Abstract
Genetically transformed plants of Cymbidium were regenerated after cocultivating protocorm-like bodies (PLB) with Agrobacterium tumefaciens strain EHA101 (pIG121Hm) that harbored genes for β-glucuronidase (gus), hygromycin phosphotransferase (hpt) and neomycin phosphotransferase II (nptII). PLB of three genotypes maintained in liquid new Dogashima medium (NDM), were subjected to transformation experiments. The PLB inoculated with Agrobacterium produced secondary PLB, 4 weeks after transfer onto 2.5 g L−1 gellan gum-solidified NDM containing 10 g L−1 sucrose, 20 mg L−1 hygromycin and 40 mg L−1 meropenem. Transformation efficiency was affected by genotype and the presence of acetosyringone during cocultivation. The highest transformation efficiency was obtained when PLB from the genotype L4 were infected and cocultivated with Agrobacterium on medium containing 100 μM acetosyringone. Transformation of the hygromycin-resistant plantlets regenerated from different sites of inoculated PLB was confirmed by histochemical GUS assay, PCR analysis and Southern blot hybridization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orchidaceae is the largest family of flowering plants, comprising more than 800 genera and 25,000 species widely distributed around the world that includes a large number of important ornamental species belonging to diverse genera such as Cymbidium, Dendrobium, Phalaenopsis, Cattleya and Oncidium. Among these, cymbidium orchid is one of the most popular orchids grown for commercial production of cut flowers and pot plants. Since Cymbidium is one of the most improved genera in the family Orchidaceae, a large number of novel cultivars with new flower colors, shapes, plant length, disease and pest resistance have been released through interspecific hybridization since the late 19th century. As a supplement to these conventional breeding programs, improvement of Cymbidium by means of genetic engineering is now expected to confer new and desirable traits such as blue or purple flowers, disease resistance and stress tolerance.
To date, a few studies of genetic transformation have been reported in orchids by using Agrobacterium-mediated method (Belarmino and Mii 2000; Chai et al. 2002; Chen et al. 2002; Liau et al. 2003a; Men et al. 2003; Mishiba et al. 2005) and biolistic-mediated method (Kuehnle and Sugii 1992; Chia et al. 1994; Anzai et al. 1996; Yang et al. 1999; Yu et al. 1999; Knapp et al. 2000; Tee et al. 2003). Among these studies, Yang et al. (1999) first reported the production of transgenic Cymbidium by particle bombardment method and obtained transformed plants that expressed nptII and gus genes from the bombarded PLB. Recently, Chen et al. (2002) described the Agrobacterium-mediated transfer of genes for nptII and gus into rhizomes of C. niveo-marginatum, which is oriental terrestrial species, using Agrobacterium tumefaciens. However, there has been no report on the successful Agrobacterium-mediated transformation of other Cymbidium species and cultivars derived from interspecific hybridization between epiphytic species that are more important for commercial production.
In recent years, introduction of disease resistance traits such as antimicrobial peptides and viral coat proteins into the genome of other orchids was achieved by genetic engineering (Liau et al. 2003b; Chan et al. 2005; Sjahril et al. 2006). Transgenic plants over-expressing those genes showed enhanced resistance to Erwinia carotovora and Cymbidium Mosaic Virus (CymMV). Hence, gene transfer approach may provide a powerful tool for improvement of disease resistance in orchid species and it may be possible to extend this approach to Cymbidium species.
In the present study, we developed a reproducible procedure for Agrobacterium-mediated transformation of cymbidium orchid by using PLB, which is derived from shoot-tip culture of a cultivar and PLB cultures derived from two seedlings, each derived from a different cross between elite clones. We examined the influences of genotypes, the presence of acetosyringone (AS) in the cocultivation medium and the concentration of sucrose in medium for maintaining PLB used on the transformation.
Materials and methods
Plant materials
PLB of the three different Cymbidium lines, RY, L4 and L23 were used in the transformation experiments. PLB of RY were obtained from a commercial orchid company, while PLB of L4 and L23 were obtained from single seedlings, each derived from a different cross combination between elite clones of Cymbidium. PLB were maintained by subculturing every month 0.7 g fresh weight of PLB after cutting transversely into ca. 0.5 mm thick segments into 70 mL liquid ND medium (Tokuhara and Mii 1993) containing 10 or 30 g L−1 sucrose (10S or 30S) without any plant growth regulators in a 200 mL flask at pH 5.4. Cultures were incubated on a reciprocal shaker by agitation at 60 rpm at 25°C under constant illumination with cool-white-fluorescent lamps (National FL30SN, Osaka, Japan). One-month-old PLB after each subculture was used for transformation experiments.
Influence of sucrose concentration and selective antibiotics on PLB proliferation
Number and fresh weight of PLB were recorded 1 month after subculture to compare the proliferation rates between the cultures with 10S and those with 30S for each genotype. Sensitivity of the PLB to kanamycin and hygromycin was also examined using these two kinds of cultures. For each culture, 0.5 g of 1-month-old PLB were inoculated onto a 2.5 g L−1 gellan gum-solidified 10S ND medium containing either kanamycin at 100, 200, and 400 mg L−1, or hygromycin at 5, 20, and 40 mg L−1. Fresh weight of PLB on media containing these antibiotics was measured 1 month after the transfer.
Plasmid vector and bacterial strain
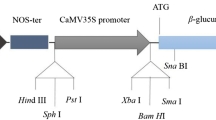
A. tumefaciens strain EHA101 (Hood et al. 1986), which harbors a binary vector pIG121Hm (Ohta et al. 1990) that contains a neomycin phosphotransferase II gene (nptII), a hygromycin phosphotransferase gene (hpt) and an intron-GUS gene in the T-DNA region were used.
Inoculation and cocultivation with Agrobacterium
Agrobacterium was grown overnight at 28°C in LB liquid medium containing 50 mg L−1 hygromycin, 50 mg L−1 kanamycin and 25 mg L−1 chloramphenicol. Before inoculation with Agrobacterium, 4 g of 1-month-old PLB were transferred to 40 mL liquid 10S ND medium with or without 100 μM acetosyringone in a 100 mL flask. Four milliliters of Agrobacterium suspension culture were added to each flask and incubated for 7 h to give a density of OD550 = 0.5. The PLB were blotted dry, and cocultivated on medium consisting of 2.5 g L−1 gellan gum-solidified 10S ND medium supplemented with or without 100 μM AS in Petri plates at 20°C in the dark.
Selection of transgenic PLB and plant regeneration
After 3 days of cocultivation, the PLB were washed with liquid 10S ND medium containing 10 mg L−1 meropenem (Ogawa and Mii 2004, Meropen; Sumitomo Pharmaceuticals Co., Ltd., Osaka, JPN), and then transferred onto a 2.5 g L−1 gellan gum-solidified 10S ND selection medium containing 20 mg L−1 hygromycin and 10 or 40 mg L−1 meropenem. PLB were subcultured every 10 days during the first month, and subsequently, every 2 weeks to a fresh medium with the same composition for 3 months. Secondary PLB showing green coloration produced on the selection medium were then proliferated by cutting transversely into several pieces and transferred to a medium either with or without 20 mg L−1 hygromycin. Plantlets regenerated from the pieces of secondary PLB were selected as putative transformants and transplanted in culture bottles containing 20 mg L−1 hygromycin. Four months after the transplanting, some of the plants with five to six fully developed leaves and several roots were transferred into vermiculite-filled pots and grown in a growth chamber at 25°C under a 16-/8-h (day/night) photoperiod without any acclimatization treatment.
GUS assay
A histochemical assay to detect GUS activity was performed on hygromycin-resistant PLB, leaves and roots. These tissues were immersed in X-Gluc solution (Jefferson et al. 1987), placed under a mild vacuum for 10 min, and then incubated overnight at 37°C. The tissues were soaked in 70% ethanol for several hours to remove chlorophyll before observation.
DNA isolation and molecular analysis
Plants regenerated from selected PLB were subjected to DNA isolation. Total genomic DNA was extracted from leaf tissues (1.5 g FW) using the CTAB method (Murray and Thompson 1980). A set of primers specific to the regions of the CaMV35S promoter 5′-GATGTGATATCTCCACTGAC-3′ and the NOS terminator 5′-CGCAAGACCGGCAACAGGAT-3′, respectively, were used to amplify both 2.2-kb fragment of the gus gene and 1.7-kb fragment of the hpt gene simultaneously. PCR amplification was carried out by using the following conditions: 30 cycles of thermal treatment with 1 min at 94°C, 1 min at 62°C and 1.5 min at 72°C.
Ten micrograms of genomic DNA from untransformed and hygromycin-resistant plants was digested with XbaI, which cuts a single site within the T-DNA, and fractionated on 0.9% agarose gels. Southern blot hybridization and detection were carried out using digoxigenin-labeled gus probe following the manufacturer’s instruction (Roche Diagnostics GmbH, Mannheim, Germany).
Results and discussion
Influence of sucrose concentration in the culture media and selective antibiotics on PLB growth
We initially induced two types of PLB from each genotype by using different sucrose concentrations (10S or 30S), i.e., relatively large PLB with dark green coloration in 10S and small-sized yellow ones in 30S, respectively. These PLB were maintained in liquid ND medium without any plant growth regulators and subcultured every month by transferring to fresh medium after cutting transversely into several pieces of approximately 5 mm in size. This process has allowed us to maintain the PLB without regeneration of plantlet under the same conditions for more than 3 years. The new PLB that formed from cut surfaces maintained their initial characteristics during the subcultures in these two media. As shown in Fig. 1a, PLB cultured on 30S ND medium showed higher numbers of PLB as well as proliferation rate (fresh weight) for all genotypes. However, proliferation of PLB was inhibited when the concentration of sucrose in the culture media was increased up to 6%, probably due to high osmotic stress (data not shown). The effect of carbon source on the growth of tissues and organogenesis has also been observed in other orchid species (Chia et al. 1988; Tokuhara and Mii 2003). Since transformation efficiency was influenced by the type of tissue used for both Agrobacterium-mediated transformation of rice (Hiei et al. 1994) and biolistic-mediated transformation of dendrobium orchid (Tee et al. 2003), we compared these two types of PLB for subsequent transformation experiments.
Effect of sucrose concentration in preculture medium on proliferation of PLB (a) and sensitivity of PLB to selective agents (b) in three different Cymbidium genotypes. a Proliferation rate (fold per month) and number (per gram) of PLB 1 month after subculture of 0.7 g PLB into a flask containing 70 mL of 10S or 30S liquid ND medium. Each value represents a mean ± SE of the three independent experiments. Bars with the same letter are not significantly different according to the Turkey HSD test at 0.05%. b Sensitivity of PLB to selective agents evaluated as the growth rates, 1 month after transfer of 1-month-old PLB to medium containing various concentrations of kanamycin or hygromycin. Each value represents a mean of the three independent experiments. The columns marked with an asterisk indicate that the cultures turned brown without further growth
Since the plasmid used in this study (pIG121Hm) contains both nptII and hpt genes as selectable markers, the optimal concentrations of selection agents were determined by transferring untransformed PLB, which had been cultured in liquid medium, onto gellan gum-solidified 10S ND medium containing various concentrations of kanamycin or hygromycin. As shown in Fig. 1b, hygromycin completely inhibited the growth of PLB in all genotypes at 20 mg L−1, while kanamycin failed to inhibit the growth even at 400 mg L−1 after 1 month of culture. These results indicate that kanamycin is not suitable as a selective agent for Cymbidium, as previously reported in Dendrobium (Chia et al. 1994) and other monocot plants (Hauptmann et al. 1988; Eady and Lister 1998). In the present study, therefore, hygromycin at 20 mg L−1 was used for the selection medium.
Selection of hygromycin-resistant PLB and plant regeneration
After transfer of PLB onto selection medium, RY and L4 successfully produced secondary PLB within 4 weeks (Fig. 2a), while L23 showed overgrowth of Agrobacterium on and around PLB within 1 week and turned brown. In our previous study, meropenem exhibited the highest activity against Agrobacterium strain EHA101 among 12 β-lactam antibiotics tested (Ogawa and Mii 2004). We also demonstrated that meropenem was suitable for eliminating Agrobacterium at a low concentration (5 mg L−1) for transformation of phalaenopsis orchid (Mishiba et al. 2005; Sjahril and Mii 2006). In the present study, however, 10 mg L−1 meropenem failed to suppress the overgrowth of Agrobacterium in L23 during the early stages of selection. This may be due to the weak anti-bacterial activity of L23 compared to L4 and RY, and PLB of L23 died more rapidly by the harmful substances produced by overgrown bacteria. Since overgrowth of bacteria in L23 was not observed when PLB were exposed to meropenem at 40 mg L−1 in the selection medium, a higher concentration of meropenem was required to suppress the growth of Agrobacterium in L23 genotype. Since the other two strains, RY and L4 showed comparably high transformation efficiency both at 10 and 40 mg L−1 meropenem, bacterial elimination could be generally achieved at 40 mg L−1 without exhibiting apparent cellular necrosis in Cymbidium (Fig. 3).
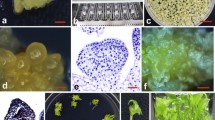
Selection and regeneration of putative transformants in Agrobacterium-mediated transformation of Cymbidium. a Hygromycin-resistant secondary PLB (arrow) of L4 proliferated on the selective medium 1 month after infection with A. tumefaciens EHA101 (pIG121Hm). b A hygromycin-resistant secondary growth of PLB of L4 (arrow) on hygromycin-containing medium 3 months after selection. c Multiple PLB of RY derived from a hygromycin-resistant PLB after cutting transversely into several pieces. d RY plantlets with developing shoots regenerated from hygromycin-resistant PLB, 8 months after inoculation with Agrobacterium on hygromycin-containing medium. e Transgenic RY plant after acclimatization. Bars 5 mm
Effects of various factors on the efficiency of putative transformation in three Cymbidium genotypes by A. tumefaciens EHA101 (pIG121Hm). Each bar represents number of hygromycin-resistant secondary PLB derived from 4 g primary PLB after 2 months of culture on selection medium containing 20 mg L−1 hygromycin. Each value represents a mean of the three independent experiments. Bars with the same letter are not significantly different according to the Turkey HSD test at 0.05%. The columns marked with an asterisk indicate that the cultures showed overgrowth of Agrobacterium
Three months after the transfer, the newly formed secondary PLB reached approximately 5 mm in diameter (Fig. 2b). These PLB were proliferated by cutting transversely into several pieces (Fig. 2c), which subsequently developed into plantlets within 4 months (Fig. 2d). When the plantlets had five to six leaves and several roots, a total of 32 plants were transferred into pots containing vermiculite (Fig. 2e). All of the plants surviving and no difference in their morphology compared to nontransgenic plants was observed. Since regenerated plants were grown in culture bottles for more than 4 months before being transferred into pots, the plants were large in size and this contributed to their high rate of survival ex vitro. As shown in Fig. 3, no significant changes were observed in transformation efficiency between PLB maintained on 10S and those on 30S ND medium for each genotype. In general, transformation efficiency was increased in the presence of AS during the inoculation and cocultivation period (Drake et al. 1997; Song and Sink 2004). The effectiveness of AS in Agrobacterium-mediated transformation has also been reported previously in Phalaenopsis (Belarmino and Mii 2000), Dendrobium (Men et al. 2003), and Oncidium (Liau et al. 2003a). In this study, 100 μM AS at inoculation and cocultivation increased the putative transformation efficiency. The highest number of hygromycin-resistant secondary PLB was obtained when PLB of L4 were treated with 100 μM AS during inoculation and cocultivation, which resulted in 19.7 independent hygromycin-resistant PLB per 4 g primary PLB following selection on hygromycin-containing medium. In contrast, the lowest number of hygromycin-resistant secondary PLB was obtained in L23, suggesting that genetic background played an important role in Agrobacterium-mediated transformation of Cymbidium.
GUS assay
Twenty hygromycin-resistant PLB from each genotype were selected at random and used for GUS assay. As shown in Table 1, more than 90% of hygromycin-resistant PLB showed blue staining of the tissues, which were not observed in the untransformed PLB. Although some of the PLB did not show GUS activity among the selected PLB, the presence of the transgenes was confirmed by PCR in these GUS negative samples. The leaves and roots of twenty putative transformants (83%; 20/24) also showed GUS activity in the regenerated plants (Fig. 4a–c).
Histochemical GUS assay of transgenic Cymbidium strain L4. a Stable GUS expression on hygromycin-resistant (right) and control (left) PLB. b Stable GUS expression on root of transformed (bottom) and control (upper) plants. c Stable GUS expression on leaf of transformed (right) and control (left) plants. Bars 5 mm
Molecular assays of transformants
The presence of the gus and hpt genes in putatively transformed plants was confirmed by PCR amplification by producing the expected 2.2- and 1.7-kb amplification products, respectively (Fig. 5a). As shown in Fig. 5b, Southern blot analysis using the gus gene as the probe revealed that three transformed plants had a single copy, while others showed multiple copies of T-DNA integrated in the plant genomes.
Molecular analysis of transgenic plants. a PCR analysis of transgenic plants for the gus (upper) and hpt (bottom) genes. Lane M molecular size marker (λDNA/HindIII, øX174/HaeIII). Lane P plasmid (pIG121Hm). Lane N nontransformed control plant. Lanes 1–6 transgenic plants. b Southern blot analysis of transgenic plants. Genomic DNA was digested with XbaI. Blot was hybridized with a digoxigenin-labeled gus gene probe. Lane N nontransformed control plant. Lane 1–7 transgenic plants
In the present study, we have succeeded in the production of transgenic plants in an important orchid, Cymbidium through establishing a protocol using A. tumefaciens, although some of the cultivars and/or genotypes may require optimization of the protocol for overcoming the low efficiency of transformation. Since Agrobacterium-mediated method has already been applied successfully for the transformation of several orchids such as Phalaenopsis (Belarmino and Mii 2000), Dendrobium and Oncidium (Yu et al. 2001; Liau et al. 2003a), it should be capable of transforming a wide range of species similar to the particle bombardment method.
In the previous study on the transformation of Cymbidium using the biolistic method, Yang et al. (1999) initiated the selection of transformants with kanamycin 1 month after bombardment. In the present study, however, we used hygromycin as a selective agent and performed the selection of transformants immediately after cocultivation of PLB with Agrobacterium. Although kanamycin is one of the most widely used antibiotics as a selective agent for transformation of plants, it was often ineffective for monocot plants (Hauptmann et al. 1988; Eady and Lister 1998). Our present results also showed that kanamycin was almost ineffective for inhibiting the continual PLB growth on all the three Cymbidium genotypes examined even at 400 mg L−1 which gave a mild damage. In contrast, all of the genotypes we have tested showed a high sensitivity to hygromycin. At the concentration that we used in transformation experiments (20 mg L−1), whole tissues of untransformed plants turned brown within 1 month. These results suggest that the use of hpt gene will be much more suitable than nptII as a selection agent for transformants in Cymbidium and possibly for many other orchids, since most of them have considerable tolerance to kanamycin (unpublished results).
Although Agrobacterium-mediated transformation system has also been reported in C. niveo-marginatum, an oriental terrestrial species, by using rhizomes as a target for gene transfer (Chen et al. 2002), there has been no report of the successful production of transgenic plants in Cymbidium by using PLB or protocorms, which are the main target material available in commercially important cymbidium orchids as targets for the transformation. In the present study, therefore, we chose PLB as a target material for Agrobacterium-mediated transformation. Our results indicate that hygromycin-resistant secondary PLB were produced from meristems of PLB. This implies that the T-DNA transfer and integration are likely to occur in unwounded site (Escudero and Hohn 1997). Since PLB derived from 30S had more apical meristems than those from 10S, it is expected that 30S-derived PLB might have an higher transformation efficiency. However, no significant increase in the transformation efficiency was observed in PLB derived from 30S. In addition, PLB derived from 30S showed less overgrowth of Agrobacterium than those derived from 10S in RY and L4 during selection of transformants, when meropenem was added to the medium at concentrations as low as 5 mg l−1 (unpublished observation). Therefore, it can be speculated that PLB in 30S medium had higher expression of antimicrobial defense system associated with higher mitotic activity compared to those in 10S medium, although the mechanisms involved in this phenomenon are unknown.
In this study, inoculation of PLB with Agrobacterium was carried out in liquid medium for 7 h, which might be also the cause for overgrowth of Agrobacterium in L23. Although inoculation of many other plant tissues with Agrobacterium has most often been carried out in liquid medium for a short period (a few minutes), no hygromycin-resistant PLB were obtained in this study, when less than 1 h inoculation of PLB in liquid medium was performed (data not shown). In Phalaenopsis, optimum inoculation periods for obtaining transformed callus and protocorms were 10 and 7 h, respectively, in Agrobacterium-mediated transformation (Belarmino and Mii 2000; Mishiba et al. 2005). These results indicate that a relatively long period might be needed for adhesion of Agrobacterium on the surface of target orchid tissues.
In Agrobacterium-mediated transformation, phenolic compounds such as AS serve as a potent inducer for the expression of the virulence (vir) genes located on the Ti plasmid of A. tumefaciens (Ashby et al. 1987). Since orchids are not natural hosts of A. tumefaciens, previous studies have shown that addition of AS to infection and coculture medium is effective in producing transgenic plants in several orchid species belonging to Dendrobium (Men et al. 2003), Phalaenopsis (Belarmino and Mii 2000) and Oncidium (Liau et al. 2003a). Our present results also indicate that the presence of AS during infection and cocultivation period improved the transformation efficiency in most of the genotypes used. Since hygromycin-resistant secondary PLB were also obtained even when AS was omitted from the infection and coculture medium, Cymbidium may have an activator for vir genes of A. tumefaciens, as reported in Dendrobium (Nan et al. 1997), which is an important factor for successful Agrobacterium-mediated transformation (Yu et al. 2001).
In conclusion, we have established a transformation procedure in Cymbidium using PLB as a material for Agrobacterium infection. The transformation system will be used efficiently for introducing genes that confer commercially important traits such as flower color and resistance to diseases into Cymbidium.
Abbreviations
- AS:
-
Acetosyringone
- GUS:
-
β-Glucuronidase
- Hm:
-
Hygromycin
- Km:
-
Kanamycin
- ND:
-
New Dogashima
- PLB:
-
Protocorm-like body
Reference
Anzai H, Ishii Y, Shichinohe M, Katsumata K, Nojiri C, Morikawa H, Tanaka M (1996) Transformation of Phalaenopsis by particle bombardment. Plant Tissue Cult Lett 13:265–271
Ashby AM, Watson MD, Shaw CH (1987) A Ti-plasmid determined function is responsible for chemotaxis of Agrobacterium tumefaciens towards the plant wound product acetosyringone. FEMS Microbiol Lett 41:189–192
Belarmino MM, Mii M (2000) Agrobacterium-mediated genetic transformation of a Phalaenopsis orchid. Plant Cell Rep 19:435–442
Chai ML, Xu CJ, Senthil KK, Kim JY, Kim DH (2002) Stable transformation of protocorm-like bodies in Phalaenopsis orchid mediated by Agrobacterium tumefaciens. Sci Hortic 96:213–224
Chan YL, Lin KH, Sanjaya, Liao LJ, Chen WH, Chan MT (2005) Gene stacking in Phalaenopsis orchid enhances dual tolerance to pathogen attack. Transgenic Res 14:279–288
Chen L, Hatano T, Niimi Y (2002) High efficiency of Agrobacterium-mediated rhizome transformation in Cymbidium. Lindleyana 17:130–134
Chia TF, Hew CS, Loh CS, Lee YK (1988) Carbon/nitrogen ratio and greening and protocorm formation in orchid callus tissues. HortScience 23:599–601
Chia TF, Chan YS, Chua NH (1994) The firefly luciferase gene as a non-invasive reporter for Dendrobium transformation. Plant J 6:441–446
Drake PMW, John A, Power JB, Davey MR (1997) Expression of the gusA gene in embryogenic cell lines of Sitka spruce following Agrobacterium-mediated transformation. J Exp Bot 48:151–155
Eady CC, Lister CE (1998) A comparison of four selective agents for use with Allium cepa L. immature embryos and immature embryo-derived cultures. Plant Cell Rep 18:117–121
Escudero J, Hohn B (1997) Transfer and integration of T-DNA without cell injury in the host plant. Plant Cell 9:2135–2142
Hauptmann RM, Vasil V, Ozias-Akins P, Tabaeizadeh Z, Rogers SG, Fraley RT, Horsch RB, Vasil IK (1988) Evaluation of selectable markers for obtaining stable transformants in the Gramineae. Plant Physiol 86:602–606
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–82
Hood EE, Helmer GL, Fraley RT, Chilton MD (1986) The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T DNA. J Bacteriol 168:1291–1304
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Knapp JE, Kausch AP, Chandlee JM (2000) Transformation of three genera of orchid using the bar gene as a selectable marker. Plant Cell Rep 19:893–898
Kuehnle AR, Sugii N (1992) Transformation of Dendrobium orchid using particle bombardment of protocorms. Plant Cell Rep 11:484–488
Liau CH, You SJ, Prasad V, Hsiao HH, Lu JC, Yang NS, Chan MT (2003a) Agrobacterium tumefaciens-mediated transformation of an Oncidium orchid. Plant Cell Rep 21:993–998
Liau CH, Lu JC, Prasad V, Hsiao HH, You SJ, Lee JT, Yang NS, Huang HE, Feng TY, Chen WH, Chan MT (2003b) The sweet pepper ferredoxin-like protein (pflp) conferred resistance against soft rot disease in Oncidium orchid. Transgenic Res 12:329–336
Men S, Ming X, Liu R, Wei C, Li Y (2003) Agrobacterium-mediated genetic transformation of a Dendrobium orchid. Plant Cell Tissue Organ Cult 75:63–71
Mishiba K, Chin DP, Mii M (2005) Agrobacterium-mediated transformation of Phalaenopsis by targeting protocorms at an early stage after germination. Plant Cell Rep 24:297–303
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight DNA. Nucleic Acids Res 8:4321–4325
Nan GL, Tang CS, Kuehnle AR, Kado CI (1997) Dendrobium orchid contain an inducer of Agrobacterium virulence genes. Physiol Mol Plant Pathol 51:391–399
Ogawa Y, Mii M (2004) Screening for highly active β-lactam antibiotics against Agrobacterium tumefaciens. Arch Microbiol 181:331–336
Ohta S, Mita S, Hattori T, Nakamura K (1990) Construction and expression in tobacco of a β-glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol 31:805–813
Sjahril R, Chin DP, Khan RS, Yamamura S, Nakamura I, Amemiya Y, Mii M (2006) Transgenic Phalaenopsis plants with resistance to Erwinia carotovora produced by introducing wasabi defensin gene using Agrobacterium method. Plant Biotechnol 23:191–194
Sjahril R, Mii M (2006) High-efficiency Agrobacterium-mediated transformation of Phalaenopsis using meropenem, a novel antibiotic to eliminate Agrobacterium. J Hortic Sci Biotechnol 81:458–464
Song GQ, Sink KC (2004) Agrobacterium tumefaciens-mediated transformation of blueberry (Vaccinium corymbosum L.). Plant Cell Rep 23:475–484
Tee CS, Maziah M, Tan CS, Abdullah MP (2003) Evaluation of different promoters driving the GFP reporter gene and selected target tissues for particle bombardment of Dendrobium Sonia 17. Plant Cell Rep 21:452–458
Tokuhara K, Mii M (1993) Micropropagation of Phalaenopsis and Doritaenopsis by culturing shoot tips of flower stalk buds. Plant Cell Rep 13:7–11
Tokuhara K, Mii M (2003) Highly-efficient somatic embryogenesis from cell suspension cultures of phalaenopsis orchids by adjusting carbohydrate sources. In Vitro Cell Dev Biol Plant 39:635–639
Yang J, Lee H, Shin DH, Oh SK, Seon JH, Paek KY, Han K (1999) Genetic transformation of Cymbidium orchid by particle bombardment. Plant Cell Rep 18:978–984
Yu H, Yang SH, Goh CJ (2001) Agrobacterium-mediated transformation of a Dendrobium orchid with the 1 knox gene DOH1. Plant Cell Rep 20:301–305
Yu ZH, Chen MY, Nie L, Lu HF, Ming XT, Zheng HH, Qu LJ, Chen ZL (1999) Recovery of transgenic orchid plants with hygromycin selection by particle bombardment to protocorms. Plant Cell Tissue Organ Cult 58:87–92
Acknowledgements
We Thank Hitaka Orchid Co., Ltd. for providing Cymbidium seeds and Mukoyama Orchids Co., Ltd. for providing PLB of RY.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. K. Kamo
Rights and permissions
About this article
Cite this article
Chin, D.P., Mishiba, Ki. & Mii, M. Agrobacterium-mediated transformation of protocorm-like bodies in Cymbidium . Plant Cell Rep 26, 735–743 (2007). https://doi.org/10.1007/s00299-006-0284-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0284-5