups differ not only in the length of their N and C termini and in several single amino acid residues but also in terms of their water channel activity (Chaumont et al. 2000). When expressed alone in Xenopus oocytes, PIP1s are inactive or have low activity, whereas PIP2 s cause a marked increase in the osmotic water permeability coefficient (Fetter et al. 2004; Moshelion et al. 2002). When coexpressed, PIP1 and PIP2 interact to regulate their activity or their subcellular localization (Fetter et al. 2004; Zelazny et al. 2007). In this study, our data revealed that the activities of both GhPIP2;1 (P f = 30.58) and GhPIP2;2 (P f = 37.26) are two- to three-fold higher than that of GhPIP1;1 (Fig. 3). By contrast, the activities of SsAQP2 (P f ≥ 130 μm/s) are much higher (about sevenfold higher) than those of SsAQP1 (Moshelion et al. 2002), and the activities of ZmPIP2s (P f ≥ 70 μm/s) are as five-fold as those of ZmPIP1s (Fetter et al. 2004). In addition, the coexpression of GhPIP1;1 with GhPIP2;1 or GhPIP2;2 did not lead to an increase in membrane P f , unlike that of ZmPIP1s with ZmPIP2s (Fetter et al. 2004). These results suggest that regulation mechanism of cotton PIP activities may show some difference from that of the known plant PIPs.
The discovery of aquaporins changed our views about how water moves rapidly across membrane and how plants might regulate water flow in different physiological conditions. Although the water transport across membrane mediated by aquaporins is not the unique approach of water transport in plants, it plays significant role in some special organs and in some certain conditions. Two GhPIP1 members (GhAQP1 and GhPIP1-2) had been reported previously in our lab (Li et al. 2006) and the other lab (Liu et al. 2008). Although both genes show high sequence identity with GhPIP1;1 at the amino acid level, the expression patterns of them exhibit completely different features. GhAQP1 is expressed specifically in 6–15 DPA ovules which might be involved in osmoregulation during cotton ovules development (Li et al. 2006), whereas GhPIP1-2 transcripts were accumulated abundantly during rapid fiber elongation (Liu et al. 2008). In this study, Northern blot analysis (Fig. 4) showed that the mRNAs of the three genes all are abundant in young roots. This confirms the general view that cotton AQPs play an important role in facilitating water movement across cell membranes in roots. Additionally, all three genes were preferentially expressed in young roots or young parts of roots, but remarkably declined with further root development, suggesting that the gene activities are developmental-regulated in cotton roots (Fig. 5). It had been reported that almost all ZmPIPs mRNAs were strongly dependant on the developmental stage of the root, with a general increase in expression towards the elongation and mature zones (Hachez et al. 2006). The increase in ZmPIP transcript abundance along the longitudinal root axis can be attributed to a decrease in symplastic continuity along the growing and maturing zones and therefore to a need for facilitated water transport through the cellular plasma membrane. GhPIP mRNAs are also abundant in the tip of the root, suggesting that these genes might play similar physiological roles in facilitating water uptake and transport during root development of cotton.
In the stress experiments, the expression of three cotton PIP genes was up- or down-regulated in response to different stresses (Fig. 6). GhPIP1;1 and GhPIP2;1 are sensitive to different concentrations of salt or PEG treatment. The expression levels of GhPIP1;1 and GhPIP2;1 are also related with the duration of treatment. Just within 6 h after salt or drought treatments, both genes were up-regulated (Fig. 6). The expression patterns of AtPIPs in response to abiotic stresses had been reported by Jang et al. (2004). The expression levels of most AtPIPs were markedly down-regulated in the roots by cold and drought treatment, while were highly up-regulated in the salt-treated roots (Jang et al. 2004). Similarly, the expression of all three genes was up-regulated in roots by higher concentrations (0.5–1%) of salt stress treatment, and down-regulated by cold treatment except GhPIP1;1. Under drought stress, however, the expression of GhPIP2;1 and GhPIP1;1 was significantly up-regulated in roots, unlike AtPIPs. In brief, our results revealed that the isolated cotton PIPs may play important roles in modulating osmotic balance of root cells when plants encountered environmental stresses. Thus, the data presented in this study may contribute to an understanding for water transport and osmotic regulation of cells in root development of cotton.
References
Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15:439–447. doi:10.1105/tpc.009225
Alexandersson E, Fraysse L, Sjovall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59:469–484. doi:10.1007/s11103-005-0352-1
Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282:1183–1192. doi:10.1074/jbc.M603761200
Bots M, Feron R, Uehlein N, Weterings K, Kaldenhoff R, Mariani T (2005) PIP1 and PIP2 aquaporins are differentially expressed during tobacco anther and stigma development. J Exp Bot 56:113–121. doi:10.1093/jxb/eri009
Chaumont F, Barrieu F, Jung R, Chrispeels MJ (2000) Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol 122:1025–1034
Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 125:1206–1215
Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Bio Cell 97:749–764
Ciavatta VT, Morillon R, Pullman GS, Chrispeels MJ, Cairney J (2001) An aquaglyceroporin is abundantly expressed early in the development of the suspensor and the embryo proper of loblolly pine. Plant Physiol 127:1556–1567
Dordas C, Chrispeels MJ, Brown PH (2000) Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol 124:1349–1362
Fetter K, Van Wilder V, Moshelion M, Chaumont F (2004) Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16:215–228. doi:10.1105/tpc.017194
Gerbeau P, Guclu J, Ripoche P, Maurel C (1999) Aquaporin Nt-TIPa can account for the high permeability of tobacco cell vacuolar membrane to small neutral solutes. Plant J 18:577–587. doi:10.1046/j.1365-313x.1999.00481.x
Guenther JF, Roberts DM (2000) Water-selective and multifunctional aquaporins from Lotus japonicus nodules. Planta 210:741–748. doi:10.1007/s004250050675
Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F (2006) Localization and quantification of plasma membrane aquaporin expression in maize primary root: a clue to understanding their role as cellular plumbers. Plant Mol Biol 62:305–323. doi:10.1007/s11103-006-9022-1
Henzler T, Steudle E (2000) Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J Exp Bot 51:2053–2066
Ishikawa F, Suga S, Uemura T, Sato MH, Maeshima M (2005) Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in Arabidopsis thaliana. FEBS Lett 579:5814–5820. doi:10.1016/j.febslet.2005.09.076
Jahn TP, Moller AL, Zeuthen T, Holm LM, Klaerke DA, Mohsin B, Kuhlbrandt W, Schjoerring JK (2004) Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett 574:31–36. doi:10.1016/j.febslet.2004.08.004
Jang JY, Kim DG, Kim YO, Kim JS, Kang H (2004) An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol Biol 54:713–725. doi:10.1023/B:PLAN.0000040900.61345.a6
Javot H, Maurel C (2002) The role of aquaporins in root water uptake. Ann Bot 90:301–303. doi:10.1093/aob/mcf199
Javot H, Lauvergeat V, Santoni V, Martin-Laurent F, Guclu J, Vinh J, Heyes J, Franck KI, Schaffner AR, Bouchez D, Maurel C (2003) Role of a single aquaporin isoform in root water uptake. Plant Cell 15:509–522. doi:10.1105/tpc.008888
Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P (1998) Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant cell 10:451–459
Johansson I, Karlsson M, Johanson U, Larsson C, Kjellbom P (2000) The role of aquaporins in cellular and whole plant water balance. Biochim Biophys Acta 1465:324–342. doi:10.1016/S0005-2736(00)00147-4
Johanson U, Gustavsson S (2002) A new subfamily of major intrinsic proteins in plants. Mol Biol Evol 19:456–461
Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjovall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126:1358–1369
Li DD, Huang GQ, Tan X, Wang J, Wang XL, Xu WL, Wu YJ, Wang H, Li XB (2006) Cloning of GhAQP1 gene and its specific expression during ovule development in cotton. Plant Physiol Mol Bio 32:543–550 in Chinese
Li XB, Cai L, Cheng NH, Liu JW (2002) Molecular characterization of the cotton GhTUB1 gene that is preferentially expressed in fiber. Plant Physiol 130:666–674
Li XB, Fan XP, Wang XL, Cai L, Yang WC (2005) The cotton ACTIN1 gene is functionally expressed in fibers and participates in fiber elongation. Plant Cell 17:859–875. doi:10.1105/tpc.104.029629
Liu DQ, Tu LL, Wang L, Li YJ, Zhu LF, Zhang XL (2008) Characterization and expression of plasma and tonoplast membrane aquaporins in elongating cotton fibers. Plant Cell Rep (in press). doi:10.1007/s00299-008-0545-6
Marin-Olivier M, Chevalier T, Fobis-Loisy I, Dumas C, Gaude T (2000) Aquaporin PIP genes are not expressed in the stigma papillae in Brassica oleracea. Plant J 24:231–240. doi:10.1046/j.1365-313x.2000.00874.x
Maurel C (2007) Plant aquaporins: novel functions and regulation properties. FEBS Lett 581:2227–2236. doi:10.1016/j.febslet.2007.03.021
Mizutani M, Watanabe S, Nakagawa T, Maeshima M (2006) Aquaporin NIP2;1 is mainly localized to the ER membrane and shows root-specific accumulation in Arabidopsis thaliana. Plant Cell Physiol 47:1420–1426
Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, Levi H, Moran N, Kaldenhoff R (2002) Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell 14:727–739. doi:10.1105/tpc.010351
Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (2000) Structural determinants of water permeation through aquaporin-1. Nature 407:599–605. doi:10.1038/35036519
Niemietz CM, Tyerman SD (2000) Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett 465:110–114. doi:10.1016/s0014-5793(99)01729-9
Schäffner AR (1998) Aquaporin function, structure, and expression: are there more surprises to surface in water relations? Planta 204:131–139. doi:10.1007/s004250050239
Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14:869–876. doi:10.1105/tpc.000901
Sui H, Han BG, Lee JK, Walian P, Jap BK (2001) Structural basis of water-specific transport through the AQP1 water channel. Nature 414:872–878. doi:10.1038/414872a
Takano J, Wada M, Ludewig U, Schaaf G, von Wiren N, Fujiwaraa T (2006) The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18:1498–1509. doi:10.1105/tpc.106.041640
Temmei Y, Uchida S, Hoshino D, Kanzawa N, Kuwahara M, Sasaki S, Tsuchiya T (2005) Water channel activities of Mimosa pudica plasma membrane intrinsic proteins are regulated by direct interaction and phosphorylation. FEBS Lett 579:4417–4422. doi:10.1016/j.febslet.2005.06.082
Tyerman SD, Niemietz CM, Bramley H (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25:173–194. doi:10.1046/j.0016-8025.2001.00791.x
Wallace IS, Choi WG, Roberts DM (2006) The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim Biophys Acta 1758:1165–1175. doi:10.1016/j.bbamem.2006.03.024
Weig AR, Jakob C (2000) Functional identification of the glycerol permease activity of Arabidopsis thaliana NLM1 and NLM2 proteins by heterologous expression in Saccharomyces cerevisiae. FEBS Lett 481:293–298. doi:10.1016/S0014-5793(00)02027-5
Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F (2007) FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. PNAS 104:12359–12364. doi:10.1073/pnas.0701180104
Zhang RB, Verkman AS (1991) Water and urea permeability properties of Xenopus oocytes: expression of mRNA from toad urinary bladder. Am J Physiol 260:C26–C34
Acknowledgments
This work was supported by National Natural Sciences Foundation of China (grant No. 30871317), and Natural Sciences Foundation of Hubei Province, China (grant No. 2006ABA181).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W.-H. Wu.
D.-D. Li and Y.-J. Wu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, DD., Wu, YJ., Ruan, XM. et al. Expressions of three cotton genes encoding the PIP proteins are regulated in root development and in response to stresses. Plant Cell Rep 28, 291–300 (2009). https://doi.org/10.1007/s00299-008-0626-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-008-0626-6