Abstract
In plants, MYB transcription factors play important roles in many developmental processes and various defense responses. AtMYB24, as a member of R2R3-MYB gene family in Arabidopsis, was found mainly expressed in flowers, especially in microspores and ovules using Northern blots and in situ hybridization. It was further found that the expression of AtMYB24 was tightly regulated during anther development. Over-expression of AtMYB24 in transgenic plants resulted in pleiotropic phenotypes, including dwarfism and flower development defects, in particular, producing abnormal pollen grains and non-dehiscence anthers. Further analysis showed that the anther development of the AtMYB24-ox lines was retarded starting from the anther developmental stages 10–11. At stages 12 and 13, the septum and stomium cells of anthers would not break, and fewer or no fibrous bands were found in the endothecium and connective cells in the AtMYB24-ox plants. Similar aberrant anther phenotype was also observed in the AtMYB24-GR-ox lines treated with dexamethasone (DEX). Quantitative real-time PCR showed expression of genes involved in phenylpropanoid biosynthetic pathway, such as CHS and DFR, and AtGTP2 were altered in AtMYB24-ox lines. These results suggest an important role of AtMYB24 in the normal development of anthers in Arabidopsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcription factors play important roles in the regulation of developmental and physiological processes by binding to promoters of the downstream target genes to control their expression (Pabo and Sauer 1992). Based on the nature of the DNA-binding domains, transcription factors can be categorized into many families, among which MYB superfamily is one of the largest families in Arabidopsis (Riechmann et al. 2000). A MYB domain is usually composed of one to three imperfect repeats, each with 51–53 amino acid residues to form a helix–turn–helix structure (Stracke et al. 2001). MYB proteins in plant are classified into three major groups: R2R3-MYB, which is plant specific, with two adjacent repeats; R1R2R3-MYB, with three adjacent repeats; and MYB-related proteins, which usually but not always contain one MYB repeat (Stracke et al. 2001; Chen et al. 2006). In plants, MYB genes are found to be involved in regulation of many physiological and biochemical processes such as cellular morphogenesis, meristem maintenance and lateral organ specification, floral and seed development, cell cycle, and biological circadian. Plant MYB genes are also involved in phytochrome and phytohormone signaling pathways (Stracke et al. 2001; Chen et al. 2006; Kranz et al. 1998).
Many MYB genes have been reported to play important roles in flower development, e.g., pollen and anther development. AtMYB32 is required for normal pollen development, since atmyb32 mutant produces more than 50% aberrant pollen grains, which are devoid of cytoplasmic contents (Preston et al. 2003). AtMYB103, specifically expressed in tapetum and trichome, is also important for pollen development. Down-regulation of AtMYB103 expression would result in distorted pollen grains with reduced or no cytoplasmic content, and cause early tapetum degeneration at anther stage 9 (Higginson et al. 2003). AtMYB26 is important for anther development because the expansion of the endothecium layer, which happens at late stages of the anther development, does not occur in atmyb26 mutant. Further analysis found that the lignification did not take place in the mutant (Steiner-Lange et al. 2003). In addition, AtMYB21 is a flower-specific gene, whose over-expression causes abnormal flower development (Shin et al. 2002).
In a previous study, we identified two groups of MYB transcription factors, which all share a conserved M/Y-MDDIW motif at their C-terminal regions, and interestingly, these genes in the group I in Arabidopsis seemed to be expressed predominantly in flowers (Li et al. 2006a). Here, we report the characterization of one gene from the group I, AtMYB24 (At5g40350). AtMYB24 is predominantly expressed in flowers, especially in microspore and ovules. Over-expression of AtMYB24 caused pleiotropic phenotype including dwarfism, aberrant pollens, and non-dehiscence anthers. Real-time PCR showed that expression of genes involved in phenylpropanoid biosynthetic pathway and AtGTP2 were altered in AtMYB24-ox lines. Our data indicated that AtMYB24 play an important role in anther development.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana plants, ecotype Columbia, were used as wild-type controls and for in situ hybridization. Plant were germinated on MS media (pH=5.7) containing 1% sucrose and grown under long-day light at 22±2°C. For continuous dexamethasone (DEX) treatment, the plant flower bud was watered with MS containing 1% sucrose and 10 μM DEX daily for 10 days (Shin et al. 2002).
Plasmid construction and transgenic plants
For AtMYB24-GFP fusion construct, AtMYB24 was amplified using a primerF: (5′-CAC CAC ACT AGT GAG AAA AGA GAA-3′) and a primerR: (5′-CCC ACT AGT ATT ACC ATT ATA TAT-3′) with Pfu DNA polymerase, and the amplified fragments were cloned into SpeI site of pCAMBIA1302 vector (CAMBIA). For AtMYB24-GR fusion construct, AtMYB24 was amplified using a primerF: (5′-CAG GAT CCA ATG GAG AAA AGA GAA-3′) and a primerR: (5′-GGA TCC GCA TTA CCA TTA TAT ATA TTC ATG G-3′) with Pfu DNA polymerase, and the amplified fragments were cloned into BamH I site of pBIΔGR vector. All plasmids were confirmed by sequencing. All constructs mentioned earlier were transformed into the Agrobacterium tumefaciens GV3101 and subsequently into Arabidopsis. More than 45 transformed lines carrying each constructs were confirmed by PCR analysis.
GFP fusion protein analysis
Particle bombardment was conducted as described (Li et al. 2006b).
Trans-activation activity assays
Trans-activation activity assay was conducted as described (Li et al. 2006a). Full-length and various deletions of the AtMYB24 coding region were amplified by PCR using Pfu DNA polymerase (Takara, Japan) and the following appropriate primers:
-
AtMYB24 (1)-F: 5′-CGT CGA CAA ATG GAG AAA AGA GAA-3′
-
AtMYB24 (104)-F: 5′-TGT CGA CGA AGA ACC GAC AAT GAG-3′
-
AtMYB24 (191)-F: 5′-AGT CGA CTA TCT GTT GAT CAA TC -3′
-
AtMYB24 (190)-R: 5′-AGT CGA CCA TTA TCT GTT GAT CAA-3′
-
AtMYB24 (214)-R: 5′-GGC GCG TCG ACC TTA ATT ACC ATT-3′
RNA isolation, Northern analysis, and real-time PCR
Total RNA was isolated from 35-day-old Arabidopsis plants using Trizol solution (Tiangen, China). The Northern blots were conducted following the methods as described (Qin et al. 2005). A fragment in the AtMYB24 coding region were amplified and labeled by PCR using primers (5′-TCA TCA AGA GCG GAG AAA CGA C-3′ and 5′-TCA TGA TCG AAC CGG ATT CAG G-3′).
For reverse transcription, 5 μg of total RNA for each samples were digested with RNase-free DNase I (Roche, Switzerland) before they were used for reverse transcription according to the manufacturer's instructions (Invitrogen GmbH, Germany). Quantitative real-time PCR was conducted as described previously (Guo et al. 2006). Primers for the quantitative real-time PCR are as follows:
-
PAL1-F: 5′-CCG GTG TGA ATG CTA GTA GTG A-3′
-
PAL1-R: 5′-CCT TGG AGG AGA GTG TTG ATT C-3′
-
PAL2-F: 5′-GGA GAC TTC AAG AGC CGG TGT G-3′
-
PAL2-R: 5′-CCG GAG TAT CCT TGG AGA AGA G-3′
-
CHS-F: 5′-GTG AAC ACA TGA CCG ACC TCA A-3
-
CHS-R: 5′-GTA GTG CAG AAG ACG ACA TGA G-3′
-
DFR-F: 5′-CTA GCC TTA TCA CCG CGC TCT C-3′
-
DFR-R: 5′-TGT CCG TCA GCT TCT TGG AAC T-3′
-
F3H-F: 5′-CCT CGG ACT CAA GCG TCA CAC T-3′
-
F3H-R: 5′-TTA GAG TTC ACC ACG GCC TGA T-3′
-
4CL3-F: 5′-GAA CGA TCC AGA AGC CAC TTC A-3′
-
4CL3- R: 5′-CAA CAG CTG CAT CGG CAA TTG A-3′
-
ANS1-F: 5′-GCG TGG AAC ATC AAG TGA TCG T-3′
-
ANS1-R: 5′-GAG AAC GGT ACT CGT CGA ACC T-3′
-
ANS2-F: 5′-TTC TAC GAG GGC AAA TGG GTC A-3′
-
ANS2-R: 5′-AAC TTA GCC GGA GAC TCA ACA C-3′
-
C4H-F: 5′-CAA CAG CTG GAA GAA GCC TGAA-3′
-
C4H-R: 5′-AGA CTG TCC TGG AGG AGG AAG A-3′
-
CcoAOMT-F: 5′-GCT ACG TCA ACT TCC ATG AGA G-3′
-
CcoAOMT-R: 5′-GAC GTC TAC AGA GCG TGA TAC C-3′
-
COMT-F: 5′-GCT CCT TCT CAT CCT GGT ATT G-3′
-
COMT-R: 5′-TTG GTT GAG AGG CTT GAG TCT G-3′
-
AtGPT2-F: 5′-AGA CCA GAT TTC GCC GTT AAC T-3′
-
AtGPT2-R: 5′-ACT GCT TCG CCT GTG AGT AGA G-3′
-
UBQ10-F: 5′-TCC GGA TCA GCA GAG GCT TA-3′
-
UBQ10-R: 5′-TCA GAA CTC TCC ACC TCA AG-3
In situ hybridization
Inflorescences were fixed with formaldehyde and embedded in paraplast. The tissues were sliced into 8-μm-thick slices and mounted on poly-Lys-coated glass slides. The in situ hybridization was conducted following the methods described previously (Qu et al. 2003). The probe used for in situ hybridizations was the 300-bp C-terminal end of AtMYB24 coding region.
Light microscopy
For pollen analysis, pollen grains were mounted by Alexander's stain (Alexander 1969). For toluidine blue stain, Arabidopsis inflorescences were fixed in 4% glutaraldehyde in 12.5 mM of phosphate buffer (pH6.8) for 2 days. The inflorescences were embedded in Spurr resin (SPI-ChemTM) according to the manufacturer's instructions and sliced into 3 μm transverse sections. All slides were observed under bright field microscopy (OLYMPUS BX51, Japan) equipped with a CCD camera (Eastman Kodak, Rochester, NY).
Results
Characterization of AtMYB24 as a transcription factor
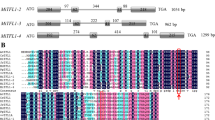
AtMYB24, which displays 68.5% sequence identity to that of AtMYB21, encodes an R2R3-MYB protein comprising 214 amino acids (Fig. 1A). Based on the conserved motifs outside the MYB domains, AtMYB24 was found sharing a W-MDDIW motif at their C-terminal regions with four other Arabidopsis members (AtMYB21, AtMYB57, AtMYB62, AtMYB116), two snapdragon proteins (AmMYB305 and AmMYB340), one protein from pea (PsMYB26) and three rice MYB proteins (9633.m04622, 9633.m00347, 9640.m03662) (Kranz et al. 1998; Li et al. 2006a).
A Amino acid alignment of AtMYB24 and other MYB proteins. B Subcellular localization of AtMYB24-GFP. Each image was inspected by both a fluorescence microscope (upper panels) and a differential–interference–contrast microscope (bottom panels). Bar=50 μm. C β-Galactosidase activity assay. Three independent transformants were shown. Empty pYF503 vector was used as the negative control, and pYF504, which harbors the full-length GAL4 gene, was used as the positive control (Ye et al. 2004)
To test whether AtMYB24 is localized to nuclei, we generated an AtMYB24-GFP fusion protein construct in which transient gene expression was driven by the strong CaMV35S promoter, and bombarded into onion epidermal cells. AtMYB24-GFP fusion protein was found exclusively localized in nuclear, as visualized by fluorescence microscopic and differential interference contrast (DIC) images (Fig. 1B). This result suggests that the AtMYB24 protein is targeted to the nuclei, consistent with the proposed role of AtMYB24 as a transcription factor.
Expression pattern of AtMYB24 in flowers. A RNA gel blot analysis of AtMYB24 in different organs. Fifteen micrograms of RNA samples were loaded per lane. B–G In situ hybridization of AtMYB24, using DIG-labeled anti-sense probe (B–F) and sense probe (G). B Inflorescences before floral stage 6. C Floral stages 7–8. D Floral stage 9. E Floral stages 11–12. F Floral stages 13–14. G Sense probe control. An, anther; Sp, sepal; T, tapetum; Msp, microspore; Ov, ovule; Cp, carpel; PG, pollen grain; P, petal. Bar=20 μm
To determine whether AtMYB24 acts as a transcriptional activator, we fused a series of deletion of AtMYB24 with the GAL4 DNA-binding domain and co-transformed them with the reporter vector (Ye et al. 2004). As shown in Fig. 1C, the entire AtMYB24 protein significantly activated the LacZ reporter gene expression in yeast cells, suggesting that AtMYB24 is a transcriptional activator. Furthermore, deletion of a 24-bp region including the W-MDDIW motif from the C-terminus completely abolished the trans-activation activity, indicating that this conserved motif is essentially important for the activation activity (Fig. 1C). This is consistent with the results of AtMYB21 (Li et al. 2006a). However, although the 110 amino acid residues at the C-terminus is with transcriptional activity and the region 104–190 amino acid has no activity, this 24-bp region alone is of no activation activity at all (Fig. 1C), suggesting that the region 104–190 amino acid is also required for the transactivation activity.
Phenotypes of AtMYB24-ox plants. A Relative expression level of AtMYB24 in AtMYB24-ox lines revealed by quantitative real-rime PCR. The number of y-axis indicates the relative expression level of AtMYB24 to that of wild-type. B A 7-week-old wild-type plant and two independent AtMYB24-ox plants. C Comparison of flowers and pollen grains of wild-type and the two AtMYB24-ox lines. Bar=0.5 mm. D Alexander's staining of pollens from wild-type and AtMYB24-ox lines. Pollen grains of wild-type are stained red inside and the outer wall is stained green (Alexander 1969). The abnormal pollen grains of AtMYB24-ox lines were stained green. Bar=20 μm
AtMYB24 is a flower-specific gene
To understand the expression characteristics of AtMYB24, we examined the expression of this gene in different tissues by Northern blots. The result showed that the transcripts of AtMYB24 were only detected in flowers, suggesting that AtMYB24 is a flower-specific gene (Fig. 2A).
To further determine the expression pattern of AtMYB24 during the flower development, in situ hybridization was adopted with a 300-bp coding region of AtMYB24 as the probe which shows low sequence homology to AtMYB21. The results showed that, in early flower development stages before floral stage 6 (Smyth et al. 1990), AtMYB24 is expressed in the primordia of the flower organs (Fig. 2B). From floral stages 7 and 8 to floral stages 9 and 10 (Smyth et al. 1990), high level expression of AtMYB24 was detected in male and female gametophytes, especially in microspores (Fig. 2C and 2D). At floral stages 11 and 12 (corresponding to anther stages 10 and 11), the expression of AtMYB24 was greatly reduced in the anthers (Fig. 2E). At about floral stages 13 and 14, transcripts of AtMYB24 were only detected in ovules but not in carpels (Fig. 2F). These results suggest that the expression of AtMYB24, although constitutively detected in the ovules, is tightly regulated with the development of anthers.
Over-expression of AtMYB24 causes dwarfism, pollen defects, and male sterility
To investigate the function of AtMYB24 in anther development, we generated transgenic plants over-expressing AtMYB24 under the control of a CaMV 35S promoter. Two independent homozygous lines, AtMYB24-ox-9 and AtMYB24-ox-16, were identified, in which the transcript level of AtMYB24 was elevated by 45- and 28-fold, respectively (Fig. 3A). These two lines were subjected to subsequent analysis.
As shown in Fig. 3B, the AtMYB24-ox lines exhibited dwarf phenotype, with severely decreased fertility, and the dwarf phenotype correlates with the expression levels of AtMYB24. The AtMYB24-ox plants had smaller flowers, with narrow petals and malformed carpels (Fig. 3C, top panel). Alexander staining of the anthers showed that large proportions of pollens (almost 100% for AtMYB24-ox-9 and 50% for AtMYB24-ox-16) were found aberrant in the AtMYB24-ox plants (Fig. 3C, bottom panel). Further analysis revealed that the aberrant pollens from both the over-expression lines were collapsed and devoid of cytoplasmic contents (Fig. 3D).
To further investigate the mechanism of sterility, we pollinated the stigmas of AtMYB24-ox plants with wild-type pollens. The result showed that the sterility phenotype of AtMYB24-ox-16 and AtMYB24-ox-9 plants were rescued by the wild-type pollens (Table 1), suggesting that the sterility phenotype was due to pollen defects of AtMYB24-ox lines. This is different from AtMYB21-ox lines, in which the sterile phenotype was not rescued either by pollinating the transgenic stigma with wild-type pollens, or by pollinating the wild-type stigma with transgenic pollens (Shin et al. 2002).
To examine at which developmental stage the pollens of the AtMYB24-ox plants become abnormal, we compared the anther development of the AtMYB24-ox lines with that of the wild-type plants. The result showed that no obvious difference was found until anther stage 9, when an exine wall was generated and microspores become vacuolated (Sander et al. 1999). At anther stages 10 and 11, mitotic divisions occur in pollens and tapetum degenerate in the wild-type plants (Sander et al. 1999). However, in the AtMYB24-ox plants, the pollen grains generated from microspore were devoid of cytoplasm and could not be stained by toluidine blue (Fig. 4). At anther stages 12 and 13 in wild-type plants (Sander et al. 1999), the septum was degenerated so that the anthers become bilocular, and the stomium of the anthers was broken so that pollens were released from the locules. At the same time, fibrous bands, which may help the anthers “flip open” at dehiscence (Sander et al. 1999), appear in the endothecium and connective cells of the anthers. However, in AtMYB24-ox, the septum and the stomium were not broken, and very few and even no fibrous bands were found in the anthers of AtMYB24-ox-16 and AtMYB24-ox-9 plants (Fig. 4). Interestingly, the septum, the stomium, and the endothecial cells underwent a cell-death program that is different from that of the wild-type plants. These aberrant pollens and anther abnormities ultimately resulted in anthers with non-dehiscent phenotype, leading to sterility of the AtMYB24-ox plants. Since, in wild-type plants, the expression of AtMYB24 was greatly reduced in the male gametophytes during anther stages 10 and 11 (Fig. 2E), the abnormal anther development of the AtMYB24-ox plants should be due to the over-expression of AtMYB24 at these stages.
To exclude the possibility that the phenotypes were the result of constitutive expression of AtMYB24, we generated transgenic plants over-expressing AtMYB24-GR fusion protein, in which, without DEX, the GR-fusion proteins would localize in the cytoplasm, whereas, when treated with DEX, the fusion protein would translocate into nucleus (Lloyd et al. 1994). Two independent homozygous lines, AtMYB24-GR-ox-1 and AtMYB24-GR-ox-9, were obtained, in which expression of AtMYB24 were elevated by 403- and 115-fold, respectively (Fig. 5A). In the absence of DEX, AtMYB24-GR-ox lines were indistinguishable from wild-type plants, suggesting that AtMYB24-GR was not functional (Figs. 5B and C). When the inflorescences of the 5-week-old plants were treated with DEX solution daily continuously for 10 days, the AtMYB24-GR-ox lines displayed smaller flower phenotype with narrower petals, aberrant anther development, and severe sterility, whereas wild-type plants grew normal. When treated with DEX, the phenotype severity of the AtMYB24-GR-ox lines was correlated with the expression level of the AtMYB24 (Figs. 5B and C). These data confirmed that AtMYB24 plays important roles in flower development, and that over-expression of AtMYB24 even for a short period could significantly affect normal flower development.
Comparison of flowers from wild-type and AtMYB24-GR-ox plants. A Relative expression level of AtMYB24 in AtMYB24-GR-ox lines revealed by quantitative real-rime PCR. The number of y-axis indicates the relative expression level of AtMYB24 to that of wild-type. B Flowers of wild-type and AtMYB24-GR-ox lines treated with DEX. Bar=0.2 mm C Pollen grains of wild-type and AtMYB24-GR-ox lines stained by Alexander's dye when treated with DEX. Bar=20 μm
Expression of genes involved in phenylpropanoid biosynthetic pathway and AtGTP2 were altered in AtMYB24-ox lines
It was reported that AmMYB305, AmMYB340, PsMYB26, and AtMYB21 could bind to the promoters and activate the expressions of several genes involved in phenylpropanoid biosynthetic pathway (Shin et al. 2002; Moyano et al. 1996; Uimari and Strommer 1997). To test whether AtMYB24 regulates genes in this pathway as well, we adopted quantitative real-time PCR to assess transcript levels of these genes both in AtMYB24-ox and wild-type flowers. As shown in Fig. 6, transcript levels of CHS (encoding chalcone synthase) and DFR (encoding dihydroflavonol 4-reductase) were substantially decreased, whereas those of PAL2, ANS1, and C4H were increased by 50% in AtMYB24-ox plants compared to the wild-type plants, suggesting that the phenylpropanoid biosynthetic pathway is affected. In the meantime, the expression of AtGPT2, coding for a glucose-6-phosphate/phosphate-translocator, was also significantly down-regulated by ninefold in AtMYB24-ox plants. Interestingly, the closely related homologue of this gene, AtGPT1, had been reported to be involved in the pollen maturation and embryo sac development in Arabidopsis (Niewiadomski et al. 2005). It would be interesting to investigate the mechanism that affects the normal development of anthers by regulating the phenylpropanoid biosynthetic pathway and other metabolic pathways by AtMYB24 in the future.
Discussion
AtMYB24 encodes a group I MYB protein. The genes in this group are all flower-specific, and the encoded proteins share a conversed W-MDDIW motif in Arabidopsis and Antirrhinum (Li et al. 2006a). For instance, AmMYB305 and AmMYB340 are found highly expressed in nectary, developing ovules and placental tissue (Moyano et al. 1996). The in situ hybridization result in this study presents a more detailed expression pattern of AtMYB24 in flowers. AtMYB24 is expressed in gynoecium from young flower buds to mature flowers, similar to those of AmMYB305 and AmMYB340 (Jackson et al. 1991) and expressed in anthers until anther stage 10, especially in microspores. In addition, the expression of AtMYB24 depends on anther development.
Anther dehiscence in Arabidopsis includes the endothecial cell expansion, degeneration of septum, stomium breaking, and releasing of pollen grains (Sander et al. 1999). Several Arabidopsis mutants that are reported have defects in anther dehiscence process. Some of these mutated genes, i.e., DELAYED DEHISCENCE1, DAD1, and AOS, are involved in jasmonic acid biosynthesis pathway, and mutants of these genes result in dehiscence-delayed anthers (Sanders et al. 2000; Ishiguro et al. 2001; von Malek et al. 2002; Park et al. 2002). Other mutants, e.g., atmyb26 mutant and nst1 nst2 double mutant, exhibit non-dehiscent anther phenotype (Steiner-Lange et al. 2003; Mitsuda et al. 2005). Nevertheless, all these mutants would have their septum degenerated and stomium broken and produced bilocular anthers from stage 12 onward. In AtMYB24-ox lines, breaking of septum and stomium did not occur, producing anthers with four locules through the development. This suggests that over-expression of AtMYB24 has greater affects on the anther dehiscence process at the earlier stages than do those mutants that are described above. It was reported that ectopic expression of PCS1 caused septum and stomium cells to survive but to die in wild-type anthers (Ge et al. 2005). It will be interesting to study whether AtMYB24 and PCS1 are involved in the same regulation route and, if so, whether they are interacted.
The expression level of CHS and DFR involved in phenylpropanoid metabolism pathway were down-regulated in flowers of AtMYB24-ox lines. Although CHS was found to be involved in pollen germination (Napoli et al. 1999), it was reported that atmyb32 mutants produced abnormal pollens devoid of cytoplasm and DFR was also down-regulated in the mutant (Preston et al. 2003), similar to the pollen phenotypes in AtMYB24-ox lines. Many products of phenylpropanoid metabolism pathway and its branch pathways are important components of pollen coat and precursors for sporopollenin, which are required for normal pollen development (Piffanelli et al. 1998), for example, knock-down the expression of PAL genes in tapetum resulted in pollen defects in tobacco (Matsuda et al. 1996). Changes in the expression of genes involved in these metabolism pathways due to the over-expression of AtMYB24 may account for the abnormal pollens and anther defects in those transgenic AtMYB24-ox lines. The evidences presented in this study suggest an important role of AtMYB24 in the development of anther in Arabidopsis.
Two atmyb24 mutants were obtained from ABRC (SALK_030452 and SALK_017221). Unfortunately, the phenotypes of these two mutants are indistinguishable with the wild-type plants (data not shown). It is probably due to the gene redundancy of AtMYB21 and/or other related MYB transcription factor genes, since AtMYB21-ox plants also exhibited malformed flower development and sterility (Shin et al. 2002). This is further supported by a recent report that atmyb21 atmyb24 double mutant displayed a more serious male sterile phenotype than atmyb21 did (Mandaokar et al. 2006). Although atmyb21 and atmyb24 double mutant had non-dehiscent anthers and closed petals, the anthers seemed to be morphologically normal up to stage 13 (Mandaokar et al. 2006). Considering the fact that both elevation of expression of either these two genes and simultaneous knock-out of these two genes would result in defects in anther development, we suggests that AtMYB24 and its homolog AtMYB21, partially redundant in their functions, play important roles in anther development, especially in anther dehiscence. Future work would be focused on dissecting the distinct function of AtMYB24 with AtMYB21 during anther development.
References
Alexander MP (1969) Differential staining of aborted and non-aborted pollen. Stain Technol 44:117–122
Chen YH, Yang XY, He K, Liu MH, Li JG, Lin ZQ, Zhang YF, Wang XX, Qiu XM, Shen YP, Zhang L, Deng XH, Luo JC, Deng XW, Chen ZL, Gu HY, Qu LJ (2006) The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol Biol 60:107–124
Ge X, Dietrich C, Matsuno M, Li G, Berg H, Xia Y (2005) An Arabidopsis aspartic protease functions as an anti-cell-death component in reproduction and embryogenesis. EMBO Rep 6:282–288
Guo L, Wang ZY, Lin H, Cui WE, Chen J, Liu MH, Chen ZL, Qu LJ, Gu HY (2006) Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res 16:277–286
Higginson T, Li SF, Parish RW (2003) AtMYB103 regulates tapetum and trichome development in Arabidopsis thaliana. Plant J 35:177–192
Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13:2191–2209
Jackson D, Culianez-Macia F, Prescott AG, Roberts K, Martin C (1991) Expression patterns of myb genes from Antirrhinum flowers. Plant Cell 3:115–125
Kranz HD, Denekamp M, Greco R, Jin H, Leyva A, Meissner RC, Petroni K, Urzainqui A, Bevan M, Martin C, Smeekens S, Tonelli C, Paz-Ares J, Weisshaar B (1998) Towards functional characterization of the members of the R2R3-MYB gene family from Arabidopsis thaliana. Plant J 16:263–276
Li JG, Yang XY, Wang Y, Li XJ, Gao ZF, Pei M, Chen ZL, Qu LJ, Gu HY (2006a) Two groups of MYB transcription factors share a motif which enhances trans-activation activity. Biochem Biophys Res Commun 341:1155–1163
Li JG, Li XJ, Guo L, Lu F, Feng XJ, He K, Wei LP, Chen ZL, Qu LJ, Gu HY (2006b) A subgroup of MYB transcription factor genes undergoes highly conserved alternative splicing in Arabidopsis and rice. J Exp Bot 57:1263–1273
Lloyd AM, Schena M, Walbot V, Davis RW (1994) Epidermal cell fate determination in Arabidopsis: patterns defined by steroid-inducible regulator. Science 266:436–439
Matsuda N, Tsuchlya T, Kishitani S, Tanaka Y, Toriyama K (1996) Partial male sterility in transgenic tobacco carrying antisense and sense PAL cDNA under the control of a tapetum specific promoter. Plant Cell Physiol 37:215–222
Mandaokar A, Thines B, Shin B, Markus Lange B, Choi G, Koo YJ, Yoo YJ, Choi YD, Choi G, Browse J (2006) Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J 46:984–1008
Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2005) The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 17:2993–3006
Moyano E, Martinez-Garcia JF, Martin C (1996) Apparent redundancy in myb gene function provides gearing for the control of flavonoid biosynthesis in Antirrhinum flowers. Plant Cell 8:1519–1532
Napoli CA, Fahy D, Wang HY, Taylor LP (1999) White anther: a petunia mutant that abolishes pollen flavonol accumulation, induces male sterility, and is complemented by a chalcone synthase transgene. Plant Physiol 120:615–622
Niewiadomski P, Knappe S, Geimer S, Fischer K, Schulz B, Unte US, Rosso MG, Ache P, Flügge UI, Schneider A (2005) The Arabidopsis plastic glucose 6-phosphate/phosphate translocator GPT1 is essential for pollen maturation and embryo sac development. Plant Cell 17:760–775
Pabo CO, Sauer RT (1992) Transcription factors: structure families and principles of DNA recognition. Annu Rev Biochem 61:1053–1095
Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31:1–12
Piffanelli P, Ross JHE, Murphy DJ (1998) Biogenesis and function of the lipidic structure of pollen grains. Sex Plant Reprod 11:65–80
Preston J, Wheeler J, Heazlewood J, Li SF, Parish RW (2003) AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J 40:979–995
Qin G, Gu H, Zhao Y, Ma Z, Shi G, Yang Y, Pichersky E, Chen H, Liu M, Chen Z, Qu LJ (2005) An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell 17:2693–2704
Qu LJ, Chen J, Liu M, Pan N, Okamoto H, Lin Z, Li C, Li D, Wang J, Zhu G, Zhao X, Chen X, Gu H, Chen Z (2003) Molecular cloning and functional analysis of a novel type of Bowman-Birk inhibitor gene family in rice. Plant Physiol 133:560–570
Riechmann JI, Heard J, Martin G, Reuber L, Jiang C, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, Creelman R, Pilgrim M, Broun P, Zhang JZ, Ghandehari D, Sherman BK, Yu G (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290:2105–2110
Sander PM, Bui A, Weterings K, Mcintire KN, Hsu YC, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, Goldberg RB (2000) The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12:1041–1061
Shin B, Choi G, Yi H, Yang S, Cho I, Kim J, Lee S, Paek NC, Kim JH, Song PS, Choi G (2002) AtMYB21, a gene encoding a flower-specific transcription factor, is regulated by COP1. Plant J 30:23–32
Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2:755–767
Steiner-Lange S, Unte US, Eckstein L, Yang C, Wilson ZA, Schmelzer E, Dekker K, Saedler H (2003) Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers. Plant J 34:519–528
Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4:447–456
Uimari A, Strommer J (1997) Myb26: a MYB-like protein of pea flowers with affinity for promoters of phenylpropanoid genes. Plant J 12:1273–1284
von Malek B, Van Der Graaff E, Schneitz K, Keller B (2002) The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 216:187–192
Ye R, Yao QH, Xu ZH, Xue HW (2004) Development of an efficient method for the isolation of factors involved in gene transcription during rice embryo development. Plant J 38:348–357
Acknowledgments
This study was supported by National Priority Basic Research Programs of People's Republic of China: Biosafety Study on GMOs of Agricultural Importance (GN 001CB10902 to Qu), National Natural Science Foundation of China (GN 30470358), and the Excellent Young Teachers Program of MOE, China (to Qu L-J). We thank Professor Hongwei Guo (Peking University) for valuable comments and Ms. Li Zhang and Professor Meihua Liu (Peking University) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. -H. Wu
Rights and permissions
About this article
Cite this article
Yang, X.Y., Li, J.G., Pei, M. et al. Over-expression of a flower-specific transcription factor gene AtMYB24 causes aberrant anther development. Plant Cell Rep 26, 219–228 (2007). https://doi.org/10.1007/s00299-006-0229-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0229-z