Abstract
RHO-like monomeric G-proteins of plants (ROPs, also called RACs), are involved in plant development and interaction with the environment. The barley (Hordeum vulgare) ROP protein HvRACB has been shown to be required for entry of the biotrophic powdery mildew fungus Blumeria graminis f.sp. hordei (Bgh) into living host cells. To get a deeper insight into evolutionarily conserved functions of ROPs in cell polarity and pathogen responses, we stably expressed constitutively activated (CA) mutant variants of different barley ROPs (HvRACB, HvRAC1, HvRAC3) in barley. CA HvROPs induced epidermal cell expansion and/or abolished polarity in tip growing root hairs. All three CA HvROPs enhanced susceptibility of barley to penetration by Bgh whereas only CA HvRAC1 supported whole cell H2O2 production in non-penetrated cells. Despite increasing penetration by Bgh, CA HvRAC1 promoted callose deposition at sites of fungal attack and resistance to penetration by Magnaporthe oryzae. The data show an involvement of ROPs in polar growth processes of the monocot barley and in responses to fungal pathogens with different life style.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small monomeric RHO-related proteins of plants (ROPs, also called RACs) are members of the Ras superfamily of small G-proteins. Plant ROPs constitute a unique plant specific group of RHO-like proteins (Berken 2006) and function as switches in signal transduction of environmental or endogenous stimuli. ROPs are active when bound to GTP and inactive when GDP-bound. Hydrolysis of GTP and GDP/GTP exchange are fine-tuned by regulator proteins and upstream signaling. ROPs operate in polar cell growth, disease resistance and susceptibility, hormone responses, hypoxia responses and secondary wall formation. RAC/ROP-regulated processes include exo- and endocytosis, cytoskeleton organization, cytosolic Ca2+ signaling, production of reactive oxygen species (ROS) and regulation of gene expression (Agrawal et al. 2003; Berken 2006; Nibau et al. 2006). There is a growing body of evidence that ROPs modulate production of ROS and hypersensitive cell death reactions. This includes regulation of activity or abundance of ROS-producing plasma membrane NADPH oxidases (Hassanain et al. 2000; Jones et al. 2007; Kawasaki et al. 1999; Moeder et al. 2005; Morel et al. 2004; Ono et al. 2001; Wong et al. 2007).
In those plant species, where ROPs have been intensively studied, 6–11 family members have been identified (Christensen et al. 2003; Schultheiss et al. 2003; Winge et al. 2000). Single rop mutants often have weak phenotypes indicating redundant functions of ROPs. ROPs might achieve specificity by complex interactions with upstream proteins (e.g. receptors) or downstream effectors and by tissue-specific expression of individual family members. Due to genetic redundancy, ectopically expressed mutants that are either constitutively activated (CA, GTP-bound) or dominantly negative (nucleotide free or GDP-bound) turned out to be valuable tools to study RAC/ROP functions.
In barley, ROPs modulate susceptibility to the powdery mildew disease caused by Blumeria graminis f.sp. hordei (Bgh) (Schultheiss et al. 2002, 2003, 2005). Susceptibility to Bgh operates at the level of accessibility to penetration by Bgh. HvRACB is involved in fungal penetration into epidermal cells of barley. Expression of CA HvRACB hinders actin polarization and penetration resistance, whereas knock-down of HvRACB promotes polarization and basal resistance. Therefore, it has been speculated that virulent Bgh might over-activate HvRACB to suppress polar defense reactions. Alternatively, ROP activity might be required for accommodation of the fungal haustorium, which also requires reorganization of the cytoskeleton and membranes to form the haustorial complex (Opalski et al. 2005; Schultheiss et al. 2002, 2003). Perhaps similarly, bacterial effectors target metazoan RHO-family proteins for induction of membrane ruffling and for uptake into host cells (Gruenheid and Finlay 2003).
The ROP protein family is organized in two subtypes and a total of four clades (Agrawal et al. 2003; Christensen et al. 2003; Schultheiss et al. 2003; Vernoud et al. 2003; Winge et al. 2000). Type I and type II ROP proteins differ insofar that an additional exon in type II ROP genes causes elongation of the carboxyterminal hypervariable region (Winge et al. 2000). In microprojectile-mediated single cell transformation assays, three out of five epidermis-expressed barley ROPs (type I CA HvRACB, type II CA HvRAC3, type II CA HvROP6) supported fungal penetration (Schultheiss et al. 2003). Stable transgenic barley plants expressing CA HvRACB showed enhanced susceptibility to powdery mildew, reduced sensitivity to abscisic acid in regard to stomata closure and reduced water retention capacity when cut off from water supply. Additionally, plants showed organ expansion defects, downward rolled leaves and reduced CO2-assimilation under saturating light intensity (Schultheiss et al. 2005). In contrast, CA HvRAC1 did not induce susceptibility to Bgh in single transformed cells. The putative rice ortholog of HvRAC1, OsRAC1, is involved in lignification and race-specific resistance to the rice blast fungus Magnaporthe oryzae (Kawasaki et al. 2006; Ono et al. 2001). Magnaporthe oryzae is pathogenic on barley and rice, and barley recessive mlo (MILDEW LOCUS O) mutants with full resistance to Bgh are super-susceptible to M. oryzae (Jarosch et al. 1999; Zellerhoff et al. 2006). Influence of HvRACB on susceptibility to Bgh depends on functional MLO (Schultheiss et al. 2003).
Here, we report about an impact of CA HvROPs on epidermal cell development in barley. Additionally, we describe the dual capability of HvRAC1 to support accommodation of fungal haustoria and the cellular defense reactions in interaction with Bgh, whereas it enhances penetration resistance in interaction with M. oryzae.
Materials and methods
Plant and pathogen material, inoculation
Donor material and transgenic plants of the barley (Hordeum vulgare L.) cultivar ‘Golden Promise’ were grown in a growth chamber at 18°C, 60% relative humidity, and a photoperiod of 16 h (150 μmol s−1 m−2 photon flux density) up to E.C. 30. As of the 5th week after germination, plants were grown in a greenhouse at 20°C. Blumeria graminis (DC) Speer f.sp. hordei Em. Marchal, race A6 (Wiberg 1974), was maintained on ‘Golden Promise’ under the same conditions. For microscopic evaluation, Bgh was inoculated onto detached second or third leaves of 14–20 days old barley plants to give a density of 30 conidia mm−2.
Magnaporthe oryzae isolate TH6772 was kindly provided by the Institute of Biochemistry, Tamagawa University, Machida-shi, Tokyo, Japan. Isolate TH6772 was grown alternately on PDA (Becton, Dickinson and Company, Sparks, USA) and rice leaf-agar (water extract of 50 g L−1 rice leaves, 2 g L−1 Faex medicinales (yeast extract, Biolabor, Germany), 10 g L−1 water soluble starch, 15 g L−1 agar–agar). It was maintained at 27°C under a 16/8 h day/night regime using black light (310–360 nm) for 2 weeks. For inoculation, conidia were harvested from plates by rinsing with distilled water and filtering through gauze. Conidia suspension was diluted 1:1 (v/v) with spraying solution [0.1% (w/v) gelatin, 0.05% (w/v) Tween-20]. The spore concentration was adjusted to 2 × 105 conidia mL−1. For microscopic studies the solution was sprayed onto primary leaves of barley plants 7 days after sowing. Additionally, third and fourth leaves were inoculated for macroscopic studies 16 days after sowing. Thereafter, plants were incubated in a dark moist chamber at 26°C and nearly 100% relative humidity for 24 h and afterwards kept under growth chamber conditions.
Recently, there was a reorganization with regard to designations within the Magnaporthe species complex and isolates compatible with rice are devoted to the species M. oryzae (Couch and Kohn 2002). This new nomenclature was used throughout this work.
Plant transformation
Stable genetic transformation of barley with pLH6000-ZmUbi::HvRACB-G15V, pLH6000-ZmUbi::HvRAC1-G23V, pLH6000-ZmUbi::HvRAC3-G17V was performed by Agrobacterium-mediated genetic transformation as described by Hensel et al. (2008). Constructs were generated as described earlier (Schultheiss et al. 2005).
Analysis of segregating populations of transgenic barley using RT-PCR and genomic PCR
Total RNA was extracted from one leaf using RNA extraction buffer (Invitrogen, Karlsruhe, Germany). To test T1 segregating individuals, mRNA expression was analyzed by one-step RT-PCR using the OneStep RT-PCR kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Primers were designed to allow amplification of expressed transgenic CA HvRAC but not endogenous RAC. For all three constructs of RACB, RAC1 and RAC3, 5′ Ubiquitin UTR1 primer (5′-AACCAGATCTCCCCCAAATC-3′, targeting a non-translated part of the maize ubiquitin promoter) was used and the 3′ primer was specific to each gene (Schultheiss et al. 2003). The same primers were taken to test segregating individuals by genomic PCR using the REDExtract-N-Amp plant PCR kit (Sigma-Aldrich Chemie GmbH, Munich, Germany). For all transgenic barley lines, transgene integration and expression was confirmed by genomic and RT-PCR, respectively. Additionally, visible phenotypes of transgenic plants were used to confirm the transgenic nature of individuals.
In most experiments (Fig. 3), three independent transgenic lines were used. The bulk of segregants that lost the transgene due to segregation was used as azygous controls in all experiments. Average azygous controls from individual lines behaved similarly. Previous experiments had shown that populations of azygous segregants behaved in development and pathogen response as wild type parent ‘Golden Promise’ plants (not shown). For statistical analysis of the transgene effect, we pooled transgenic individuals from different lines and corresponding controls, respectively. Lines used were B18-1-6, B18-1-10, 17-1-11, B17-1-15 and B17-1-22 for ZmUbi::CA HvRACB, B14-1-8, B16-1-1, B16-1-2, B16-1-33 and B19-3-3 for ZmUbi::CA HvRAC3, B27-2-32, B27-3-7, and B27-3-17 for ZmUbi::CA HvRAC1.
Histological staining and microscopic analysis
The outcome of the interaction of transgenic barley plants with Bgh was evaluated 24 and 48 h after inoculation. Penetration of attacked cells was ascertained by detection of haustoria formation. Staining with 3,3-diaminobenzidine (DAB) was taken as reliable markers of H2O2 accumulation (Görg et al. 1993; Thordal-Christensen et al. 1997; Hückelhoven et al. 1999). Bright-field and fluorescence microscopy was performed as described by Hückelhoven and Kogel (1998). Because the rate of fungal penetration into short and long epidermal cells is different, only short cells (cell types A and B, see Koga et al. 1990) were evaluated. To avoid misinterpretation due to the effects of induced accessibility or induced inaccessibility (Lyngkjær and Carver 1999), we exclusively evaluated interaction sites where only one fungus per cell attempted to penetrate. For callose staining, we cleared green leaves in ethanol:acetic acid (6:1 v/v) overnight and washed them with water. Subsequently, cleared leaves were incubated in 0.05% aniline blue (w/v) in 0.067 M K2HP04 buffer at pH 9.2 overnight and washed in water for microscopy. Accumulation of autofluorescent material was observed using epi-fluorescence microscopy as described by Jarosch et al. (1999).
Epidermal cell and root hair phenotypes in barley
To calculate the average cell length, we removed 5 cm from the tip of fully expanded leaves of similar length, and took the next 5 cm to count the numbers of B-cells (see Koga et al. 1990) in a middle row between two rows of stomata (see Fig. 1d). The number of cells present in a row on either side of the mid rib was counted and the average was taken. Each data set derived from four to five leaf samples taken from individual plants (approximately 200 cells per transgenic line).
Phenotype of wild type, CA HvRACB (line 18-1-6), CA HvRAC3 (line 17-3-16) or CA HvRAC1 (line 27/2-29) plants. a Shoot development of 7-week-old barley genotypes. b Root phenotype of 1-week-old barley. Pictures show representative sectors of the differentiation zone of barley roots. c Root hair phenotypes. Pictures show details of wild type or aberrant root hair development in CA HvROP barley. Bar 30 μm. d Confocal laser scanning micrographs of cell walls of the adaxial epidermis of fourth leaves of indicated barley genotypes after propidium iodide staining. Middle row of B-cells is marked with asterisks. Bar = 150 μm
To visualize epidermal cell shapes, sample fixation and modified pseudo-Schiff propidium iodide (mPS-PI) staining was performed exactly as described by Truernit et al. (2008). Propidium iodide staining was allowed for 2 h. The samples were then transferred onto microscope slides and covered with a chloral hydrate solution (4 g chloral hydrate, 1 mL glycerol, and 2 mL water). Epidermal cells were examined by using a confocal laser scanning microscope (Leica SP5, Mannheim, Germany). Samples were excited with a 488 nm laser lane. Emission was detected between 560 and 675 nm. The epidermal cell layer between two rows of stomata was scanned in xy direction in 10 z-sections at 1.5 μm increments.
Root hair phenotypes were observed on solid (0.6% gelrite) half-MS medium (Murashige and Skoog 1962) containing 5% sucrose.
Statistical analysis
For all data, 95% confidence intervals were calculated and plotted into figures as error bars. For normally distributed data, Student’s t tests were performed. For percentages, we carried out χ2 testing.
Results
CA HvROPs affect plant and cell architecture
We generated transgenic barley with stable expression of CA HvROPs to get a deeper insight into the physiological and pathogenesis-related function of HvRACB, HvRAC3 and HvRAC1 (referred to as barley ROPs, where more than one of them is meant). We expressed CA HvROPs under the control of the maize ubiquitin promoter, which is constitutively active in barley root and leaf epidermal tissue (Hensel et al. 2008; Schultheiss et al. 2005). All CA HvRACB, CA HvRAC3 and CA HvRAC1 genotypes were similar in macroscopic appearance. They appeared wild type like until the three-leaf stage. Later, plants consistently showed stunted shoots, less tillering (Fig. 1a) and strongly reduced formation of ears demonstrating developmental failure when barley ROPs are CA. In contrast, azygous sibling plants, which had lost the transgene due to segregation, set ears and developed normally. Because HvRACB is involved in cell polarity in plant defense (Opalski et al. 2005) and HvRACB-like Arabidopsis ROPs are crucial for pavement cell development (Fu et al. 2002, 2005), we wondered whether barley ROPs interfere with cell polarity when over-activated. Therefore, we inspected root hair development in transgenic barley plants expressing CA HvROPs in two independent experiments. When barley seedlings grew on artificial solid medium containing 5% sucrose (w/v), which appears to enhance root hair defects (Yang et al. 2007), root hairs of both CA HvRACB and CA HvRAC3 were short, swollen and irregular when compared to those of azygous controls or wild type barley. CA HvRAC1 barley had also deformed root hairs but the phenotype was less obvious when compared to CA HvRACB or CA HvRAC3 (Fig. 1b, c). Additionally, barley plants expressing CA HvROPs showed a more irregular shape of leaf epidermal cells when compared to wild type (Fig. 1d).
Results from heterologous expression in tobacco, where CA HvROPs induced strong epidermal cell size effects (unpublished data of I.P.P.), stimulated us to carefully evaluate effects of CA barley ROPs on barley epidermal cell size. We measured length of epidermal B-cells covering mesophyll tissue between longitudinal vessels (Fig. 1d; Koga et al. 1990). To confirm that T-DNA insertion or somatic mutations during regeneration would not be the cause of potential CA barley ROP-induced phenotypes, we worked with at least three independent transgenic lines for each construct (see “Materials and methods”). We used segregating T1 and T2 populations expressing the transgene as checked by both genomic and RT-PCR. In all experiments, a subpopulation of all individuals checked by PCR had lost the transgene due to segregation. These azygous individuals have a wild type genotype combined with a tissue-culture background. Therefore, they constitute ideal genetic controls, and were used to demonstrate CA HvROP effects (see “Materials and methods”). According to Student’s t tests or χ2 tests, respectively, independent transgenic lines behaved similarly, and thus data from different lines with the same barley ROP construct were pooled and treated as a metapopulation (Figs. 2, 3). Expression of CA HvRACB and CA HvRAC1 clearly enhanced B-cell length by about 20%, whereas CA HvRAC3 enhanced cell length by 8% only (Fig. 2).
Sizes of barley epidermal cells of transgenic plants expressing distinct CA barley ROPs. Average cell sizes were measured in fully expanded leaves of 3-week-old barley plants. Data show average size of epidermal cells covering mesophyll tissue in between two rows of longitudinal main vessels without having direct contact to stomata complexes. Data were pooled from three independent segregating transgenic lines for each CA barley ROP construct. Error bars show 95% confidence intervals. **, ***; Student’s t test: P < 0.01; P < 0.001 when compared to azygous control. Repetition of the experiments led to similar results (lines used: CA HvRACB 18-1-10, 17-1-11, 18-1-6; CA HvRAC1 27-3-17, 27-2-2; and CA HvRAC3 14-1-8, 17-3-16)
Microscopic interaction phenotype of metapopulations of transgenic barley lines expressing distinct CA barley ROPs with Bgh. a Penetration site at 24 HAI on wild type barley. The fungus produced a primary germ tube (pgt), an appressorium (app), a haustorium (hau) and small elongated secondary hyphae (esh) from its conidium (c). Plant reacted with H2O2 production as indicated by DAB staining in association with the pgt. Bar = 10 μm. b Non-penetrated cell shows HR associated with whole cell DAB staining at 24 HAI on wild type barley. Bar = 30 μm. c DAB staining in association with formation of a CWA at a site where the fungus failed in penetration on wild type barley. Bar = 20 μm. d Average fungal penetration success displayed as percentage of all interaction sites where Bgh was able to establish a visible haustorium in epidermal cells of barley at 24 HAI (see a). e Average frequency of interaction sites where the fungus failed in penetration and the attacked cell responded with hypersensitive cell death accompanied by whole cell DAB staining (see b). f Average frequency of interaction sites where the fungus failed in penetration, the attacked cell survived and displayed local DAB staining beneath the fungal appressorium (see c). Thirty-two testings revealed significant differences between CA HvRACB (P < 0.001) or CA HvRAC1 (P < 0.001) and azygous controls whereas HvRAC3 was not significantly different from controls (P > 0.05). Error bars show 95% confidence intervals. Each column represents data summarized from a metapopulation of three to four independent transgenic lines for each CA barley ROP construct (lines used: CA HvRACB 18-1-10, 18-1-6, 17-1-15, 17-1-22; CA HvRAC3 14-1-8, 16-1-1, B19-3-3; CA HvRAC1 27-3-17, 27-3-7, 27-2-32). Repetition of the experiments led to similar results
CA barley ROPs support penetration by Bgh or the hypersensitive reaction or both
To understand potentially overlapping and distinct functions of barley ROPs in development and pathogenesis, we inoculated third leaves of transgenic barley plants expressing CA HvROPs with Bgh and performed a microscopic analysis of the interaction outcome at 24 h after inoculation (HAI). All three CA HvROP-barley genotypes were more susceptible to fungal penetration than azygous controls (Fig. 3a, d). In contrast to results from transient transformation assays (Schultheiss et al. 2003), the effect on CA HvRAC1 plants was similar to that on CA HvRACB plants (Fig. 3a, d). We also inspected attacked but non-penetrated cells for the frequency of H2O2 accumulation as evidenced by DAB staining (Thordal-Christensen et al. 1997). Whole cell DAB staining is indicative of a hypersensitive cell death reaction (HR), which is part of basal resistance in barley (Hückelhoven et al. 1999; Koga et al. 1990; Thordal-Christensen et al. 1997). CA HvRAC1 promoted whole cell H2O2 accumulation. However, this was observed only in cells where a fungal attempt to penetrate failed, i.e. where neither a fungal haustorium initial nor an elongated secondary hyphae of the fungus developed (Fig. 3b, e). In contrast, neither CA HvRACB nor CA HvRAC3 influenced the frequency of whole cell DAB staining. However, no spontaneous cell death was observed on non-inoculated CA HvRAC1 barley.
Because ROPs are regulators of ROS production in plants, we also evaluated the number of living cells showing localized DAB staining beneath the fungal appressorium (Fig. 3c, f), which is typically associated with the formation of non-penetrated cell wall appositions (CWAs, Thordal-Christensen et al. 1997; Hückelhoven et al. 1999). This revealed that all three CA HvROPs reduced the frequency of local DAB staining at sites of fungal attack with CA HvRAC1 having the strongest effect. This relates to enhanced fungal penetration, which was not accompanied by local DAB staining in CWAs. The particular strength of this effect in CA HvRAC1 barley is additionally explained by frequent whole cell DAB staining.
Inoculation experiments on detached leaves can yield quantitative or qualitative differences in interaction outcome when compared to attached leaf assays (Liu et al. 2007). To confirm that enhanced susceptibility to fungal penetration would also occur on intact plants, wild type Golden Promise and each two independent lines of CA HvRAC1 and CA HvRACB were inoculated and microscopically analyzed at 48 HAI. Both CA HvRAC1 and CA HvRACB supported fungal penetration into epidermal cells of barley, however, at a higher basal level when compared to detached leaves. The absolute frequency of whole cell DAB staining was lower on intact plants when compared to detached leaves but on both detached and attached leaves CA HvRAC1 nearly doubled the frequency of whole cell H2O2 accumulation (Fig. 3 and data not shown).
CA HvRAC1 enhances pathogen-induced callose deposition
Because CA HvRAC1 barley displayed little H2O2 accumulation at CWAs, we observed aniline blue stainable callose deposition in CWAs, which is another hallmark of cell wall-associated plant defense (Hückelhoven 2007; Schmelzer 2002). In wild type parent and azygous segregants of the T2 generation, we observed little penetration and differently strong callose deposits at sites of fungal attack. However, we did not observe any spatial association of strength or pattern of callose depositions and local penetration resistance (see Fig. 4a, b). Consistent with our previous results, CA HvRAC1 barley allowed for more penetration than wild type and azygous controls. Additionally, two independent lines of CA HvRAC1 barley showed more CWAs with strong callose deposits when compared to either control (Fig. 4c). Together, CA HvRAC1 appears to support local callose deposition.
CA HvRAC1 supports pathogen-induced callose deposition. a Strong callose depositions beneath sites of attempted fungal penetration at 24 HAI. CWA Cell wall apposition; APP appressorium, size bar 30 μm. b Strong callose depositions beneath a site of fungal penetration at 40 HAI. CWA Cell wall apposition; APP appressorium; ESH elongated secondary hyphae, size bar 45 μm. c Columns represent the average frequency of fungal penetration success and total interaction sites with strong callose staining in CWAs beneath fungal appressoria at 24 HAI. WT Wild type parent Golden Promise. Data were obtained from analysis of the segregating transgenic line 27-3-17 and parent wild type. Experiments were repeated with independent T2 plants (line 27-2-32) and gave similar results. χ2 testings revealed significant differences between CA HvRAC1 and both wild type and azygous controls (p < 0.001). Error bars show 95% confidence intervals
CA HvRAC1 barley is less susceptible to M. oryzae
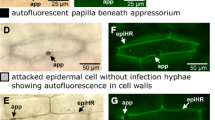
We reasoned that CA HvRAC1 barley might differ from wild type in resistance to M. oryzae. Indeed, CA HvRAC1 barley showed less formation of blast symptoms than wild type and azygous controls after inoculation with M. oryzae in three independent experiments (Fig. 5). A microscopic analysis revealed clear differences in the interaction outcome of CA HvRAC1 barley when compared to wild type or to azygous controls. CA HvRAC1 reduced the success of fungal penetration attempts on barley when compared to either control (Fig. 6). Stronger resistance to fungal penetration resulted in less epidermal cell death, as revealed by whole cell autofluorescence, and in less invasive growth into the mesophyll. M. oryzae invasive growth into the mesophyll was accompanied by mesophyll cell death, which precedes blast symptom formation. However, localized mesophyll cell death was also observed without invasion of the mesophyll. Accordingly, CA HvRAC1 showed less mesophyll cell death in association with invasive growth than wild type genotypes. Mesophyll cell death without invasive growth was unchanged (Fig. 6).
Numbers of blast symptoms caused by M. oryzae per leaf segment of CA HvRAC1 barley, wild type cultivar Golden Promise (WT) and azygous controls. Data were obtained from metaanalysis of the segregating transgenic lines 27-3-17, 27-3-7 and 27-2-32. Asterisk indicates average that shows a significant difference from the azygous control (Student’s t test, P < 0.05). Error bars show 95% confidence intervals
Microscopic interaction phenotype of transgenic barley expressing CA HvRAC1 with M. oryzae. Leaves of barley cultivar Golden Promise (WT), CA HvRAC1 barley and corresponding azygous controls were inoculated with M. oryzae and were fixed 96 HAI. Cellular plant–fungus interaction sites were classified according to the formation of non-penetrated CWA formed beneath a fungal appressorium or the detection of autofluorescent material in the epidermis (E) and the mesophyll (M). Columns represent mean values from 4 leaves with approximately 100 interaction sites inspected per genotype and per leaf. Data were obtained from metaanalysis of the segregating transgenic lines 27-3-17, 27-3-7 and 27-2-32. Significant differences (χ2 testing) between both controls and CA HvRAC1 were determined for the interaction outcome (P < 0.0001). Error bars show 95% confidence intervals. Invasive growth Infection hyphae in the mesophyll associated with cell collapse
Discussion
The data presented here support a function of barley ROPs in modulation of susceptibility or basal resistance to at least two fungal leaf pathogens and suggest an involvement in root hair and epidermal cell development.
Role of barley ROPs in epidermal cell development
To address the question whether ROPs might affect tip growth in monocots, we observed root hairs of transgenic barley expressing CA HvROPs on a solid medium. CA barley ROPs and in particular CA HvRACB and CA HvRAC3 induced shortening and ballooning of root hairs. It had been demonstrated before, that ROPs need to be fine-tuned in spatial abundance and in activity for initiation and maintenance of root hair tip growth in dicots (Bloch et al. 2005; Carol et al. 2005; Hwang et al. 2005; Jones et al. 2002; Jones et al. 2007; Klahre and Kost 2006; Molendijk et al. 2001). Ectopic over-activation of ROPs in Arabidopsis leads to loss of spatial control and of polarity resulting in isotropic growth instead of tip growth. The fact that CA HvROPs can also interfere with root hair tip growth in the monocot barley indicates an evolutionarily conserved function of ROPs in polar growth processes. Polarized ROP activity at sites of fungal penetration is also indicated by spatial accumulation of HvRIC171, a protein which both interacts with and is recruited to the cell periphery by activated HvRACB (Schultheiss et al. 2008).
HvRACB homologous Arabidopsis ROP2 and ROP4 operate in pavement cell development, organizing both actin and microtubule cytoskeleton in lobe and neck formation. CA AtROP2 causes aberrant cell shape effects in epidermal pavement cells of Arabidopsis (Fu et al. 2002, 2005). Similarly, ectopic expression of CA AtRAC10, a homolog of CA HvRAC3, causes elimination of epidermal cell lobes in Arabidopsis (Bloch et al. 2005). In contrast, barley plants expressing CA HvROPs had not such a dramatic cell shape phenotype but more irregular cell shapes. Apparently, the brick-like shape of barley epidermal cells did not allow for extremely aberrant cell shapes as observed in CA AtROP2-expressing Arabidopsis plants. However, CA HvRACB and HvRAC1 induced epidermal cell size effects in barley (Fig. 2). CA AtROP2 induces lateral and radial cell expansion in Arabidopsis (Fu et al. 2002). However, in contrast to HvRACB and HvRAC1, AtROP2 does not significantly affect cell length in Arabidopsis leaf longitudinal orientation (Fu et al. 2002). Hence barley and Arabidopsis ROPs might share conserved functions in epidermal cell growth but also differ in the pattern of growth processes they are involved in.
CA HvROPs support susceptibility to powdery mildew fungi
All the three CA HvROPs enhanced fungal penetration. Both leaf epidermal cell size effects and enhanced susceptibility were strong in CA HvRACB and CA HvRAC1 plants and weaker in CA HvRAC3 plants (Figs. 2, 3d). Importantly, the fact that transient expression of CA HvRACB and CA HvRAC3 induced super-susceptibility in fully expanded leaf cells (Schultheiss et al. 2003) shows that cell size or cell shape not directly determined susceptibility. However, the association of cell size and susceptibility in stable transgenic barley suggests that cell growth and susceptibility to cell wall penetration by Bgh share common elements.
For CA HvRAC1 barley, enhanced susceptibility was unexpected from transient transformation experiments (Schultheiss et al. 2003). Our inspection of transiently transformed CA HvRAC1-cells supports that CA HvRAC1 reduces vital movement of the cytoplasm when over expressed in single cells, whereas CA HvRACB does not (unpublished results of I.P.P.). Hence, transient multicopy expression of CA HvRAC1 in single cells seems to limit cell survival, which might have superimposed CA HvRAC1-induced susceptibility to biotrophic Bgh. However, we never observed spontaneous cell death in stable CA HvRAC1 barley.
Potential roles of HvROPs in interaction with Bgh or M. oryzae
CA HvRAC1 enhanced local callose deposition but reduced the number of CWAs with H2O2 accumulation and enhanced susceptibility (Figs. 3, 4). This shows that glucan synthase dependent callose deposition can be uncoupled from other wall-associated defenses, and that strong callose deposition is not indicative of penetration resistance to Bgh. Arabidopsis CA ROP1 binds UDP-glucose transferase, which is part of the callose forming complex at the cell plate (Hong et al. 2001). The Arabidopsis pmr4 mutant, which is defective in CWA-associated callose deposition due to mutation of glucan synthase 5, displays enhanced salicylic acid-dependent resistance to powdery mildew. This indicates that plant glucan synthases can negatively control callose-independent plant defense (Jacobs et al. 2003; Nishimura et al. 2003). However, callose could be involved in penetration resistance of barley to M. oryzae as it is in resistance of Brachypodium distachyon to adapted Magnaporthe (Routledge et al. 2004). In a preliminary histochemical analysis of CA HvRAC1 barley, CWAs often showed positive callose staining with aniline blue at sites of attack by M. oryzae.
In contrast to CA HvRACB and CA HvRAC3, CA HvRAC1 supported HR-like whole cell H2O2 accumulation in barley when attacked by Bgh (Fig. 3e). Phylogenetic analysis of plant ROPs revealed that HvRACB, HvRAC3 and HvRAC1 belong to three different subclades of barley ROPs (Schultheiss et al. 2003). Because all three activated barley ROPs promoted penetration success of Bgh but only CA HvRAC1 promoted whole cell H2O2 accumulation, these barley ROPs might have overlapping and distinct functions in plant–microbe interactions. The closest relative of HvRAC1 in rice, OsRAC1, promotes production of ROS and HR-like cell death in response to M. oryzae. Additionally, dominant negative OsRAC1 or Medicago sativa RAC1 antisense RNA suppress hypersensitive cell death in tobacco and rice (Kawasaki et al. 1999; Moeder et al. 2005; Ono et al. 2001; Schiene et al. 2000). OsRAC1 boosts HR by interaction with and activation of ROS-producing plasma membrane NADPH oxidase and by down-regulation of metallothionein, a reactive oxygen scavenger in rice (Kawasaki et al. 1999; Ono et al. 2001; Wong et al. 2004, 2007). Interestingly, ROS and ROP-related spatial control of ROS production is also required for root hair initiation and polarity (Carol et al. 2005; Foreman et al. 2003; Jones et al. 2007).
All CA barley ROPs induced a drop in local CWA-associated DAB staining beneath fungal appressoria (Fig. 3). This might indicate different sources of ROS during HR and during formation of CWAs, respectively. Accordingly, transient knock-down of a barley NADPH oxidase did not limit the cell wall-associated production of H2O2 (Trujillo et al. 2006). Alternatively, CA HvROPs caused depolarization of attacked cells inhibiting local secretion for cell wall-associated H2O2 production.
How can one explain that CA HvRAC1 supported biotrophic Bgh at the penetration level but also promoted HR? Whole cell H2O2 accumulation was exclusively observed in cells where Bgh failed to penetrate. HR of non-penetrated cells occurs at a low to medium frequency in basal resistance to virulent Bgh (Fig. 3, Hückelhoven and Kogel 1998; Hückelhoven et al. 1999; Koga et al. 1990). However, once virulent Bgh succeeds in penetrating an epidermal cell of barley, such a cell extremely rarely undergoes HR. Hence, biotrophic Bgh may be able to suppress host cell death after penetration. Therefore, a potential influence of CA HvRAC1 on the threshold for HR-like cell death might become obvious only in non-penetrated cells (Fig. 3e). The observation that ROPs can modulate both susceptibility and cellular defense responses suggests that ROPs are potential interfaces of different pathogen signals.
In contrast to what was observed in interaction with Bgh, CA HvRAC1 barley has reduced susceptibility to M. oryzae (Fig. 5). This underlines that ROPs, similar as the receptor-like HvMLO (Jarosch et al. 1999; Zellerhoff et al. 2006), have distinct functions in interaction with pathogens of different life style and further supports that MLO and ROPs could be functionally linked (Miklis et al. 2007; Schultheiss et al. 2002, 2003). It was shown previously, that transgenic CA OsRAC1 rice displays resistances and HR-like cell death when challenged by virulent M. oryzae. In contrast, dominant negative OsRAC1 blocked race-specific resistance to M. oryzae but did not enhance susceptibility to virulent M. oryzae. Hence, OsRAC1 is required for race-specific immunity to M. oryzae (Ono et al. 2001). CA OsRAC1 further induces resistance to virulent Xanthomonas oryzae pv. oryzae and multiple defense reactions in rice cell cultures, which are greatly overlapping with non-specific elicitor-induced responses (Fujiwara et al. 2006; Ono et al. 2001). Our microscopic evaluation of the interaction of CA HvRAC1 barley with M. oryzae (Fig. 6) revealed a picture differing from what was observed in CA OsRAC1 rice (Ono et al. 2001). We did not observe enhanced fungus-induced cell death in interaction with M. oryzae but enhanced resistance to fungal penetration, which was associated with formation of local CWAs. Similarly, it was reported that both basal resistance of barley against adapted isolates of M. oryzae and non-host resistance to non-adapted Magnaporthe isolates rely on restriction of fungal penetration (Zellerhoff et al. 2006). Furthermore cell death and other defense reactions such as callose deposition appear uncoupled in the interaction of CA HvRAC1 barley with M. oryzae. Astonishingly, both CA HvRAC1 and CA OsRAC1 enhance resistance to M. oryzae, but defense mechanisms clearly differ in both grass species. Potentially, RAC1 is involved in basal resistance to M. oryzae in barley whereas it is involved in race-specific resistance to M. oryzae in rice. Nevertheless, both cell death in rice and penetration resistance in barley resulted in reduced invasive growth of M. oryzae.
Abbreviations
- CA:
-
Constitutively activated
- CWA:
-
Cell wall apposition
- DAB:
-
3,3-Diaminobenzidine
- HAI:
-
Hours after inoculation
- HR:
-
Hypersensitive reaction
- MLO:
-
MILDEW LOCUS O
- ROS:
-
Reactive oxygen species
- RHO:
-
Rat sarcome oncogene product (RAS) homologue
- ROP:
-
RHO of plants
- RAC:
-
Ras related C3 botulinum toxin substrate
References
Agrawal GK, Iwahashi H, Rakwal R (2003) Small GTPase ‘Rop’: molecular switch for plant defense responses. FEBS Lett 546:173–180
Berken A (2006) ROPs in the spotlight of plant signal transduction. Cell Mol Life Sci 63:2446–2459
Bloch D, Lavy M, Efrat Y, Efroni I, Bracha-Drori K, Abu-Abied M, Sadot E, Yalovsky S (2005) Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling. Mol Biol Cell 16:1913–1927
Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L (2005) A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438:1013–1016
Christensen TM, Vejlupkova Z, Sharma YK, Arthur KM, Spatafora JW, Albright CA, Meeley RB, Duvick JP, Quatrano RS, Fowler JE (2003) Conserved subgroups and developmental regulation in the monocot rop gene family. Plant Physiol 133:1791–1808
Couch BC, Kohn LM (2002) A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 94:683–693
Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Fu Y, Li H, Yang Z (2002) The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14:777–794
Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z (2005) Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120:687–700
Fujiwara M, Umemura K, Kawasaki T, Shimamoto K (2006) Proteomics of Rac GTPase signaling reveals its predominant role in elicitor-induced defense response of cultured rice cells. Plant Physiol 140:734–745
Görg R, Hollricher K, Schulze-Lefert P (1993) Functional analysis and RFLP-mediated mapping of the Mlg resistance locus in barley. Plant J 3:857–866
Gruenheid S, Finlay BB (2003) Microbial pathogenesis and cytoskeletal function. Nature 422:775–781
Hassanain HH, Sharma YK, Moldovan L, Khramtsov V, Berliner LJ, Duvick JP, Goldschmidt-Clermont PJ (2000) Plant Rac proteins induce superoxide production in mammalian cells. Biochem Biophys Res Commun 272:783–788
Hensel G, Valkov V, Middlefell-Williams J, Kumlehn J (2008) Efficient generation of transgenic barley: the way forward to modulate plant-microbe interactions. J Plant Physiol 165:71–82
Hong Z, Zhang Z, Olson JM, Verma DP (2001) A novel UDP-glucose transferase is part of the callose synthase complex and interacts with phragmoplastin at the forming cell plate. Plant Cell 13:769–779
Hückelhoven R (2007) Cell wall-associated mechanisms of disease resistance and susceptibility. Annu Rev Phytopathol 45:101–127
Hückelhoven R, Kogel KH (1998) Tissue-specific superoxide generation at interaction sites in resistant and susceptible near-isogenic barley lines attacked by the powdery mildew fungus (Erysiphe graminis f.sp. hordei). Mol Plant Microbe Interact 11:292–300
Hückelhoven R, Fodor J, Preis C, Kogel KH (1999) Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with H2O2 but not with salicylic acid accumulation. Plant Physiol 119:1251–1260
Hwang JU, Gu Y, Lee YJ, Yang Z (2005) Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell 16:5385–5399
Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, Fincher GB (2003) An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15:2503–2513
Jarosch B, Kogel KH, Schaffrath U (1999) The ambivalence of the barley Mlo Locus: mutations conferring resistance against powdery mildew (Blumeria graminis f.sp. hordei) enhance susceptibility to the rice blast fungus Magnaporthe grisea. Mol Plant Microbe Interact 12:508–514
Jones MA, Shen JJ, Fu Y, Li H, Yang Z, Grierson CS (2002) The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14:763–776
Jones MA, Raymond MJ, Yang Z, Smirnoff N (2007) NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot 58:1261–1270
Kawasaki T, Koita H, Nakatsubo T, Hasegawa K, Wakabayashi K, Takahashi H, Umemura K, Umezawa T, Shimamoto K (2006) Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc Natl Acad Sci USA 103:230–235
Kawasaki T, Henmi K, Ono E, Hatakeyama S, Iwano M, Satoh H, Shimamoto K (1999) The small GTP-binding protein Rac is a regulator of cell death in plants. Proc Natl Acad Sci USA 96:10922–10926
Klahre U, Kost B (2006) Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell 18:3033–3046
Koga H, Bushnell WR, Zeyen RJ (1990) Specificity of cell type and timing of events associated with papilla formation and the hypersensitive reaction in leaves of Hordeum vulgare attacked by Erysiphe graminis f.sp. hordei. Can J Bot 68:2344–2352
Liu G, Kennedy R, Greenshields DL, Peng G, Forseille L, Selvaraj G, Wei Y (2007) Detached and attached Arabidopsis leaf assays reveal distinctive defense responses against hemibiotrophic Colletotrichum spp. Mol Plant Microbe Interact 20:1308–1319
Lyngkjær MF, Carver TLW (1999) Modification of mlo5 resistance to Blumeria graminis attack in barley as a consequence of induced accessibility and inaccessibility. Physiol Mol Plant Pathol 55:163–174
Miklis M, Consonni C, Bhat RA, Lipka V, Schulze-Lefert P, Panstruga R (2007) Barley Mlo modulates actin-dependent and actin-independent antifungal defence pathways at the cell periphery. Plant Physiol 144:1132–1143
Moeder W, Yoshioka K, Klessig DF (2005) Involvement of the small GTPase Rac in the defense responses of tobacco to pathogens. Mol Plant Microbe Interact 18:116–124
Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, Gilroy S, Palme K (2001) Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J 20:2779–2788
Morel J, Fromentin J, Blein JP, Simon-Plas F, Elmayan T (2004) Rac regulation of NtrbohD, the oxidase responsible for the oxidative burst in elicited tobacco cell. Plant J 37:282–293
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Nibau C, Wu HM, Cheung AY (2006) RAC/ROP GTPases: ‘hubs’ for signal integration and diversification in plants. Trends Plant Sci 11:309–315
Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301:969–972
Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K (2001) Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Adad Sci USA 98:759–764
Opalski KS, Schultheiss H, Kogel KH, Hückelhoven R (2005) The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f.sp. hordei. Plant J 41:291–303
Routledge APM, Shelley G, Smith JV, Talbot NJ, Draper J, Mur LAJ (2004) Magnaporthe grisea interactions with the model grass Brachypodium distachyon closely resemble those with rice (Oryza sativa). Mol Plant Pathol 5:253–265
Schiene K, Puhler A, Niehaus K (2000) Transgenic tobacco plants that express an antisense construct derived from a Medicago sativa cDNA encoding a Rac-related small GTP-binding protein fail to develop necrotic lesions upon elicitor infiltration. Mol Gen Genet 263:761–770
Schmelzer E (2002) Cell polarization, a crucial process in fungal defence. Trends Plant Sci 7:411–415
Schultheiss H, Dechert C, Kogel KH, Hückelhoven R (2002) A small GTP-binding host protein is required for entry of powdery mildew fungus into epidermal cells of barley. Plant Physiol 128:1447–1454
Schultheiss H, Dechert C, Kogel KH, Hückelhoven R (2003) Functional analysis of barley RAC/ROP G-protein family members in susceptibility to the powdery mildew fungus. Plant J 36:589–601
Schultheiss H, Hensel G, Imani J, Broeders S, Kumlehn J, Kogel KH, Sonnewald U, Hückelhoven R (2005) Ectopic expression of constitutively activated RACB in barley enhances susceptibility to powdery mildew and abiotic stress. Plant Physiol 139:353–362
Schultheiss H, Preuss J, Pircher T, Eichmann R, Hückelhoven R (2008) Barley RIC171 interacts with RACB in planta and supports entry of the powdery mildew fungus. Cell Microbiol 10:1815–1826
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localisation of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Trujillo M, Altschmied L, Schweizer P, Kogel KH, Hückelhoven R (2006) Respiratory burst oxidase homologue A of barley contributes to penetration by the powdery mildew fungus Blumeria graminis f.sp. hordei. J Exp Bot 57:3781–3791
Truernit E, Bauby H, Dubreucq B, Grandjean O, Runions J, Barthélémy J, Palauqui JC (2008) High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis. Plant Cell 20:1494–1503
Vernoud V, Horton AC, Yang Z, Nielsen E (2003) Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol 131:1191–1208
Wiberg A (1974) Genetical studies of spontaneous sources of resistance to powdery mildew in barley. Hereditas 77:89–148
Winge P, Brembu T, Kristensen R, Bones AM (2000) Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics 156:1959–1971
Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T, Shimamoto K (2007) Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 19:4022–4034
Wong HL, Sakamoto T, Kawasaki T, Umemura K, Shimamoto K (2004) Down-regulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol 135:1447–1456
Yang G, Gao P, Zhang H, Huang S, Zheng ZL (2007) A mutation in MRH2 kinesin enhances the root hair tip growth defect caused by constitutively activated ROP2 small GTPase in Arabidopsis. PLoS ONE 2:e1074
Zellerhoff N, Jarosch B, Groenewald JZ, Crous PW, Schaffrath U (2006) Nonhost resistance of barley is successfully manifested against Magnaporthe grisea and a closely related Pennisetum-infecting lineage but is overcome by Magnaporthe oryzae. Mol Plant Microbe Interact 19:1014–1022
Acknowledgments
We are grateful to Cornelia Marthe (IPK Gatersleben) for technical assistance. This work was funded by the German Research Foundation (HU886/1-3), by BMBF-GABI-Agrotec, by the German Academic Exchange Service, and by the BASF AG.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Feher.
Rights and permissions
About this article
Cite this article
Pathuri, I.P., Zellerhoff, N., Schaffrath, U. et al. Constitutively activated barley ROPs modulate epidermal cell size, defense reactions and interactions with fungal leaf pathogens. Plant Cell Rep 27, 1877–1887 (2008). https://doi.org/10.1007/s00299-008-0607-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-008-0607-9