Abstract
In screening for potent antimicrobial proteins (AMPs) from plant seeds, we had purified a heat-stable AMP, LJAMP2, from the seeds of a medicine herb, motherwort (Leonurus japonicus Houtt). In an in vitro assay, the protein can inhibit the growth of both fungi and bacteria. Then a cDNA encoding LJAMP2 was cloned by the rapid amplification of cDNA ends based on the N-terminal amino acid sequence determined. The deduced amino acid sequences of this cDNA show similarity to plant non-specific lipid transfer proteins. Northern blotting assay revealed that this nsLTP-like gene, designated LJAMP2, was expressed in seeds. Overexpression of LJAMP2 in tobacco enhanced resistance to the fungal pathogen Alternaria alternata and the bacterial pathogen Ralstonia solanacearum, significantly, while no visible alteration in plant growth and development. Our data confirm the antifungal and antibacterial function of LJAMP2 from motherwort seeds and suggest the potential of LJAMP2 in improving disease resistance in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytopathogens cause enormous losses in cultivated and stored crops worldwide and thereby threaten human sustenance (Oard and Enright 2006; Osusky et al. 2000). Since cultural practices, agrochemicals, and conventional breeding for disease resistance have not been sufficient, or permanently successful in keeping phytopathogens under control, alternative strategies for sustainable agriculture have attracted great attention during recent years (Langen et al. 2006). Plant genetic engineering in combination with antimicrobial proteins (AMPs) is regarded as an effective means (Gao et al. 2006; Oard and Enright 2006).
An increasing amount of defense-related genes have been characterized in some plant–pathogen systems (Lee et al. 2002). Proteins encoded by these genes are usually pathogenesis-related proteins or low-molecular-weight AMPs that may play an important role in the growth of pathogens in infected hosts. In screening of AMPs from plants, we have discovered an AMP, termed LJAMP2, in motherwort seeds (Yang et al. 2006). The partial N-terminal sequence determined has shown that the sequence revealed similarity with known plant non-specific lipid transfer protein (nsLTPs) sequences in data bank.
Plant nsLTPs, reported in various organs and tissues in many mono- and dicotyledonous species, form a multigene family of basic protein with 91–95 amino acid residues in their primary structure and a approximately 9 kDa molecular mass (Blein et al. 2002; Jung et al. 2005). They share several structural features, e.g. eight strictly conserved cysteine residues forming four disulfide bridges, which are responsible for the nsLTPs’ compact folding (Blein et al. 2002). NsLTPs, as a member of plant AMPs, hold an internal hydrophobic cavity, which serves as the binding site for lipid-like molecule in structure (Carvalho and Gomes 2007; Lin et al. 2005; Samuel et al. 2002). The structural, biochemical, and physiological data have confirmed that nsLTPs can bind not only lipids but also sterol molecules (Wang et al. 2007). Furthermore, these nsLTP/sterol complexes may interact with receptors at plant plasma membranes to trigger plant defense responses (Blein et al. 2002; Carvalho and Gomes 2007; Cheng et al. 2004). Evidence is accumulating that nsLTPs or nsLTP-like proteins display roles in plant defense against viral, bacterial, and fungal pathogens (Gomes et al. 2003; Maldonado et al. 2002; Molina and Garcia-Olmedo 1997; Park et al. 2002; Terras et al. 1995). However, it was also found that not all nsLTPs possess antimicrobial properties and accordingly defensive roles (Cammue et al. 1995). No antimicrobial activity was detected for Ta-nsLTP from Triticum aestivum seeds in the test at high concentration (200 μg/ml) (Cammue et al. 1995).
In previous work, we had purified a heat-stable AMP, termed LJAMP2, from seeds of motherwort (Leonurus japonicus Houtt), a medicine herb. In vitro experiments showed that this protein possesses inhibition activity on the growth of both fungi and bacteria (Yang et al. 2006). To further investigate the function of LJAMP2, in this paper, we cloned a cDNA encoding LJAMP2 from motherwort, and validated its antimicrobial roles by challenging the transgenic tobacco with a fungal pathogen, Alternaria alternata and a bacterial pathogen, Ralstonia solanacearum, respectively.
Materials and methods
Plants and microbes
Motherwort (L. japonicus Houtt) seeds were obtained from a local medicine herb market, and plants were kept under natural condition. Tobacco plants (Nicotiana tabacum var. Xanthi) and its transgenic lines were grown in a greenhouse at 26–28°C under natural and additional artificial light (14/10 h photoperiod at 150 μmol m−2 s−1). A. alternata and R. solanacearum were prepared as previously described (Peng et al. 2004).
Isolation of LJAMP2 cDNA and Northern blotting
Based on the N-terminal amino acid sequence of mature LJAMP2 (Yang et al. 2006), a degenerate primer, 5′-GCA ATA GGT TG(T/C) AA(T/C) AC(A/G/C/T) GT(A/G/C/T) GC-3′, was designed to amplify the cDNA using the rapid amplification of cDNA ends (3′-RACE) method with a 3′ full RACE kit (Takara Bio., Japan). Amplified DNA was purified and ligated into pGEM-T (Promega, WI, USA) plasmid for sequencing. To amplify the 5′-flanking sequence of the gene, a Y-shaped adaptor-dependent extension (YADE) method (Xiao et al. 2002) was employed. According to the nucleotide sequence of the 3′ RACE products, two nested primers (primer 1: 5′-GTAAGGAATGCTGACACCAC-3′ and primer 2: 5′-AGTACGACTTGGCAAGCGTT-3′) were designed for the amplification of 5′ part sequence of the gene. The YADE product was purified and cloned into pGEM-T (Promega, WI, USA) for sequencing. The products of YADE and the 3′ RACE sequence were overlapped with SeqMan program of DNAStar (DNASTAR, Madison, WI, USA), and the contig sequence was further used to perform similarity search with BLASTX program to determine the putative initiator ATG. The coding region of LJAMP2 gene was further amplified from the genomic DNA using the primers of 5′-TCCAATGGCTGCCTTGATCA-3′ and 5′-TCAGCCGGAGTACGACTTGGCAA-3′. The protein sequence of LJAMP2 was deduced using the Editseq program of DNASTAR software (DNASTAR, Madison, WI, USA) and it was aligned with 9 nsLTPs sequences from other plant species (http://www.ncbi.nlm.nih.gov/blast/) using ClustalW (v. 1.83) program provided online by DDBJ (http://clustalw.ddbj.nig.ac.jp) and GENEDOC computer programs. Analysis of posttranslational modifications was done using the tools and software packages in ExPASy provided online by the Swiss Institute of Bioinformatics (http://ca.expasy.org/) (Blom et al. 2004). The three-dimensional (3D) structure of LJAMP2 was simulated using the software package SWISS-MODEL provided online by SIB (Schwede et al. 2003).
Total RNAs were isolated from various motherwort tissues and tobacco leaves using a guanidinium thiocyanate method (Logemann et al. 1987). mRNA expression in motherwort tissues and tobacco were assessed by hybridizing a multiple tissue Northern blotting (containing 2 μg of polyadenylated RNA per lane) with a 32P-labeled LJAMP2 cDNA probe (Sambrook and Russell 2001).
Vector construction and plant transformation
The cDNA fragment encoding LJAMP2 was cloned downstream of cauliflower mosaic virus (CaMV) 35S promoter. The nos transcription terminator was placed downstream. Then the expression cassette with the CaMV 35S promoter-LJAMP2-nos terminator and CaMV 35S promoter-gus-nos terminator and nos promoter-npt II-nos terminator were cloned in the binary vector pBIN19 (Frisch et al. 1995). The construct was delivered to Agrobacterium tumefaciens LBA4404 by freeze-thawing method (Hoekema et al. 1983) and the resulting Agrobacterium strain was used for transformation of tobacco leaf-disk by co-cultivation (Li et al. 1992). The transgenic plants were regenerated under kanamycin selection (100 mg/l, Sigma). GUS histochemical analysis was preformed as previously described (Jefferson et al. 1987)
Analyses on transgenic tobacco
For proteins analysis, after incubation at 26°C in a rotary shaker (170 rpm) for 3 days, the germinating tobacco seeds were grown in sterile soil in multi-well plastic containers that were kept in a growth chamber at 80% relative humidity, 25–28°C, under a 14/10 h photoperiod at 150 μmol m−2 s−1. Fresh leaf tissues (10 g) of tobacco were homogenized with 10 ml of 20 mM sodium phosphate buffer (pH 6.2), containing 50 mM KCl, 5 mM EDTA, 1 mM aprotinin and 20 mM thiourea. To identify the transgene products, the samples were analyzed by SDS-PADE, and HPLC on a Source 5RPC column (4.6 × 150 mm, 15 μm, Amersham Biosciences, Sweden) equilibrated with 0.05% TFA using ÄKTA Explorer 10S (Amersham Biosciences, Sweden) with 0.05% TFA over 5 min, and a linear gradient of 0–45% acetonitrile in 0.05% TFA over 35 min at a flow rate of 1.0 ml/min.
To test the resistance of transgenic tobacco to infection by A. alternata, the bioassay was performed. A. alternata strain was grown on potato dextrose agar (PDA) at room temperature for 2–3 weeks. Hyphae, fragmented and combined with spores were harvested in sterile tap water with 0.05% (v/v) Tween 80. Phytopathogenic fungus inoculation of tobacco was carried out by placing 20 μl of the aqueous on the leaves of 4-week-old plants. The inoculated plants were placed in a growth chamber at 90% relative humidity and 28°C for 3 days. Then the inoculated plants were further incubated at 80% relative humidity and 28°C for 7 days. The development of symptoms was monitored and a five-class disease-severity-scale (DS) was evaluated by the previously described method (Yang et al. 2007). Disease resistance was expressed using a disease-index (DI). The DI was calculated using the following formula: DI (%) = (Σi × j/4 × n) × 100, where i is the DS-class, j is the number of disease leaves in each class, and n is the total number of leaves. The experiments were repeated three times, and each replicate contained 18 plants.
To determine whether the overexpression of LJAMP2 in tobacco can provide resistance to the bacterial pathogen, we infected the tobacco plants with a R. solanacearum isolate, which causes bacterial wilt disease on tobacco. The bacterium was grown for 48 h at 30°C on Luria–Bertani medium. The bacterial suspension for inoculation was prepared by washing the medium surface with sterile tap water, and the bacterial population was adjusted to 1 × 107 bacteria/ml. Tobacco plants (4 weeks old) were inoculated by dipping the roots into the bacterial suspensions. The plants were kept in a growth chamber at 95% relative humidity and 28°C for 2 days and then at 80% relative humidity and 28°C for 12 days. The number of wilting leaves was recorded for each plant daily, and a five-class DS was calculated by the previously described method (Cary et al. 2000). Each treatment was replicated three times, and each replicate contained 20 plants.
All data were analyzed by t test at P ≤ 0.05 using the software of Origin v6 (OriginLab Co. MA, USA).
Results
Molecular cloning and analysis of the gene encoding LJAMP2
Based on the N-terminal amino acid sequence of LJAMP2 purified from motherwort seeds (Yang et al. 2006), a degenerate primer corresponding to the sequence of AIGCNTVA was designed for amplification of the 3′ cDNA. The nucleotide sequence of the fragment was determined and the N-terminal of the deduced amino acid sequence matched perfectly with the N-terminal amino acid sequence of LJAMP2 previously determined. To extend the 5′ part of the cDNA, two new primers were designed, and the YADE method (Xiao et al. 2002) was employed. The full-length cDNA of LJAMP2 was constructed from the nucleotide sequences of the 5′ and 3′ parts. The gene contains a 345-bp open reading frame coding for 115 amino acids and a 3′ untranslated region of 181-bp up to the poly (A) tail (Fig. 1). The C-terminal portion of the deduced protein from the 25th to the 85th residue was identical to the amino acid sequence determined for the LJAMP2 protein. The first 24 residues had characteristic features of a signal peptide. The mature form of LJAMP2 is composed of 91 residues. According to the cDNA sequence, a genome DNA fragment was generated by PCR. By Comparing the PCR product sequence with the cDNA sequence, we found that the gene encoding LJAMP2, named LJAMP2, is an intronless gene. To determine LJAMP2 transcript accumulations in various organs of motherwort, Northern blotting analysis was performed by using the cDNA probe and total RNA isolated from roots, stems, leaves, flowers and seeds. The hybridization signal was strong in seeds, but was not detected in roots, stems, leaves and flowers (Fig. 2), implying that LJAMP2 is a seed-specific expression gene in motherwort.
Nucleotide sequence and deduced amino acid sequence of LJAMP2 cDNA clone. The sequence corresponding to the determined N-terminal amino acid sequence of mature LJAMP2 is italic. The deduced signal peptide sequence is bold. The initiation codon (ATG) is bold underlined, and the poly (A) tail is underlined
RNA gel-blot analysis of the LJAMP2 transcripts in different tissues of motherwort. Total RNAs were extracted from leaves, stems, flowers, roots of 5-week-old plants, and from the seeds at the 30th day after anthesis. RNAs electrophoresed in a 1% agarose gel containing 6% formaldehyde were hybridized with a 32P-labeled LJAMP2 cDNA probe. Hybridizing signals were visualized by exposing the membrane to X-ray film (top). The gel was stained with ethidium bromide to detect rRNAs as control (bottom)
The amino acid sequence of deduced mature LJAMP2 shows similarity to the family of plant nsLTPs-like proteins by comparing with the previously sequenced proteins from other plant species in the BLAST search (http://www.ncbi.nlm.nih.gov/BLAST), sharing 76% identity to a LTP from Avicennia marina, 70% identity to the LTP from Sesamum indicum (sesame), 67% to Salvia miltiorrhiza, 63% to N. tabacum (common tobacco) and Oryza sativa, and 61% to Zea mays, respectively. Thirty-three amino acids conserved residues, including eight cysteine residues, are present in all regions of the proteins (Fig. 3). Analyses of their evolutionary relationship indicated that the LJAMP2 from L. japonicus (Labiatae) has a close evolutionary relationship to nsLTPs from A. marina (Verbenaceae) and S. indicum (Pedaliaceae).
Multiple sequence alignment of the deduced amino acid sequence of LJAMP2 with other nsLTPs from plants, available in the data banks. Protein sequences were aligned with the ClustalW algorithm. Gene bank numbers corresponding to these sequences are as follows: Am A. marina (AAK01293), Ca C. annuum (AAX20049), Lp Lycopersicon pennellii (AAB07487), Nt N. tabacum (AAM74206), Os O. sativa (EAY79716), Si S. indicum (ABQ53933) Sm S. miltiorrhiza (ABP01768), Zm Z. mays (A31779). The last line is the conserved amino acids residues
Analyses of posttranslational modifications using software programs in ExPASy provided online by (SIB) indicated that five potential phosphorylation sites lie in LJAMP2 at positions Y17, T19, 40, 41, and S57 of the mature protein. Using the software package SWISS-MODEL provided online by SIB (Schwede et al. 2003), the 3D structure of LJAMP2 (Fig. 4a) was simulated. The 3D model suggests a global fold similar to the structure of NLTP1 (Fig. 4b) from tobacco (accession number: Q42952) (Da Silva et al. 2005), including an internal cavity and four large helixes involving residues Cys4-Thr19 (H1), Leu24 to Ala37 (H2), Thr41 to Ser57 (H3), and Leu63 to Cys73 (H4). The four helixes were stabilized by four disulfide bridges between the eight conserved cysteine residues (residue 4 with 50, 14 with 27, 28 with 73, 48 with 87, respectively).
The three-dimensional structure of LJAMP2 simulated using SWISS-MODEL software package. The structure was drawn with PYMOL software package (http://www.pymol.org/). a, LJAMP2; b, NLTP1 from tobacco (accession number: Q42952). C C-terminal; N N-terminal. Numbers indicate the sequence number of amino acid residues
Overexpression of LJAMP2 in tobacco
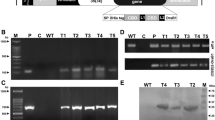
To express LJAMP2 in tobacco, a transgene expression plasmid was constructed for constitutive expression (Frisch et al. 1995), in which the LJAMP2 coding region was driven by a CaMV 35S promoter. The npt ІІ and gus were used as the selectable marker gene and the reporter gene, respectively (Fig. 5a). This plasmid was introduced into tobacco via an Agrobacterium-mediated transformation method. As a control (Ct), tobacco plants were transformed with the empty vector. Kanamycin-resistant calli were regenerated and the T0 plants were screened by GUS histochemical analysis (Jefferson et al. 1987). The GUS-positive and kanamycin-resistant plantlets were grown in a greenhouse. Transgenic plants were self-pollinated to produce the second generation (T2).
The expression analysis of LJAMP2 in transgenic tobacco seedlings. a Construct of plant expression vector pBIN-LJAMP2. RB right border, NosP nos promoter, NPT II neomycin phosphotransferase II, NosT nos terminator, 35S cauliflower mosaic virus 35S promoter, GUS β-glucuronidase, LB left border. b RNA gel blot analysis of LJAMP2 transcripts in four independent tobacco T1 transgenic lines and the control. Total RNA was prepared from leaves of transgenic plants. RNA samples were separated by denaturing formaldehyde-agarose gel electrophoresis, blotted, and hybridized with a 32P-labeled LJAMP2 cDNA probe. Hybridizing signals were visualized by exposing the membrane to X-ray film (top). The gel was stained with ethidium bromide to detect rRNAs as control (bottom). c HPLC profiles of proteins from transgenic lines control (Ct), L17, L5, L4, and the purified LJAMP2. The samples were analyzed by HPLC on a Source 5RPC column (4.6 × 150 mm, 15 μm) using ÄKTA Explorer 10S system. Arrows indicate the position of detected LJAMP2 peak. Rt retention time (min)
We randomly selected GUS-expressing plantlets for subsequet molecular analyses. Hybridization with an LJAMP2 cDNA probe indicated that the level of LJAMP2 mRNA varied in the independent transgenic plants (Fig. 5b). A strong hybridization signal was seen in the transgenic line L5, a moderate signal in line L4, and weak signals in the lines L2 and L17. To detect the translation levels of LJAMP2 in transgenic tobacco plants, the protein extracts from the leaves of all transgenic lines were subjected to HPLC, and the profiles were compared with that of the purified LJAMP2. The LJAMP2-specific peak, which displayed inhibition activity against R. solanacearum (data not shown), was detected in the lines L5 and L4, while this peak was not found in the line L2 (data not shown), L17 and Ct (Fig. 5c).
Disease resistance of transgenic tobacco
To assess the disease resistance of the transgenic tobacco, transgenic lines (T2), L2, L4, L5, L17, Ct (transformed with the empty vector), and wild-type tobacco (Wt) were challenged by the fungal pathogen, A. alternata. The resistance levels were evaluated by DI shown in Fig. 6a. Transgenic lines L4 and L5 displayed higher disease resistance than L2, L17, and Ct, and the difference was statistically significant. The L2, L17, and Ct lines demonstrated increased disease resistance in comparison with that of Wt, but no significant difference among them was observed. When challenged, the tobacco plants with the bacterial pathogen, R. solanacearum, the results were very similar to that observed in that of A. alternata (Fig. 6b). The lines L4 and L5 showed significant enhanced resistance to R. solanacearum, while the resistance of lines L2 and L17 exhibited similarity with that of line Ct, which showed no significant difference in comparison with Wt. Furthermore, no visible phenotype alteration was observed in all transgenic tobacco (Fig. 7), suggesting that over-expression of LJAMP2 might not bring about morphological changes in plants.
Resistance of transgenic T2 tobacco plants inoculated with the fungal pathogen A. alternata and the bacterial pathogen R. solanacearum. a Fungal infection assays of tobacco plants holding LJAMP2. Mean values of disease index (%) estimated from three independent infection assays (18 plants per line) are shown. b Bacterial infection assays of tobacco transformed with LJAMP2. Mean values of disease index (%) estimated from three independent infection assays (20 plants per line) are shown. Asterisks indicate that the DI in transgenic lines are significantly different (P ≤ 0.05 by t test) compared with that in the control. Error bars indicate standard deviation
Phenotype of transgenic and control tobacco plants infected with A. alternata and with P. solanacearum, respectively. a Tobacco leaves infected with A. alternata were photographed at 10 days after inoculation. b Tobacco plants were infected with P. solanacearum by dipping roots and photographed at 12 days after infected. L4 line was used as the representative plants to show the resistance against P. solanacearum. 1, Wild-type tobacco infected with P. solanacearum; 2, transgenic line L4, infected with P. solanacearum; 3, wild-type tobacco (uninfected)
Discussion
In the previous work on screening for potent AMPs from plant seeds, we have isolated a novel heat-stable nsLTP-like AMP, LJAMP2, from motherwort (Yang et al. 2006). In the present study, cloning and characterization of this protein (LJAMP2) have further confirmed that it belongs to a member of plant nsLTP-like proteins. The deduced amino acid sequence of LJAMP2 possesses a number of characteristics common to all other plant nsLTPs, such as low molecular mass, basic pI value, eight cysteine residues at the conserved positions and an N-terminal signal peptide. Analysis of the deduced amino acid sequence of LJAMP2 indicates that the alanine residue at position 24, where the A-X(E)-A motif is located, is most likely cleaved to become a mature protein, which corresponds to the determined N-terminal sequence of LJAMP2.
It has been reported that the expression of nsLTP genes in various plants was different and most of these genes were temporally and spatially controlled (Gausing 1994; Molina et al. 1993). Expression of the LJAMP2 gene also appears to be organ-specific. LJAMP2 gene transcripts are detected in seeds but are undetected in roots, stems, leaves and flowers of motherwort, indicating that the LJAMP2 protein may function mainly in the resistance of seeds against pathogens.
NsLTP’s role in defense is mainly based on the antimicrobial activities that they exert in vitro and in planta (Jung et al. 2005; Jung et al. 2003; Molina and Garcia-Olmedo 1997). For instance, the overexpression of the barley LTP2 protein under the control of a constitutive promoter in tobacco and Arabidopsis enhanced resistance to Pseudomonas syringae pv. tabaci and P. syringae pv. tomato, respectively (Molina and Garcia-Olmedo 1997). Furthermore, expression of nsLTP-like AMP, Ace-AMP1, from onion in scented geranium shows transgenic plants have increased resistance to both fungal and bacterial pathogens (Bi et al. 1999; Patkar and Chattoo 2006). Considerable evidence suggests that the nsLTPs have a defensive role in the resistance of plants. The results reported in the previous work demonstrate in vitro, a potent inhibition of fungal and bacterial growth exerted by LJAMP2 (Yang et al. 2006).
To confirm the antimicrobial function of LJAMP2 in planta, we let the gene overexpression in transgenic tobacco. Bioassays for fungal and bacterial pathogens indicated that transgenic tobacco plants had increased resistance to A. alternata and R. solanacearum (Figs. 6, 7). This evidence strongly supports the role of LJAMP2 in plant defense. LJAMP2 could be a good candidate for transgenic overexpression in plants. One interesting finding is that the level of LJAMP2 mRNA was inconsistent with the level of LJAMP2 protein found in the lines L4 and L5. This discrepancy may be explained as a result of transgenic suppression, which often was observed in plants, when the insertion of a transgene resulted in suppression (Cao et al. 1998; Stam et al. 1997). Here, we speculate that in L5 line, suppression takes place at the level of translation by an unknown mechanism. In addition, clearly, transgenic tobacco lines L4 and L5 differed in their levels of LJAMP2 mRNA transcription and LJAMP2 protein expression, but the disease resistance was no significant difference. Instability and limitation of the transgenic protein may account for this phenomenon.
In conclusion, LJAMP2 cloned from motherwort exerted significant homology to nsLTPs-like protein from plants. The LJAMP2 transgenic tobacco exhibited resistance to the bacterial pathogen, R. solanacearum, as well as the hyphomycete fungus, A. alternata. Furthermore, no visible changes were found in transgenic tobacco, implying that the over-expression of LJAMP2 does not alter the plant growth and development. These results suggest that the role of LJAMP2 in motherwort seeds is against phytopathogens and LJAMP2 has potential application in plant protection by gene engineering.
References
Bi YM, Cammue BPA, Goodwin PH, KrishnaRaj S, Saxena PK (1999) Resistance to Botrytis cinerea in scented geranium transformed with a gene encoding the antimicrobial protein Ace-AMP1. Plant Cell Rep 18:835–840
Blein J-P, Coutos-Thévenotb P, Marionc D, Ponchet M (2002) From elicitins to lipid-transfer proteins: a new insight in cell signalling involved in plant defence mechanisms. Trends Plant Sci 7:293–296
Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S (2004) Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4:1633–1649
Cammue BPA, Thevissen K, Hendriks M, Eggermont K, Goderis IJ, Proost P, Van Damme J, Osborn RW, Guerbette F, Kader JC, Broekaert WF (1995) A potent antimicrobial protein from onion seeds showing sequence homology to plant lipid transfer proteins. Plant Physiol 109:445–455
Cao H, Li X, Dong X (1998) Generation of broad-spectrum disease resistance by overexpression of an essential regulatory gene in systemic acquired resistance. Proc Natl Acad Sci USA 95:6531–6536
Carvalho AO, Gomes VM (2007) Role of plant lipid transfer proteins in plant cell physiology—a concise review. Peptides 28:1144–1153
Cary JW, Rajasekaran K, Jaynes JM, Cleveland TE (2000) Transgenic expression of a gene encoding a synthetic antimicrobial peptide results in inhibition of fungal growth in vitro and in planta. Plant Sci 154:171–181
Cheng CS, Samuel D, Liu YJ, Shyu JC, Lai SM, Lin KF, Lyu PC (2004) Binding mechanism of nonspecific lipid transfer proteins and their role in plant defense. Biochemistry 43:13628–13636
Da Silva P, Landon C, Industri B, Marais A, Marion D, Ponchet M, Vovelle F (2005) Solution structure of a tobacco lipid transfer protein exhibiting new biophysical and biological features. Proteins: Struct, Funct, Genet 59:356–367
Frisch DA, Harris-Haller LW, Yokubaitis NT, Thomas TL, Hardin SH, Hall TC (1995) Complete sequence of the binary vector Bin 19. Plant Mol Biol 27:405–409
Gao Y, Fencil KJ, Xu X, Schwedler DA, Gilbert JR, Herman RA (2006) Purification and characterization of a chimeric Cry1F δ-endotoxin expressed in transgenic cotton plants. J Agric Food Chem 54:829–835
Gausing K (1994) Lipid transfer protein genes specifically expressed in barley leaves and coleoptiles. Planta 192:574–580
Gomes E, Sagot E, Gaillard C, Laquitaine L, Poinssot B, Sanejouand YH, Delrot S, Coutos-Thevenot P (2003) Nonspecific lipid-transfer protein genes expression in grape (Vitis sp.) cells in response to fungal elicitor treatments. Mol Plant-Microbe Interact 16:456–464
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jung HW, Kim KD, Hwang BK (2005) Identification of pathogen-responsive regions in the promoter of a pepper lipid transfer protein gene (CALTPI) and the enhanced resistance of the CALTPI transgenic Arabidopsis against pathogen and environmental stresses. Planta 221:361–373
Jung HW, Kim W, Hwang BK (2003) Three pathogen-inducible genes encoding lipid transfer protein from pepper are differentially activated by pathogens, abiotic, and environmental stresses. Plant Cell Environ 26:915–928
Langen G, Imani J, Altincicek B, Kieseritzky G, Kogel K-H, Vilcinskas A (2006) Transgenic expression of gallerimycin, a novel antifungal insect defensin from the greater wax moth Galleria mellonella, confers resistance to pathogenic fungi in tobacco. Biol Chem 387:549–557
Lee YH, Yoon IS, Suh SC, Kim HI (2002) Enhanced disease resistance in transgenic cabbage and tobacco expressing a glucose oxidase gene from Aspergillus niger. Plant Cell Rep 20:857–863
Li Y, Hagen G, Guilfoyle TJ (1992) Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Dev Biol 153:386–395
Lin KF, Liu YN, Hsu STD, Samuel D, Cheng CS, Bonvin AMJJ, Lyu PC (2005) Characterization and structural analyses of nonspecific lipid transfer protein 1 from mung bean. Biochemistry 44:5703–5712
Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163:16–20
Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK (2002) A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419:399–403
Molina A, Garcia-Olmedo F (1997) Enhanced tolerance to bacterial pathogens caused by the transgenic expression of barley lipid transfer protein LTP2. Plant J 12:669–675
Molina A, Segura A, Garcia-Olmedo F (1993) Lipid transfer proteins (nsLTPs) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett 316:119–122
Oard S, Enright F (2006) Expression of the antimicrobial peptides in plants to control phytopathogenic bacteria and fungi. Plant Cell Rep 25:561–572
Osusky M, Zhou G, Osuska L, Hancock RE, Kay WW, Misra S (2000) Transgenic plants expressing cationic peptide chimeras exhibit broad-spectrum resistance to phytopathogens. Nat Biotechnol 18:1162–1166
Park CJ, Shin R, Park JM, Lee GJ, Youl JS, Paek KH (2002) Induction of pepper cDNA encoding a lipid transfer protein during the resistance response to tobacco mosaic virus. Plant Mol Biol 48:243–254
Patkar RN, Chattoo BB (2006) Transgenic indica rice expressing ns-LTP-like protein shows enhanced resistance to both fungal and bacterial pathogens. Mol Breed 17:159–171
Peng JL, Bao ZL, Ren HY, Wang JS, Dong HS (2004) Expression of harpin(Xoo) in transgenic tobacco induces pathogen defense in the absence of hypersensitive cell death. Phytopathology 94:1048–1055
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Samuel D, Liu YJ, Cheng CS, Lyu PC (2002) Solution structure of plant nonspecific lipid transfer protein-2 from rice (Oryza sativa). J Biol Chem 277:35267–35273
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Stam M, Mol JNM, Kooter JM (1997) The silence of genes in transgenic plants. Ann Bot 79:3–12
Terras FRG, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, Rees SB, Torrekens S, Leuven FV, Vanderleyden J, Cammue BPA, Broekaert WF (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7:573–588
Wang C, Xie W, Chi F, Hu W, Mao G, Sun D, Li C, Sun Y (2007) BcLTP, a novel lipid transfer protein in Brassica chinensis, may secrete and combine extracellular CaM. Plant Cell Rep 27:159–169
Xiao Y, Luo M, Fang W, Luo K, Hou L, Li D, Pei Y (2002) PCR walking in cotton genome using YADE method. Acta Genet Sin 29:62–66
Yang X, Li J, Li X, She R, Pei Y (2006) Isolation and characterization of a novel thermostable non-specific lipid transfer proteinlike antimicrobial protein from motherwort (Leonurus japonicus Houtt) seeds. Peptides 27:3122–3128
Yang X, Xiao Y, Wang X, Pei Y (2007) Expression of a novel small antimicrobial protein from the seeds of motherwort (Leonurus japonicus) confers disease resistance in tobacco. Appl Environ Microbiol 73:939–946
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 30370916 to X. Yang, and grant no. 30671327 to X. Li).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Y. Lu.
Rights and permissions
About this article
Cite this article
Yang, X., Wang, X., Li, X. et al. Characterization and expression of an nsLTPs-like antimicrobial protein gene from motherwort (Leonurus japonicus). Plant Cell Rep 27, 759–766 (2008). https://doi.org/10.1007/s00299-008-0506-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-008-0506-0