Abstract
We analysed the endogenous cytokinin levels of Dendrobium Madame Thong-In seedlings grown in vitro during vegetative and flowering-inductive periods. HPLC was used to fractionate the extracts and radioimmunoassay (RIA) was used for assay of zeatin (Z), dihydrozeatin (DZ), N6-(Δ2-isopentenyl)-adenine (iP) and their derivatives. Coconut water used in experiments was found to contain high level (>136 pmol ml−1) of zeatin riboside (ZR). Protocorms and seedlings cultured in medium with coconut water were found to contain 0.5–3.9 pmol g−1 FW of the cytokinins analysed. Seedlings (1.0–1.5 cm) cultured in flowering-inductive liquid medium containing 6-benzyladenine (BA, 4.4 μM) and coconut water (CW, 15%) contained up to 200 and 133 pmol g−1 FW of iP and iPA, respectively. These levels were significantly higher than all other cytokinins analysed in seedlings of the same stage and were about 80- to 150-folds higher than seedlings cultured in non-inductive medium. During the transitional (vegetative to reproductive) stage, the endogenous levels of iP (178 pmol g−1 FW) and iPA (63 pmol g−1 FW) were also significantly higher than cytokinins in the zeatine (Z) and dihydrozeatin (DZ) families in the same seedlings. Seedlings that grew on inductive medium but remained vegetative contained lower levels of iPA. The importance of the profiles of iP and its derivatives in induction of in vitro flowering of D. Madame Thong-In is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orchid seeds are usually germinated in vitro on a simple medium containing minerals and sugars (Knudson 1946). The period of vegetative growth from seed to flowering varies with species and hybrids. In the commercially important genus Dendrobium, juvenile periods of hybrids can range from 2 to 4 years. We have successfully developed a method to induce in vitro flowering of the self-pollinated seedlings of a tropical orchid hybrid, Dendrobium Madame Thong-In about 5 months after seed germination (Sim et al. 2007). Thus, the juvenile period was shortened to 1/5 or less after the treatment in tissue culture system (Sim et al. 2007). This method was also successfully applied to induce in vitro flowering of another Dendrobium hybrid, Chao Praya Smile (Hee et al. 2007).
The transition from vegetative growth to flowering is of importance in agriculture, horticulture and plant breeding. The nature of the signal that induces flowering, however, remains unclear (Corbesier and Coupland 2006). The physiological study of the floral transition has led to the identification of several putative floral signals such as cytokinins, sucrose, gibberellin and reduced N-compounds (Corbesier and Coupland 2006). Cytokinins are considered to be one of the most important physiological signals in flowering (Bernier et al. 1993; Lejeune et al. 1994; Bonhomme et al. 2000). In many plant species other than orchids, addition of cytokinins to the culture medium was also found to induce early flowering in vitro (Bernier 1988; Peeters et al. 1991). For example, in Arabidopsis, the addition of the cytokinin iPA was effective in inducing early bolting and flower bud formation in vitro (He and Loh 2002). The induction of vegetative shoot apical meristem to inflorescence meristem was observed when D. Madame Thong-In protocorms were cultured in modified Knudson C medium (KC, Knudson 1946) liquid medium containing BA and coconut water (Sim et al. 2007). Coconut water (CW) was found to be essential to trigger the transitional shoot apical meristem and 6-benzyladenine (BA) was found to enhance inflorescence initiation and flower bud formation (Sim et al. 2007).
The implication of endogenous cytokinins in the control of flowering processes has been investigated, but so far without converging results (Corbesier et al. 2003). In order to have an insight to the hormonal regulation of flowering in orchids, it is essential to understand the changes of endogenous cytokinins present at different stages of development. In this paper, we report our analysis on the endogenous levels and types of cytokinins present in tissues of D. Madame Thong-In cultures (Sim et al. 2007) under both vegetative and in vitro flowering stages. Cytokinins in the coconut water used for experiments were also analysed.
Materials and methods
Establishment of in vitro cultures
Dendrobium Madame Thong-In vitro cultures were established according to Sim et al. (2007). Briefly, seeds were cultured in 9 cm petri dishes with 25 ml of modified KC medium (Knudson 1946) supplemented with 2% (w/v) sucrose, 15% (v/v) coconut water (CW, obtained from local green coconuts) and 0.3% (w/v) Gelrite (Kelco, San Diego, USA). KC medium was used throughout the experiments as the basal medium.
To induce in vitro flowering, 2-month-old seedlings (2–3 mm height with 2 to 3 tiny leaves) were transferred to 40 ml basal liquid medium supplemented with BA (4.4 μM) and CW (15%) in 100 ml Erlenmeyer flasks on rotary shakers at 120 rpm. Subsequently, the seedlings were transferred to a two-layered medium in Magenta GA7™ vessels (Magenta Corporation, Chicago) according to Sim et al. (2007).
All the media were adjusted to pH 5.3 before autoclaving at 1 kg cm−2 (121°C) for 20 min. Cultures were incubated at 26 ± 2°C under a 16-h photoperiod of 35 μmol m−2 s−1 from daylight fluorescent lamps.
Harvesting and extraction of endogenous cytokinins
Seedlings at different stages were harvested for analysis of cytokinin contents. Cytokinins were extracted according to the methods of Jones (1990). Frozen tissues ground to a fine powder and extracted with 10 volumes of chilled (−20°C) 90% (v/v) ethanol (typically 5 g tissue + 50 ml ethanol). Extraction was continued on ice with occasional stirring, for 90–120 min. After centrifuging (1,000g, 10 min) and recovery of the supernatant, the samples were re-extracted with chilled (−20°C) 80% ethanol (v/v, 20 ml) for another hour, and again centrifuged. The supernatants were combined in a silylated 500 ml round bottom flask and reduced to small volume (1–5 ml approx. or < 0.5 ml g−1 FW) on a rotary vacuum evaporator (Eyela, Tokyo Rikakikai Co. Ltd.) at 35°C.
The SEP-PAK® C18 cartridge (Waters, Millipore Co., Milford, Massachusetts, USA) was first activated with 4 ml of methanol followed by 10 ml of 0.01 M triethylammonium acetate (TEAA) at pH 7.0. The crude extract was then loaded to the SEP-PAK® C18 cartridge, washed with 9 ml of 0.01 M TEAA, and eluted with 6 ml of 50% methanol and collected in a silylated 100 ml round bottom flask. The methanol was then removed and reduced to small volume (approx. 0.5–1 ml) on a rotary vacuum evaporator. Sample was frozen in liquid nitrogen and stored at −80°C until required.
Coconut water was boiled, filtered and kept frozen at 0°C until used for media preparation. Three samples of 10 ml CW (unautoclaved and autoclaved) were purified in pretreated SEP-PAK® C18 cartridge, and reduced to small volume as described for analysis.
HPLC separation of cytokinins
A HPLC, HP 1050-Ti Series of LC modules LC/2D MS-DOS ChemStation, was used with a HP 1050 Series variable wavelength detector at 265 nm on the column outflow. The samples were analysed by reversed-phase ODS-H-2101 Senshu Pak column (6 mm ID × 100 mm L) with a guard column filled with ODS 13 ~ 24 μm (Whatman). The moving phases were pure methanol (BDH, HPLC grade) and 0.2 mM TEAA at pH 7.0.
The column was equilibrated with 25% (v/v) methanol/TEAA at a constant flow rate of 0.7 ml min−1. One ml standard cytokinins (containing 4 nmol of each member) or samples were separated by a gradient 25–40% methanol/TEAA for 18 min, followed by continued elution with 40% methanol/TEAA for 4 min. At 23 min, eluted with 58% methanol/TEAA for 8 min. At 32 min, the final elution was with 100% methanol. Fractions (1 ml) were collected from the column effluent to a Pharmacia Fraction Collector. The individual column fractions were evaporated under vacuum on a Univapo 150H Evaporator Centrifuge at 45°C. The dried samples were redissolved in 350 μl distilled water and stored at −20°C.
Radioimmunoassay (RIA)
Radioimmunoassays have been typically ascribed a measuring range from 0.1 to 10 pmol of the riboside form of cytokinin. The procedure of Turnbull and Hanke (1985) was used in our studies. Antibodies were raised against ZR, iPA and DZR. The cytokinins in each HPLC fraction were assayed by a modification of the RIA system of Turnbull and Hanke (1985). Standards and samples were processed in duplicates. Assays were carried out in 1.5 ml microfuge tubes. In the assay, a 50 μl sample was mixed with 100 μl antibody diluted 100× with PBS containing 2 mg ml−1 γ-globulin. To each mixture in 1.5 ml microfuge tube, 50 μl tritium-labelled cytokinin riboside-[3H]-diol (approx. 4,000 dpm) was added and the mixture allowed to equilibrate for approximately 2 h. The protein was precipitated by adding 200 μl saturated ammonium sulphate and allowed 10–20 min for the precipitation to take place. The pellet was sedimented by 10 min centrifuging in a Jouan M14.11 Microfuge. The supernatant (200 μl) was pipetted into 2 ml of scintillation solution (Ecoscint™) in a 6 ml Mini Poly-Q™ vial (Beckman, USA). The radioactivity was counted in a Beckman Liquid Scintillation Counter (LS6000LL). The data files were captured by a Data Capture LS6000 software and processed on a PC using ImmunoFit™ EIA/RIA software (Beckman, USA). In each assay, the experimental unknowns were preceded by a calibration series containing cytokinin ribosides at doses from 0.2 to 25.6 pmol per assay, doubling the concentration at each step. The program plots a calibration curve using a Spline Fit, and calculates the levels of cytokinin in the original sample. The HPLC fractions were assayed with each of the three antibodies [i.e. antisera raised against the conjugated forms of zeatin riboside (ZR), dihydrozeatin riboside (DZR) and iPA].
Levels of cytokinins present in the tissues analysed were given as riboside equivalents after correction for differences in cross reactivity.
Statistical analysis
All statistical analyses were carried out to compare the levels of cytokinins in each stage using One-way ANOVA Tukey’s test at 95% confidence level.
Results
Separation and assay of cytokinins
Figure 1 shows the HPLC separation profile of the 12 cytokinins. Zeatin-family and dihydrozeatin family were eluted within 20 min whereas iP family eluted between 25 and 40 min. The iP and iPA peaks were very close, while zeatin-9-glucoside (Z9G) and dihydrozeatin-9-glucoside (DZ9G) peaks could not be separated under the HPLC conditions employed.
In each assay, 5 pmol standard of the appropriate riboside, ribotide, free base and 9-glucoside were added. Generally within each group, the antisera showed high cross reactivity [the average activity to that of the riboside (%)] with the 9-glucoside, free base and ribotide (Table 1). Antisera of iPA showed low cross reactivity with antisera of ZR and DZR. Antiserum of ZR showed 46% cross reactivity with 5 pmol of DZR. Antiserum of DZR showed 27% cross reactivity with 5 pmol of ZR (Table 1). The losses of cytokinins during extraction, purification and assay procedures were estimated by addition of cocktail of standard cytokinins to the frozen tissues before grinding in liquid nitrogen. The overall recovery was about 79%.
Cytokinins in coconut water
Coconut water was found to contain high level of ZR (>136 pmol ml−1) (Table 2). The levels did not show any significant difference when the coconut water was autoclaved. The levels of all other cytokinins were less that 3.5 pmol ml−1 (Table 2).
Cytokinin contents in seedlings (≤ 5 mm and about 2 cm in height) cultured in BA-free medium
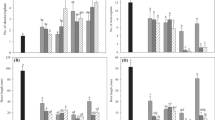
Cytokinins of the Z-, iP- and DZ families were detected in the seedlings of less than 5 mm (Fig. 2a; Table 3). Level of Z was about 1.2 pmol g−1 FW and Z5P was 2.0 pmol g−1 FW. The levels of iPA and iP were about 1.5 and 2.8 pmol g−1 FW, respectively. The four cytokinins in the DZ family tested ranged from 0.9 to 3.9 pmol g−1 FW (Table 3). All the levels of cytokinins are not significantly different.
In the seedlings of about 2 cm height (Fig. 2b), the endogenous levels of Z- family ranges from 0.7 pmol g−1 FW for Z9G and ZR to 2.4 pmol g−1 FW for Z5P (Table 3). Level of iPA was found to be at 1.2 pmol g−1 FW. Levels of DZ-family in the seedlings ranged from 0.5 pmol g−1 FW for DZR to 2.3 pmol g−1 FW for DZ9G (Table 3). The levels of cytokinins are not significantly different.
Cytokinin contents in seedlings cultured in flowering-inductive liquid medium containing BA and CW
Seedlings (2–3 mm height) were transferred to liquid medium with BA (4.4 μM) after 4 weeks as mentioned above. In this medium, they developed into bigger seedlings (Fig. 3a) which had the potential of producing flowers in culture or remained vegetative.
In seedlings of about 1.0 cm height, the levels of iP (145 pmol g−1 FW), iPAMP (46 pmol g−1 FW) and iPA (69 pmol g−1 FW) were found to be significantly higher than the level of other types of cytokinin (Table 4).
The profile of cytokinin levels in seedlings of about 1.1–1.5 cm height revealed that the level of iP (200 pmol g−1 FW) was significantly higher than other cytokinins in the same seedlings but not significantly different from iPA (133 pmol g−1 FW, Table 4). The level of iPA (133 pmol g−1FW) was significantly higher than other cytokinins tested but not significantly different from iP, iPAMP and DZ9G (Table 4). The levels of cytokinins in Z- and DZ- families were below 3 pmol g−1 FW in both seedlings of 1.0 cm and 1.1–1.5 cm height, except for DZ9G which was present at a range of 10–20 pmol g−1 FW (Table 4).
Seedlings that showed early sign of bolting (narrowing of the leaves towards the apex) (Fig. 3b) indicated that they were induced to a reproductive mode and were considered to be at transitional stage (vegetative to reproductive). Seedlings with elongated shoot tips were considered to be bolted (Fig. 3c) and seedlings with floral buds (Fig. 3d) were assayed for cytokinins. These floral buds eventually bloom in cultures (Fig. 3e).
In the transitional stage, the profile of cytokinins tested again showed that the levels of iP (178 pmol g−1 FW) and iPA (63 pmol g−1 FW) were significantly higher than other types of cytokinin analysed (Table 4). Unlike the two stages prior to the transitional stage, the levels of iPAMP and iPA in seedlings of the transitional stage were not significantly different (Table 4).
For seedlings with bolted shoot-tips, both iP (73 pmol g−1 FW) and iPA (71 pmol g−1 FW) levels were found to be significantly higher than all other cytokinins analysed (Table 4). For seedlings with flower buds, only the level of iP (270 pmol g−1 FW) was significantly higher than other cytokinins (Table 4).
Both the levels of Z and DZ families in the seedlings at transitional and flowering stages were quite constant and ranged from 1.0 to 3.4 pmol g−1 FW except for DZ9G which showed higher levels at 8.8–20.5 pmol g−1 FW (Table 4).
Some seedlings in the inductive medium failed to produce flowers and remained vegetative. Analyses of these seedlings (1.6–2.0 cm and 2.1- 3.0 cm height) revealed that the levels of iP were significantly higher than all other types of cytokinin analysed (Table 4). However, the levels of iPA were not significantly higher from other cytokinins in seedlings of the same stage. The level of iPA was reduced to 15 pmol g−1 FW in plants of 2.1–3.0 cm height (Table 4).
Both Z and DZ families were present at significantly lower amount and did not vary much as the seedlings increased in height. Only the contents of DZ9G showed slight decline in levels (Table 4).
Discussion
More than 30 naturally occurring free cytokinins had been identified in plants (Palni et al. 1990). The biological activity of naturally occurring cytokinins ranges from highly active molecules such as Z, to inactive or weakly active conjugates like zeatin-7-glucoside (Z7G) and Z9G (Letham et al. 1983). With the use of HPLC to separate individual cytokinins prior to immunoassay (MacDonald et al. 1981), it is possible, for example, to analyse cytokinin-containing mixtures by RIA with the fractions from a single HPLC run. In this project, we used HPLC to separate and collect at least 11 out of the 12 different types of cytokinins (Fig. 1). When coupled with RIA we could analyse derivatives of the three main groups of cytokinins namely Z, iP and DZ during the development of D. Madame Thong-In leading to in vitro flowering (Sim et al. 2007).
The HPLC/RIA techniques were also employed in the analysis of endogenous cytokinins in the CW used in our experiments. CW has been widely used in plant tissue culture but the precise composition of cytokinins in the content remains unknown (Ge et al. 2006). The CW used for our experiments contained more than 136 pmol ml−1 ZR, whereas the concentration of other cytokinins was relatively low at 0.1–3.5 pmol ml−1 (Table 2). Using capillary zone electrophoresis-tandem mass spectrometry, Ge et al. (2006) also found that ZR was one of the major cytokinins in CW and the level was estimated to be approximately 76.2 pmol ml−1.
The method we used to induce in vitro flowering in D. Madame Thong-In includes three essential stages. Step 1 involved the germination of seeds in a basal medium for protocorm development. In step 2, seedlings (2–3 mm) with tiny leaves were transferred to a liquid medium containing BA (4.4 μM) and CW; in this inductive medium, seedlings grew and many were rootless or with very short root(s); some of these seedlings would bolt and turn reproductive with elongated shoot tips, which eventually produced flower buds (Fig. 3b–d). Step 3 of the operation involved transfer of these bolted seedlings and seedlings with flower buds to a two-layered (liquid over Gelrite) medium containing BA and CW for promoting further development of the flower buds (Sim et al. 2007). Seedlings with fully open flower(s) could be obtained in culture (Fig. 3e). Nadgauda et al. (1990) suggested that cytokinins might be involved in flowering perhaps with CW supplying the inositol and cytokinin oxidase inhibitors, which promote cytokinin responses. Both BA and CW are sine qua non for successful induction of in vitro flowering (Sim et al. 2007) at steps 2 and 3 in D. Madame Thong-In using the method we developed. BA alone in the medium was not effective for floral induction in D. Madame Thong-In. However, in the presence of CW, BA enhanced earlier formation of inflorescence stalks and promoted flower bud induction but normal flower development did not occur in the liquid medium, as the flower buds obtained were abnormal (Sim et al. 2007).
We analysed the cytokinin content of the coconut water and the endogenous content of cytokinins of these cultures at steps 1 (vegetative phase) and 2 (for induction of flowering) in order to have an insight into the changes of cytokinins from vegetative to reproductive phase.
Our analysis revealed that seedlings grown in BA-free KC medium contained relatively low levels (from 0.5 to 3.9 pmol g−1 FW) of endogenous cytokinins (Table 3). This indicated that there were very little fluctuations in these 12 types of cytokinins during the development of D. Madam Thong-In from small 5 mm seedlings to 2 cm seedlings in vitro. This is comparable to analysis in Dendrobium hybrid Second Love which showed that the endogenous levels of Z, Z9R, iP and iP9R were about 5 pmol g−1 FW or below when cultured in basal Vacin and Went medium (Ferreira et al. 2006).
Our results showed that up to 200 and 133 pmol g−1 FW of iP and iPA, respectively, were detected in D. Madame Thong-In seedlings of about 1.1–1.5 cm height prior to the transitional stage (Table 4). These levels were about 80- and 110-folds higher than the level of the same cytokinin in seedlings cultured in non-inductive medium. During the transitional stage, the endogenous iP and iPA levels were about 178 and 63 pmol g−1 FW, respectively (Table 4) and the levels were about 80- and 50-folds higher than seedlings cultured in non-inductive medium.
The importance of iP and iPA in induction of in vitro flowering in D. Madame Thong-In could be realised from statistical analyses. The profiles of cytokinins analysed at different stages of seedlings indicated that both the levels of iP and iPA in seedlings prior to transitional stage were significantly higher than other types of cytokinins in seedlings of the same stage (Table 4). During the transitional stage, the endogenous levels of iP (178 pmol g−1 FW) and iPA (63 pmol g−1 FW) were also significantly higher than cytokinins of the zeatine (Z) and dihydrozeatin (DZ) families in the same seedlings. For seedlings that failed to flower in the inductive medium, the levels of iP (132 and 80 pmol g−1 FW for seedlings of 1.6–2.0 cm and 2.1–3.0 cm, respectively) continued to be significantly higher than other types of cytokinin analysed, however, the level of iPA was not significantly different from other cytokinins, except for iP (Table 4). This suggests that for in vitro flowering to occur in D. Madame Thong-In, either (a) both the endogenous iP and iPA levels in the seedling are required to be significantly higher than all other types of cytokinin, or (b) only the endogenous iPA level has to be significantly higher than other cytokinins in the same seedling. Further investigations are required to elucidate these possibilities. In addition, we cannot rule out the possibility that there could be a threshold value for the endogenous iPA level or for both the iP and iPA levels to reach before the seedlings could be diverted from vegetative to reproductive development in culture (Table 4).
The importance of the members of iP family and other types of cytokinin in flowering was observed by many authors. Ferreira et al. (2006) found that after culturing D. Second Love shoots in medium (containing thidiazuron) inductive to in vitro flowering for 5 days, endogenous levels of iP9G, Z9G and Z increased to 10–20 pmol g−1 FW. The level of iP, however, did not change much and was found to be at around 5 pmol g−1 FW although in vitro flowering could be achieved in D. Second Love (Ferreira et al. 2006). In Sinapis alba, the activity of the iPA in the root exudates increased in the flowering-induced plants and the levels of Z and ZR in the induced plant was only a little higher than the control (Lejeune et al. 1988). In Arabidopsis thaliana, iP forms of cytokinins increased, in both the leaf tissues and leaf exudate, from 16 h after the start of the long day. At 30 h, the shoot apical meristem of induced plants was found to contain more iP and Z than vegetative controls. These increases in cytokinins correlate well with the early events of floral transition (Corbesier et al. 2003). When early flowering was induced (by triacontanol, lanthanum and cerium) in cytokinin-free medium, a significant increase in endogenous levels of the iP subfamily in the root and leaf tissues of Arabidopsis seedlings was observed (He and Loh 2002). In Nicotiana tabacum, no free cytokinin bases were detected in pre-floral transition apices (Dewitte et al. 1999). In Chenopodium rubrum and C. murale, the increase of cytokinin levels (Z, ZR, iP and iPA) in apical parts of both photoperiodic species, during floral induction suggested a role (increased cell division and branching) for cytokinins in apex evocation (Machácková et al. 1993).
The development of an early, in vitro flowering method allows earlier assessment of certain desired characteristics of the flowers and has significant impact on the orchid industry (Sim et al. 2007; Hee et al. 2007). In addition, significant earliness of flowering can provide a model system for studying flowering initiation and development (Sim et al. 2007). As part of the program to understand mechanism regulating early flowering of plants in culture, we analysed the cytokinin contents of D. Madame Thong-In seedling cultured in non-inductive and inductive media. We showed that in D. Madame Thong-In, seedlings cultured in flowering-inductive medium resulted in very significant increase in endogenous iP and iPA (Table 4) level leading to flowering in vitro (Fig. 3e). Thus, we provided strong evidence that cytokinins, especially the iP family, play an important role in early in vitro flowering of D. Madame Thong-In. Further investigation in the role of endogenous cytokinins in flowering of field-grown tropical orchids is in progress.
Abbreviations
- BA:
-
6-Benzyladenine
- CW:
-
Coconut water
- DZ:
-
Dihydrozeatin
- DZR:
-
Dihydrozeatin riboside
- DZ9G:
-
Dihydrozeatin-9-glucoside
- DZ5P:
-
Dihydrozeatin riboside 5′-monophosphate
- HPLC:
-
High performance liquid chromatography
- iP:
-
N6-(Δ2-isopentenyl)-adenine
- iPA:
-
N6-(Δ2-isopentenyl)-adenosine
- iPAMP:
-
N6-(Δ2-isopentenyl)-adenoside-5′-monophosphate
- iP9G:
-
N6-(Δ2-isopentenyl)-adenine-9-glucoside
- KC:
-
Knudson C (1946) medium
- PSB:
-
Phosphate buffer
- iP:
-
N6-(Δ2-isopentenyl)-adenine
- RIA:
-
Radioimmunoassay
- TEAA:
-
Triethylammonium acetate
- Z:
-
Zeatin
- ZR:
-
Zeatin riboside
- Z9G:
-
Zeatin-9-glucoside
- Z5P:
-
Zeatin riboside 5′-monophosphate
References
Bernier G (1988) The control of floral evocation and morphogenesis. Annual Rev Plant Physiol Mol Biol 39:175–219
Bernier G, Havelange A, Housa C, Petitjean A, Lejeune P (1993) Physiological signals that induce flowering. Plant Cell 5:1147–1155
Bonhomme F, Kurz B, Melzer S, Bernier G, Jacqmard A (2000) Cytokinin and gibberellin activate SaMADSA, a gene apparently involved in regulation of the floral transition in Sinapis alba. Plant J 24:103–111
Corbesier L, Coupland G (2006) The quest for florigen: a review of recent progress. J Exp Bot 57:3395–3403
Corbesier L, Prinsen E, Jacqmard A, Lejeune P, Van Onckelen H, Perilleux C, Bernier G (2003) Cytokinin levels in leaves, leaf exudate and shoot apical meristem of Arabidopsis thaliana during foral transition. J Exp Bot 54:2511–2517
Dewitte W, Chiappetta A, Azmi A, Witters E, Strnas M, Rembur J, Noin M, Chriqui D, Van Onckelen H (1999) Dynamics of cytokinins in apical shoot meristems of a day-neutral tobacco during floral transition and flower formation. Plant Physiol 119:111–121
Ferreira WM, Kerbauy BK, Kraus JE, Pescador R, Suzuki RM (2006) Thidiazuron influences the endogenous levels of cytokinins and IAA during the flowering of isolated shoots of Dendrobium. J Plant Physiol 163:1126–1134
Ge L, Yong JWH, Tan SN, Ong ES (2006) Determination of cytokinins in coconut (Cocos nucifera L.) water using capillary zone electrophoresis-tandem mass spectrometry. Electrophoresis 27:2171–2181
He YW, Loh CS (2002) Induction of early bolting in Arabidopsis thaliana by triacontanol, cerium and lanthanum is correlated with increased endogenous concentration of isopentyl adenosine (iPAdos). J Exp Bot 53:505–512
Hee KH, Loh CS, Yeoh HH (2007) In vitro flowering and rapid in vitro embryo production in Dendrobium Chao Praya Smile (Orchidaceae). Plant Cell Rep 26:2055–2062
Jones LH (1990) Endogenous cytokinins in oil palm (Elaeis guineensis L.) callus, embryoids and regenerant plants measured by radioimmunoassay. Plant Cell Tissue Organ Cult 20:201–209
Knudson L (1946) A new nutrient solution for germination of orchid seed. Am Orchid Soc Bull 15:214–217
Lejeune P, Bernier G, Requier MC, Kinet JE (1994) Cytokinins in phloem and xylem saps of Sinapis alba during floral induction. Physiol Plant 90:522–528
Lejeune P, Kinet JE, Bernier G (1988) Cytokinin fluxes during floral induction in the long day plant Sinapis alba L. Plant Physiol 86:1095–1098
Letham DS, Palni LMS, Tao GQ, Gollnow BI, Bates CM (1983) Regulators of cell divisions in plant tissues XXIX. The activities of cytokinin glucosides and alanine conjugates in cytokinin bioassay. J Plant Growth Regul 2:103–115
MacDonald EMS, Akiyoshi DE, Morris RO (1981) Combined high-performance liquid chromatography-radioimmunoassay for cytokinins. J Chromatogr 214:101–109
Machácková I, Krekule J, Strnad M, Eder J (1993) Cytokinins in photoperiodic induction of flowering in Chenopodium species. Physiol Plant 87:160–166
Nadgauda RS, Parasharmi VA, Mascarenhas AF (1990) Precocious flowering and seedling behaviour in tissue-cultured bamboos. Nature 344:335–336
Palni LMS, Nandi SK, Singh S (1990) Physiology and biochemistry of cytokinins. In: Purohit SS (ed) Hormonal regulation of plant growth and development, vol 5. Agro Botanical Publishers, Bikaner
Peeters AJM, Gerards W, Barendse GWM, Wullems GJ (1991) In vitro flower bud formation in tobacco: interaction of hormones. Plant Physiol 97:402–408
Sim GE, Loh CS, Goh CJ (2007) High frequency early in vitro flowering of Dendrobium Madame Thong-In (Orchidaceae). Plant Cell Rep 26:383–393
Turnbull CGN, Hanke DE (1985) The control of bud dormancy in potato tubers. Measurement of seasonal pattern of changing concentrations of zeatin-cytokinins. Planta 165:366–376
Acknowledgments
Sim GE was supported by a research scholarship from the National University of Singapore (NUS). Loh CS thanks the NUS for a research grant to carry out this project. Loh CS is grateful to Dr David Hanke of the Department of Plant Sciences, University of Cambridge, UK, for an opportunity to learn cytokinin analysis techniques in his lab. We thank Dr. L. H. Jones, University of Cambridge, for providing the tracers used in RIA. We wish to thank Professor Prakash Kumar of NUS for critical reading of the manuscript and Ms Tan Wee Kee and Mr Koh Teng Seah for advice in statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Kamo.
Rights and permissions
About this article
Cite this article
Sim, G.E., Goh, C.J. & Loh, C.S. Induction of in vitro flowering in Dendrobium Madame Thong-In (Orchidaceae) seedlings is associated with increase in endogenous N6-(Δ2-isopentenyl)-adenine (iP) and N6-(Δ2-isopentenyl)-adenosine (iPA) levels. Plant Cell Rep 27, 1281–1289 (2008). https://doi.org/10.1007/s00299-008-0551-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-008-0551-8