Abstract
Cell-wall invertase (CIN) catalyzes the hydrolysis of sucrose into glucose and fructose for the supply of carbohydrates to sink organs via an apoplastic pathway. To study the CIN genes in rice (Oryza sativa L.), we isolated cDNA clones showing amino acid similarity to the plant cell wall invertase proteins from a search of rice sequence databases. Profile analyses revealed that the cloned genes are expressed in unique patterns in various organs. For example, transcripts of OsCIN1, OsCIN2, OsCIN4, and OsCIN7 were detected in immature seeds whereas OsCIN3 gene expression was flower-specific. Further transcript analysis of these genes expressed in developing seeds indicated that OsCIN1, OsCIN2, and OsCIN7 might play an important role involving sucrose partitioning to the embryo and endosperm. Sucrose, a substrate of CINs, induced the accumulation of OsCIN1 transcripts in excised leaves and OsCIN2 in immature seeds, while the level of OsCIN5 was significantly down-regulated in excised leaves treated with sucrose. Infecting the tissues with rice blast (Magnaporthe grisea) as a biotic stressor increased the expression of OsCIN1, OsCIN4, and OsCIN5, suggesting that these genes may participate in a switch in metabolism to resist pathogen invasion. These results demonstrate that OsCIN genes play diverse roles involving the regulation of metabolism, growth, development, and stress responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most plants, assimilates, generally sucrose, are transported from the source leaves to the developing sink organs. Uptake in these sinks can occur either directly or through the cleavage of sucrose. Plants contain two types of enzymes capable of this cleavage: (1) sucrose synthase, which catalyzes a readily reversible reaction; and (2) invertase, which catalyzes the irreversible hydrolysis of sucrose to glucose and fructose (Copenald 1990).
The invertases of higher plants are classified according to their solubility, localization, and pH optima, and include three types of enzymes: cytoplasmic, vacuolar, and cell-wall. Vacuolar and cell-wall invertases (CINs) have similar enzymatic properties and two conserved sequence motifs, i.e. the β-fructosidase motif (NDPNG/A) and the cysteine catalytic site (Kim et al. 2000). These two classes of invertases can be reliably distinguished by a single amino acid difference in their cysteine catalytic sites (MWECP/V), with CINs having a proline residue in the sequence motif, and vacuolar invertases possessing a valine residue (Goetz and Roitsch 1999; Tymowska-Lalanne and Kreis 1998).
CINs are most active in the apoplast of sink organs, and can be involved in sucrose partitioning, control of cell differentiation and plant development, and responses to wounding and pathogen infection (Roitsch et al. 2003; Sturm 1999; Sturm and Tang 1999; Tymowska-Lalanne and Kreis 1998). This class of invertase has been isolated from several plant species, e.g. carrot (Lorentz et al. 1995; Unger et al. 1994), Arabidopsis (Tymowska-Lalanne and Kreis 1998), maize (Xu et al. 1996), potato (Maddison et al. 1999), tobacco (Goetz et al. 2001), tomato (Godt and Roitsch 1997), and Japanese pear (Hashizume et al. 2003). In the Arabidopsis genome, six putative CIN genes have been identified (Sherson et al. 2003), while four, Incw1–Incw4, have been reported in maize (Kim et al. 2000; Shanker et al. 1995; Taliercio et al. 1999). For example, the maize miniature-1 seed mutant, associated with aberrant pedicel and endosperm development, has decreased invertase activity in these organs and reduced expression of Incw2 (Cheng et al. 1996; Miller and Chourey 1992). Finally, two barley CINs, HvCWINV1 and HvCWINV2, have been isolated and shown to play important roles in controlling sugar ratios in maternal and filial tissues during the early development of caryopses (Weschke et al. 2003).
OsCIN1, a rice CIN gene, is active in developing seeds (Hirose et al. 2002). During the grain-filling stage in the caryopsis, its expression is detectable only at 1–4 days after flowering (DAF). In situ localization of 1-DAF caryopses has shown that OsCIN1 mRNA is expressed mainly in the maternal tissues. These results suggest that this gene has a critical role during the pre-storage phase, being involved in the proliferation of endosperm cells and longitudinal growth of immature seeds, rather than during the starch-filling phase. Therefore, it is most probable that the function of another invertase is required for the remaining activity after 5 DAF, when the caryopses are developing.
CIN activity can be increased by such phytohormones as auxins (Morris and Arthur 1984), gibberellic acids (Wu et al. 1993), cytokinins (Ehneß and Roitsch 1997), brassinosteroids (Goetz et al. 2000), and abscisic acid (Richings et al. 2000). In contrast, ethylene represses the expression of CIN genes (Linden et al. 1996). Activity of these genes is regulated by sugars (Godt and Roitsch 1997; Krausgrill et al. 1996; Tymowska-Lalanne and Kreis 1998) or by several stress-related factors, including pathogens (Hall and Williams 2000; Herbers et al. 2000; Sturm and Chrispeels 1990), wounding (Matsushita and Uritani 1974), osmotic stress (Wang et al. 2000), or cold (Balk and de Boer 1999).
In the present study, we sought to identify CIN genes from the recently published sequence of the rice genome. To do so, we isolated all their cDNA clones and grouped them with known genes to investigate an evolutionary relationship. Their genomic structures were then determined by comparing the cloned cDNAs with published genomic sequences. To understand the possible roles of CIN genes, we also investigated temporal and spatial expression of these cloned genes in various rice organs, including the leaves, roots, flowers, and developing seeds. Finally, we examined the responses of these rice CIN (OsCIN) genes to increased levels of sucrose as well as to pathogen attack.
Materials and methods
Plant materials
Greenhouse-grown japonica rice (Oryza sativa L.) variety Jinmi was used in all experiments, except for the rice blast tests, in which we infected 3-week-old leaves of variety RIL260. Immature seeds were harvested at different developing stages and flowers were also gathered prior to heading. The flag leaves of mature plants were collected to represent a source organ while roots were taken at the four-leaf seedling stage. All samples were frozen in liquid nitrogen and kept at −80°C until needed.
cDNA cloning
Full-length cDNAs of the OsCIN genes were isolated by RT–PCR analysis, using gene-specific primers encompassing the translation start codon and 3′-untranslated regions. Primer pairs included: for OsCIN1, 5′-TCTAGTACAAAACAATGGGGACTC-3′ and 5′-CGGAAAACCTCTTTATTATCTGTA-3′; for OsCIN2, 5′-CTCTCCTCTTCTCGCTCTCACTTC-3′ and 5′-TGACTGAAACCATTTTACACAAGG-3′; for OsCIN3, 5′-AGCGAGGTAAGTAGTGTGTTAGTG-3′ and 5′-GTGTTAAAACTGGACTTATTTTGG-3′; for OsCIN4, 5′-CAAGTTAACAAAGTCTTTCACACA-3′ and 5′-GATTTCCATCTCCTTTCTACAAGT-3′; for OsCIN5, 5′-AAAGTCTCGTCCTGTTCATCTTCT-3′ and 5′-TTTTATCCTGGGAATAAGGCAATA-3′; for OsCIN6, 5′-AATTTGCAAACTCTACTGTTTTGT-3′ and 5′-TGCTAAAAAGTCAATAGTGTCAAA-3′; and for OsCIN7, 5′-ACCAAACAGGCGTTTTCTTCAGAG-3′ and 5′-GCAAAACATATAAAGCACGTGACG-3′. The cDNAs synthesized with mRNA isolated from the leaf, root, flower, and immature seed were used in the PCR reactions.
Sequence alignment and phylogenetic tree construction
Deduced amino acid sequences of the rice CINs were aligned with reported genes from other species, using the CLUSTAL W program (Thompson et al. 1994). Phylogenetic and molecular evolutionary analyses were conducted with MEGA version 2.1 (Kumar et al. 2001). The accession numbers of the sequences used to construct the phylogenetic tree are: AtcwINV1–AtcwINV6 (NM_112232, NM_115120, NM_104385, NM_129177, NM_112231, NM_121230, respectively) from Arabidopsis; DcInv1 (M58362) and DcInv2 (X78424) from carrot; ZmIncw1–ZmIncw4 (U17695, AF050631, AF043346, AF043347, respectively) from maize; StInv1 (Z22645) from potato; Psbfruct1 (X85327) from pea; Ntbfruct1 (X81834) and NtNin88 (AF376773) from tobacco; and HvCWINV1 (AJ534447) and HvCWINV2 (AF155121) from barley.
Analysis of genomic structure and chromosomal location
The genomic structure of each OsCIN gene was determined by aligning the cDNA sequences and genomic sequences of BAC/PAC clones obtained from the NCBI database. The accession numbers of those clones used to identify structure and chromosomal location are: for OsCIN1, AP004156; for OsCIN2 and OsCIN3, AL662945; for OsCIN4, AP004365; for OsCIN5 and OsCIN6, AL606646; and for OsCIN7 and OsCIN8, AP005738. The OsCIN genes were located on a high-density genetic map (Wu et al. 2002), using the Integrated Rice Genome Explorer (INE) database (http://rgp.dna.affrc.go.jp/giot/INE.html), and the fingerprinted contig (FPC) map (http://www.genome.arizona.edu/fpc/rice)
RNA isolation and semiquantitative RT–PCR analysis
Total RNA was prepared from various organs using Trizol reagent (Invitrogen, http://www.invitrogen.com). The isolated RNA was reverse-transcribed with an oligo-dT primer and the First Strand cDNA synthesis kit for RT–PCR (Roche, http://www.roche-applied-science.com). First-strand cDNA was used in PCR reactions with gene-specific primers and control primers, including OsGBSSII (Dian et al. 2003), Act1 (McElroy et al. 1990), 18S rRNA (Kim et al. 2003), and the pathogenesis-related probenazole-inducible (PBZ1) genes (Midoh and Iwata 1996). Gene-specific primers were designed in the region encompassing at least one intron per gene to exclude any influence by genomic DNA contamination. These primers were: for OsCIN1, 5′-CGACCCTACCAAGTCTTCTCTTAG-3′ and 5′-CCCATTGTTGAAGACGTAAAGATG-3′; for OsCIN2, 5′-GAAGATATCTCTGAGGAGCCTGAT-3′ and 5′-TAGGCTCCATTCATCATGACC-3′); for OsCIN3, 5′-CATCAAGAAGGGCAACTACTTC-3′ and 5′-GTTGTTGAAGACGAAGAGGTG-3′; for OsCIN4, 5′-GAGATCAAGGGAACTGATACTTTA-3′ and 5′-CATGTACTTCTGCTCTGATTTGTA-3′; for OsCIN5, 5′-ATGGAAAGTACAAGGTCTTCATGT-3′ and 5′-ATTGTTGAACACGTACAGGTGACT-3′; for OsCIN6, 5′- ATTCGTGGACATAGACATAGAGAA-3′ and 5′-ATACAGGACCACCATACGAAAT-3′; for OsCIN7, 5′-AGGAGCAGGTCCAGAACGTC-3′ and 5′-CCGTTGTTGAACACGTACAAAT-3′; for OsGBSSII, 5′-TAGGTGTCACTGAATGGCTAGAAC-3′ and 5′-TGGCCCACATCTCTAAGTAACATC-3′; for Act1, 5′-GGAACTGGTATGGTCAAGGC-3′ and 5′-AGTCTCATGGATACCCGCAG-3′; for 18S rRNA, 5′-ATGATAACTCGACGGATCGC-3′ and 5′-CTTGGATGTGGTAGCCGTTT-3′; and for PBZ1, 5′-ACCATCTACACCATGAAGCTTAAC-3′ and 5′-GTATTCCTCTTCATCTTAGGCGTA-3′. In the PCR analysis of seed coat and endosperm, each total cDNA isolated by mass in vivo excision from seed coat- and endosperm-specific libraries was used. These cDNA libraries were made with mRNA from the seed coats and endosperm of 6- to 10-DAF rice (Jun et al. 2004; S.-H. Jun et al., unpublished data). For PCR, the amplification program consisted of an initial 94°C for 5 min followed by 28–35 cycles of 94°C, for 1 min; 56°C, for 1 min; 72°C, for 1 min, and a final extension at 72°C for 5 min. Experiments were repeated at least 3 times to obtain similar results.
Sucrose treatment
Seedlings at the four-leaf stage were maintained for 48 h under dark conditions to deplete their endogenous sugars. Leaves were then excised in 1-cm portions and treated in the dark at 28°C with MS media containing either 175 mM sucrose or mannitol (Dian et al. 2003).
Rice blast infection
We inoculated 3-week-old rice leaves with Magnaporthe grisea avirulent isolate PO6-6, according to the method of Jeon et al. (2003). These infected leaves were then collected periodically for RT–PCR analysis.
Results
cDNA cloning of rice cell wall invertase genes
To identify all the CIN genes in the rice genome, we carried out systematic BLAST searches of its published sequence (Feng et al. 2002; Goff et al. 2002; Sasaki et al. 2002; Yu et al. 2002), using the nucleotide and amino acid sequences of the rice OsCIN1 gene (Hirose et al. 2002), maize Incw2 (Cheng et al. 1996; Miller and Chourey 1992), and Arabidopsis CINs (Sherson et al. 2003). Eight putative CIN genes in rice were identified as OsCIN2–OsCIN8 to follow the previously isolated rice CIN gene OsCIN1. Full-length cDNAs of seven of these OsCIN genes were isolated by RT–PCR analysis using gene-specific primers that encompassed the translation start codon and 3′-untranslated regions. Endogenous transcript for the OsCIN8 gene in the various samples, including the sucrose-treated and the rice blast-infected tissues, was not detectable, indicating that the gene is inactive in the tissues examined.
Based on our CLUSTAL W alignment of the deduced amino acid sequences, all the identified OsCIN genes showed two conserved sequence domains—the β-fructosidase motif (NDPNG/A) and the cysteine catalytic site (Kim et al. 2000)—and all possessed a proline residue in the latter (Fig. 1). Therefore, we grouped these OsCIN genes in the classification of CINs.
Comparison of deduced amino acid sequences for rice cell-wall invertases (CINs). Conserved β-fructosidase motifs, cysteine catalytic sites, and conserved glycosylation sites are shown in shaded regions and are underlined. Bold letters indicate the proline residue of the cysteine catalytic site. Identical and conserved residues are marked with asterisks and dots/colons, respectively. Accession numbers of OsCIN cDNAs and one gene are: for OsCIN1, AY578158; for OsCIN2, AY578159; for OsCIN3, AY578160; for OsCIN4, AY578161; for OsCIN5, AY578162; for OsCIN6, AY578163; for OsCIN7, AY578164; and for OsCIN8, AY578165
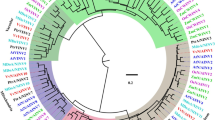
Phylogenetic and molecular evolutionary analysis
To establish the phylogenetic relationship of these OsCIN genes with CIN genes from different plant species, we constructed an unrooted phylogenetic tree using the neighbour-joining method (Fig. 2). This phylogenetic tree displayed four major groups: monocot-I, monocot-II, dicot-I and dicot-II. The OsCIN1–OsCIN3 genes belonged to monocot group I along with maize ZmIncw1–ZmIncw3 and barley HvCWINV1. The group IV genes, OsCIN4–OsCIN7 and OsCIN8, could be classified with other monocot-II genes, such as maize ZmIncw4 and barley HvCWINV2.
Genomic structure and chromosomal location of OsCIN genes
The inferred gene structure (Fig. 3) of the eight OsCIN genes was determined by aligning the cDNA sequences and genomic sequences of the BAC/PAC clones obtained from the NCBI database. Interestingly, all the second exons of our genes were 9-bp long. These exons are the smallest known to function among plant species, and encode three amino acids (DPN) of the β-fructosidase motif (NDPNG/A). Furthermore, they are highly conserved in both monocots and dicots, indicating that this 9-bp exon is a prerequisite for invertase activity.
The genomic sequence and structure of OsCIN8 was quite similar to those of OsCIN7, with both containing a comparatively larger second intron, i.e. 3.1 kb in OsCIN7 and 6.1 kb in OsCIN8. The latter gene has an insertion of a putative transposable element similar to the maize transposon MuDR within the second intron, which may cause inactivation of OsCIN8. That gene has only four exons for the deletion of a region corresponding to a portion of Exon 3, Intron 3, and Exon 4 of the OsCIN7 gene.
The chromosomal location of the OsCIN genes was based on a high-density genetic map (http://rgp.dna.affrc.go.jp; Wu et al. 2002). We used INE, a database integrating a genetic map, physical map, and sequencing information of the rice genome (Sakata et al. 2000), and the rice FPC map (Chen et al. 2002). Here, OsCIN4 was mapped to chromosome 1; OsCIN1 to chromosome 2; OsCIN2, OsCIN3, OsCIN5, and OsCIN6 to chromosome 4; and OsCIN7 and OsCIN8 to chromosome 9 (Fig. 4). OsCIN2 and OsCIN3, OsCIN5 and OsCIN6, and OsCIN7 and OsCIN8 were each located in pairs, which suggests that the OsCIN genes may have been duplicated during the evolution of the rice CIN genes. This hypothesis is further supported by the fact that each of the two linked genes in a pair retains a relatively higher similarity.
Expression profile analysis of OsCIN genes
Spatial expression of the OsCIN genes was examined by RT–PCR, using specific primers. Transcripts were detected in particular patterns within the various organ types, i.e. leaf, root, flower, and immature seed (Fig. 5a). OsCIN1, OsCIN4, and OsCIN7 were expressed in all tested organs, including a relatively low level measured in the seeds. OsCIN1 and OsCIN7 exhibited similar patterns. OsCIN2 transcript was not detected in the leaves, whereas OsCIN5 and OsCIN6 were expressed in the leaves and roots and in the roots and flowers, respectively. Four of the genes, OsCIN1, OsCIN2, OsCIN4, and OsCIN7, were expressed in the immature seeds while OsCIN3 was flower-specific. OsCIN8 gene was not expressed in any organs.
Expression profiles of OsCIN genes. For each gene, transcript levels in different tissue samples are comparable but the comparison of transcript levels among genes is not appropriate in the PCR reactions. a Spatial expression patterns of OsCIN genes in rice tissues, including leaf (L), root (R), flower (F), and immature seeds (S) mixed from 1- to 12-days after flowering (DAF) caryopses. b Expression patterns of OsCIN genes in endosperm and seed coat. c Changes in gene expression levels of OsCIN1, OsCIN2, OsCIN4 and OsCIN7 in rice seeds during development. Rice seeds were harvested over a time course of 1–15 DAF
Because CIN genes are thought to play an important role during seed development in rice and barley (Hirose et al. 2002; Weschke et al. 2003), we further analysed the four genes, OsCIN1, OsCIN2, OsCIN4 and OsCIN7, that were expressed in immature seeds. Three of these—OsCIN1, OsCIN4, and OsCIN7—were expressed specifically in the seed coats, while transcript of OsCIN2 was detected in both endosperms and seed coats (Fig. 5b). Our experiment also confirmed that OsCIN1 mRNA is localized mainly in the maternal tissues of the developing caryopsis (Hirose et al. 2002).
RT–PCR analysis was also performed using a series of seeds collected at different development stages (1–15 DAF) as well as from the ovaries prior to pollination (Fig. 5c). OsCIN1 was highly expressed in the ovary and at the very early developmental stage of the seed (1–2 DAF). Levels of OsCIN1 transcript dramatically decreased during 5–8 DAF and were weak thereafter. The expression pattern for OsCIN1 was similar to that previously reported by Hirose et al. (2002). Likewise, the expression of OsCIN4 and OsCIN7 was lowest during 5–8 DAF. OsCIN7 transcripts levels were high in the starch-filling phase (9–15 DAF), suggesting that this gene may play a role in the sucrose uptake to the endosperm during that phase rather than in the pre-storage phase. In contrast, expression of OsCIN2 was highest at 1–4 DAF and decreased only slowly afterwards. Notably, this gene maintained a relatively steady level of expression from 5 to 8 DAF, during which time transcripts of OsCIN1, OsCIN4, and OsCIN7 were barely detectable. Therefore, we believe that the OsCIN2 gene has a role distinct from that of the other three genes during endosperm development and longitudinal growth of the seed coat.
Effects of sucrose on expression of OsCIN genes
To test the effects of sucrose on OsCIN gene expression, excised leaves were treated with 175 mM of either sucrose or mannitol. We included this mannitol treatment in order to eliminate the usual induction caused by osmotic stress. RT–PCR analysis for four genes, OsCIN1, OsCIN4, OsCIN5, and OsCIN7, expressed in leaf samples demonstrated that the transcript level of OsCIN1 was significantly increased by sucrose treatment, while that of OsCIN5 was down-regulated (Fig. 6a). This result indicates that OsCIN1 may be involved in catalyzing the hydrolysis of sucrose into glucose and fructose for the supply of carbohydrates. Furthermore, the reduced expression of OsCIN5 because of a higher sucrose concentration suggests that the role of this gene may vary from one of sucrose partitioning.
Effects of sucrose on expression of OsCIN genes. Total RNAs collected after 12 and 24 h were used in the PCR reaction. a RT–PCR analysis of excised leaves supplied with sugars. b RT–PCR analysis of rice caryopses supplied with sugars. One- to 2-DAF and 9- to 10-DAF caryopses were treated with sugars. MS MS media, MS+S MS media+175 mM sucrose, MS+M MS media+175 mM mannitol
We also examined the effects of sucrose on gene expression in caryopses at two different stages: 1–2 DAF and 9–10 DAF (Fig. 6b). Our profile analysis showed that sucrose significantly induced expression of OsCIN2 at both immature stages whereas OsCIN1, OsCIN4, and OsCIN7 were not affected. Therefore, we postulate that OsCIN2 plays a predominant role in establishing metabolic sink strength during seed development. The fact that mannitol at the same molarity as used for sucrose had no influence on OsCIN activity implies that the change in expression caused by sucrose treatment was not a result of osmotic stress. Dian et al. (2003) have also reported that the expression of OsGBSSII, when used as a positive control, can be induced by such sucrose treatment.
Response of OsCIN genes to infection by Magnaporthe grisea
CIN genes may be directly involved in various plant-pathogen interactions (Hall and Williams 2000; Herbers et al. 2000; Sturm and Chrispeels 1990). In fact, we found considerable EST clones corresponding to OsCIN1 and OsCIN4 from cDNA libraries of leaves infected with rice blast (data not shown). Therefore, to determine whether expression in the OsCIN gene family is affected by pathogen attack, we examined four genes, OsCIN1, OsCIN4, OsCIN5, and OsCIN7, that were expressed in leaf samples (Fig. 7). Here, transcript levels of OsCIN1 rapidly increased at 4 h after blast infection. In contrast, OsCIN4 transcripts rose slightly 4 h after infection, peaked at 24 h, and then slowly declined to a low level. While OsCIN5 expression reached a maximum level at 48 h after infection, that of OsCIN7 showed no remarkable change after such treatment. Transcript of the pathogen-inducible gene PBZ1 was significantly increased at 24 h after rice blast infection while the Act1 gene, used as a PCR control, did not alter its expression in response to the inoculation. Therefore, we believe that three OsCIN genes—OsCIN1, OsCIN4, and OsCIN5—may be involved in regulating carbon metabolism so that rice plants are enabled to resist disastrous pathogen attacks.
Discussion
Identification of the rice CIN gene family
In the present study, we identified and characterized the OsCIN genes, members of a rice CIN gene family. Analysis of the deduced amino acid sequences predicted from OsCIN cDNAs revealed that they all contain the highly conserved β-fructosidase motifs and cysteine catalytic sites. These genes possess a proline in the fifth residue of the cysteine catalytic site, the presence of which is a reliable, distinctive indicator of the CINs (Goetz and Roitsch 1999). Therefore, we conclude that the genes isolated here are of the rice CIN type.
Our phylogenetic tree revealed that plant CIN are classified into four groups: monocot-I, monocot-II, dicot-I, and dicot-II. This suggests that each of the two types of monocot or dicot plants were delineated early, prior to the monocot–dicot divergence.
We used the sequenced BAC/PAC clones to locate the OsCIN genes on the rice chromosomes, and observed a number of transposable elements within the BAC/PAC clones encompassing these genes (data not shown). From this we could infer that the regions surrounding the OsCIN genes are the preferred target loci of transposable elements.
Diverse possible functions of OsCIN genes
In the profile analysis, we identified four genes—OsCIN1, OsCIN2, OsCIN4, and OsCIN7—that are expressed in immature seeds and which may have a role in carbon metabolism during seed development. Among them, OsCIN1, OsCIN4, and OsCIN7 were expressed in the seed coats, while OsCIN2 was expressed in both endosperms and seed coats. Our temporal examination revealed that these four genes were highly expressed in the ovaries or immature seeds, e.g. at 1–2 DAF (OsCIN1), 1–4 DAF (OsCIN2), and 9–15 DAF (OsCIN7). In addition, the high level of transcript for OsCIN2 was maintained even at 5–8 DAF. This suggests that each gene has a distinctive role in carbon partitioning during rice seed development.
Similar expression patterns have been reported for OsCIN1 in various organs, such as the roots, sink-leaves, source-leaves, and panicles (Hirose et al. 2002). Our data were broadly consistent with those previous results, except that OsCIN1 continued its, albeit weak, expression to 9–15 DAF. This may have been because we used a more sensitive RT–PCR analysis, rather than the Northern blot analysis conducted in that previous study. We also cannot exclude the possibility that the japonica rice genotype Jinmi tested in our study may have shown a slightly different expression during the seed development phase. As previously stated, we believe that OsCIN1 plays an important role during the pre-storage phase, where it is involved in the proliferation of endosperm cells and the longitudinal growth of immature seeds, rather than during the starch-filling phase.
Two CINs, HvCWINV1 and HvCWINV2, have been reported in barley (Weschke et al. 2003). These genes control sugar ratios in maternal and filial tissues during the early development of caryopses. In particular, HvCWINV1 is critical for the development and differentiation of endosperm during the pre-storage phase. Its expression is found in both maternal and filial tissues, such as the endosperm and seed coat, whereas HvCWINV2 is almost entirely seed coat-specific. HvCWINV1 shares high homology and similar expression patterns with OsCIN2 and ZmIncw2 rather than with OsCIN1.
The maize miniature-1 mutant, which manifests loss-of-function by the ZmIncw2 gene, shows decreased invertase activity in the pedicel and endosperm, resulting in their aberrant development (Cheng et al. 1996; Miller and Chourey 1992). Our expression profile and phylogenetic analyses suggest that the OsCIN2 gene plays an important role in endosperm development and longitudinal growth of seed coats, similar to the function of HvCWINV1 and ZmIncw2. Its expression in both organs of rice further supports the hypothesis that OsCIN2 shares a similar function with barley HvCWINV1 and maize ZmIncw2.
Hirose et al. (2002) have reported that CIN activity is maintained at a considerably high level after 5 DAF. Here, we also demonstrated that OsCIN2, OsCIN4, and OsCIN7 contributed, in part, to the activity of invertase after 5 DAF. In particular, OsCIN7 was highly expressed during 9–15 DAF, suggesting that it has a major role in starch-filling, rather than pre-storage, for sucrose uptake to the endosperm during seed development.
The OsCIN3 gene is expressed exclusively in the flowers. Such floral-specific CIN genes have also been identified in other species, such as tobacco (Goetz et al. 2001), Arabidopsis (Tymowska-Lalanne and Kreis 1998), and maize (Xu et al. 1996). Goetz et al. (2001) have reported that the CIN Nin88 of tobacco has a critical role in pollen development, and that expression of the Nin88 antisense construct induces male sterility. The importance of CIN during pollen development has been supported by the identification of an anther-specific monosaccharide transporter (Truernit et al. 1999; Ylstra et al. 1998). Therefore, we suggest that OsCIN3 may play a role during the development of pollen with that transporter. However, it remains to be determined whether the gene is expressed specifically in rice anthers.
Regulation of OsCIN genes by sucrose and pathogens
Sugar, especially sucrose, serves not only as the carbon source for metabolism during plant growth and development, but also as a signalling molecule that regulates the expression of genes involved in photosynthesis and heterotrophic metabolism (Koch 1996; Roitsch 1999; Sheen et al. 1999). Induction of CIN genes by sucrose has been described for several species, including tobacco (Krausgrill et al. 1996), Arabidopsis (Tymowska-Lalanne and Kreis 1998), and tomato (Godt and Roitsch 1997). In the present study, the expression level of OsCIN2 was significantly increased when caryopses were treated with sucrose, which leads us to believe that this gene has a function in carbohydrate partitioning between source and sink organs as well as in establishing metabolic sink strength during seed development. In contrast, OsCIN1 was only slightly increased in sucrose-treated, excised leaves, while OsCIN2 showed no sucrose-inducible expression in leaf samples (data not shown). Therefore, because transcript was not present in our untreated rice leaves, it is unlikely that OsCIN2 has a role in that organ.
Expression of CIN genes is markedly increased by wounding (Matsushita and Uritani 1974) or by infection with fungi (Benhamou et al. 1991; Hall and Williams 2000), bacteria (Sturm and Chrispeels 1990), or viruses (Herbers et al. 2000). Among the EST clones detected in our rice blast-infected leaves, we noted that some contained partial sequences corresponding to OsCIN1 and OsCIN4. Rice blast, caused by Magnaporthe grisea, is one of the most destructive rice fungal diseases. Here, we identified three genes—OsCIN1, OsCIN4, and OsCIN5—whose expression was enhanced after inoculation. This suggests that these genes, especially OsCIN1 and OsCIN4 (which responded within 4 h of treatment), may be directly involved in the plant–pathogen interaction of switching from a housekeeping to a defence metabolism. The role of CINs in the plant–pathogen interaction remains to be unravelled. Recent studies have reported that sugar transporters play an important role in certain plant–microorganism interactions to fulfil an increased demand for carbohydrate by cells under various biotic or abiotic stress conditions (Hall and Williams 2000; Williams et al. 2000). Therefore, together with sugar transporters, CINs may help increase carbohydrate import into infected tissues.
In summary, we have now isolated OsCIN genes that are expressed in various rice tissues and which respond in unique patterns to sucrose and fungal stressors. We believe that these genes may function in the regulation of metabolism, growth, development, and stress responses. Therefore, it will be invaluable to determine what roles they play in gain-of-function and loss-of-function in rice plants.
References
Balk PA, de Boer D (1999) Rapid stalk elongation in tulip (Tulipa gesneriana L. cv. Apeldoorn) and the combined action of induced invertase and the water-channel protein r-TIP. Planta 209:346–354
Benhamou N, Grenier J, Chrispeels MJ (1991) Accumulation of β-fructosidase in the cell walls of tomato roots following infection by a fungal wilt pathogen. Plant Physiol 97:739–750
Chen M, Presting G, Barbazuk WB, Goicoechea JL, Blackmon B, Fang G, Kim H, Frisch D, Yu Y, Sun S, Higginbottom S, Phimphilai J, Phimphilai D, Thurmond S, Gaudette B, Li P, Liu J, Hatfield J, Main D, Farrar K, Henderson C, Barnett L, Costa R, Williams B, Walser S, Atkins M, Hall C, Budiman MA, Tomkins JP, Luo M, Bancroft I, Salse J, Regad F, Mohapatra T, Singh NK, Tyagi AK, Soderlund C, Dean RA, Wing RA (2002) An integrated physical and genetic map of the rice genome. Plant Cell 14:537–545
Cheng WH, Taliercio EW, Chourey PS (1996) The miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 8:971–983
Copenald L (1990) Enzyme of sucrose metabolism. Methods Plant Biochem 3:73–85
Dian W, Jiang H, Chen Q, Liu F, Wu P (2003) Cloning and characterization of the granule-bound starch synthase II gene in rice: gene expression is regulated by the nitrogen level, sugar and circadian rhythm. Planta 218:261–268
Ehneß R, Roitsch T (1997) Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J 11:539–548
Feng Q, Zhang Y, Hao P, Wang S, Fu G, Huang Y, Li Y, Zhu J, Liu Y, Hu X, Jia P, Zhang Y, Zhao Q, Ying K, Yu S, Tang Y, Weng Q, Zhang L, Lu Y, Mu J, Lu Y, Zhang LS, Yu Z, Fan D, Liu X, Lu T, Li C, Wu Y, Sun T, Lei H, Li T, Hu H, Guan J, Wu M, Zhang R, Zhou B, Chen Z, Chen L, Jin Z, Wang R, Yin H, Cai Z, Ren S, Lu G, Gu W, Zhu G, Tu Y, Jia J, Zhang Y, Chen J, Kang H, Chen X, Shao C, Sun Y, Hu Q, Zhang X, Zhang W, Wang L, Ding C, Sheng H, Gu J, Chen S, Ni L, Zhu F, Chen W, Lan L, Lai Y, Cheng Z, Gu M, Jiang J, Li J, Hong G, Xue Y, Han B (2002) Sequence and analysis of rice chromosome 4. Nature 420:316–320
Godt DE, Roitsch T (1997) Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol 115:273–282
Goetz M, Roitsch T (1999) The different pH optima and substrate specificities of extracellular and vacuolar invertases from plants are determined by a single amino-acid substitution. Plant J 20:707–711
Goetz M, Godt DE, Roitsch T (2000) Tissue-specific induction of the mRNA for an extracellular invertase isoenzyme of tomato by brassinosteroids suggests a role for steroid hormones in assimilate partitioning. Plant J 22:515–522
Goetz M, Godt DE, Guivarc’h A, Kahmann U, Chriqui D, Roitsch T (2001) Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc Natl Acad Sci USA 98:6522–6527
Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, Hadley D, Hutchison D, Martin C, Katagiri F, Lange BM, Moughamer T, Xia Y, Budworth P, Zhong J, Miguel T, Paszkowski U, Zhang S, Colbert M, Sun WL, Chen L, Cooper B, Park S, Wood TC, Mao L, Quail P, Wing R, Dean R, Yu Y, Zharkikh A, Shen R, Sahasrabudhe S, Thomas A, Cannings R, Gutin A, Pruss D, Reid J, Tavtigian S, Mitchell J, Eldredge G, Scholl T, Miller RM, Bhatnagar S, Adey N, Rubano T, Tusneem N, Robinson R, Feldhaus J, Macalma T, Oliphant A, Briggs S (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. japonica) . Science 296:92–100
Hall JL, Williams LE (2000) Assimilate transport and partitioning in fungal biotrophic interactions. Aust J Plant Physiol 27:549–560
Hashizume H, Tanase K, Shiratake K, Mori H, Yamaki S (2003) Purification and characterization of two soluble acid invertase isozymes from Japanese pear fruit. Phytochemistry 63:125–129
Herbers K, Takahata Y, Melzer M, Mock HP, Hajirezaei M, Sonnewald U (2000) Regulation of carbohydrate partitioning during the interaction of potato virus Y with tobacco. Mol Plant Pathol 1:51–59
Hirose T, Takano M, Terao T (2002) Cell wall invertase in developing rice caryopsis: molecular cloning of OsCIN1 and analysis of its expression in relation to its role ingrain filling. Plant Cell Physiol 43:452–459
Jeon JS, Chen D, Yi GH, Wang GL, Ronald PC (2003) Genetic and physical mapping of Pi5(t), a locus associated with broad-spectrum resistance to rice blast. Mol Genet Genomics 269:280–289
Jun SH, Han MJ, Lee S, Seo YS, Kim WT, An G (2004) OsEIN2 is a positive component in ethylene signaling in rice. Plant Cell Physiol 45:281–289
Kim BR, Nam HY, Kim SU, Kim SI, Chang YJ (2003) Normalization of reverse transcription quantitative-PCR with housekeeping genes in rice. Biotechnol Lett 25:1869–1872
Kim JY, Mahe A, Guy S, Brangeon J, Roche O, Chourey PS, Prioul JL (2000) Characterization of two members of the maize gene family, Incw3 and Incw4, encoding cell-wall invertases. Gene 245:89–102
Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47:509–540
Krausgrill S, Sander A, Greiner S, Weil M, Rausch T (1996) Regulation of cell-wall invertase by a proteinaceous inhibitor. J Exp Bot 47:1193–1198
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245
Linden JC, Ehneß R, Roitsch T (1996) Regulation by ethylene of apoplastic invertase expression in Chenopodium rubrum tissue culture cells. Plant Growth Regul 19:219–222
Lorentz K, Lienhard S, Sturm A (1995) Structural organization and differential expression of carrot ß-fructofuranosidase genes: identification of a gene coding for a flower bud-specific isoenzyme. Plant Mol Biol 28:189–194
Maddison AL, Hedeley PE, Meyer RC, Aziz N, Davidson D, Machray GC (1999) Expression of tandem invertase genes associated with sexual and vegetative growth cycles in potato. Plant Mol Biol 41:741–751
Matsushita K, Uritani I (1974) Change in invertase activity of sweet potato in response to wounding and purification and properties of its invertases. Plant Physiol 54:60–66
McElroy D, Zhang W, Cao J, Wu R (1990) Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2:163–171
Midoh N, Iwata M (1996). Cloning and characterization of a probenazole-inducible gene for an intracellular pathogenesis-related protein in rice. Plant Cell Physiol 37:9–18
Miller ME, Chourey PS (1992) The maize invertase-deficient miniature-1 seed mutation is associated with aberrant pedicel and endosperm development. Plant Cell 4:297–305
Morris DA, Arthur ED (1984) Invertase and auxin-induced elongation in internodal segments of Phaseolus vulgaris. Phytochemistry 23:2163–2167
Richings EW, Cripps RF, Cowan AK (2000) Factors affecting ‘Hass’ avocado fruit size: carbohydrate, abscisic acid and isoprenoid metabolism in normal and phenotypically small fruit. Physiol Plant 109:81–89
Roitsch T (1999) Source—sink regulation by sugars and stress. Curr Opin Plant Biol 2:198–206
Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK (2003) Extracellular invertase: key metabolic enzyme and PR protein. J Exp Bot 54:513–524
Sakata K, Antonio BA, Mukai Y, Nagasaki H, Sakai Y, Makino K, Sasaki T (2000) INE: a rice genome database with an integrated map view. Nucleic Acids Res 28:97–101
Sasaki T, Matsumoto T, Yamamoto K, Sakata K, Baba T, Katayose Y, Wu J, Niimura Y, Cheng Z, Nagamura Y, Antonio BA, Kanamori H, Hosokawa S, Masukawa M, Arikawa K, Chiden Y, Hayashi M, Okamoto M, Ando T, Aoki H, Arita K, Hamada M, Harada C, Hijishita S, Honda M, Ichikawa Y, Idonuma A, Iijima M, Ikeda M, Ikeno M, Ito S, Ito T, Ito Y, Ito Y, Iwabuchi A, Kamiya K, Karasawa W, Katagiri S, Kikuta A, Kobayashi N, Kono I, Machita K, Maehara T, Mizuno H, Mizubayashi T, Mukai Y, Nagasaki H, Nakashima M, Nakama Y, Nakamichi Y, Nakamura M, Namiki N, Negishi M, Ohta I, Ono N, Saji S, Sakai K, Shibata M, Shimokawa T, Shomura A, Song J, Takazaki Y, Terasawa K, Tsuji K, Waki K, Yamagata H, Yamane H, Yoshiki S, Yoshihara R, Yukawa K, Zhong H, Iwama H, Endo T, Ito H, Hahn JH, Kim HI, Eun MY, Yano M, Jiang J, Gojobori T (2002) The genome sequence and structure of rice chromosome 1. Nature 420:312–316
Shanker S, Salazar RW, Taliercio EW, Chourey PS (1995) Cloning and characterization of full-length cDNA encoding cell-wall invertase from maize. Plant Physiol 108:873–874
Sheen J, Zhou L, Jang JC (1999) Sugars as signaling molecules. Curr Opin Plant Biol 2:410–418
Sherson SM, Alford HL, Forbes SM, Wallace G, Smith SM (2003) Roles of cell wall invertase and monosaccharide transporter in the growth and development of Arabidopsis. J Exp Bot 54:525–531
Sturm A (1999) Invertase. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol 121:1–7
Sturm A, Chrispeels MJ (1990) cDNA cloning of carrot extracellular ß-fructosidase and its expression in response to wounding and bacterial infection. Plant Cell 2:1107–1119
Sturm A, Tang GQ (1999) The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci 4:401–407
Taliercio EW, Kim JY, Mahé A, Shanker S, Choi J, Cheng WH, Prioul JL, Chourey PS (1999) Isolation, characterization and expression analyses of two cell wall invertase genes in maize (Incw1 and Incw2). J Plant Physiol 155:197–204
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Truernit E, Stadler R, Baier K, Sauer N (1999) A male gametophyte-specific monosaccharide transporter in Arabidopsis. Plant J 17:191–201
Tymowska-Lalanne Z, Kreis M (1998) Expression of the Arabidopsis thaliana invertase gene family. Planta 207:259–265
Unger C, Hardegger M, Lienhard S, Sturm A (1994) cDNA cloning of carrot (Daucus carota) soluble acid β-fructofuranosidases and comparison with the cell wall isoenzyme. Plant Physiol 104:1351–1357
Wang HL, Lee PD, Chen WL, Huang DJ, Su JC (2000) Osmotic stress-induced changes of sucrose metabolism in cultured sweet potato cells. J Exp Bot 51:1991–1999
Weschke W, Panitz R, Gubatz S, Wang Q, Radchuk R, Weber H, Wobus U (2003) The role of invertases and hexose transporters in controlling sugar ratios in maternal and filial tissues of barley caryopses during early development. Plant J 33:395–411
Williams LE, Lemoine R, Sauer N (2000) Sugar transporters in higher plants—a diversity of roles and complex regulation. Trends Plant Sci 5:283–290
Wu J, Maehara T, Shimokawa T, Yamamoto S, Harada C, Takazaki Y, Ono N, Mukai Y, Koike K, Yazaki J, Fujii F, Shomura A, Ando T, Kono I, Waki K, Yamamoto K, Yano M, Matsumoto T, Sasaki T (2002) A comprehensive rice transcript map containing 6591 expressed sequence tag sites. Plant Cell 14:525–535
Wu LL, Mitchel JP, Cohn NS, Kaufman PB (1993) Gibberellin (GA3) enhances cell wall invertase activity and mRNA levels in elongating dwarf pea (Pisum sativum) shoots. Int J Plant Sci 154:280–289
Xu J, Avigne WT, McCarty DR, Koch KE (1996) A similar dichotomy of sugar modulation and developmental expression affects both paths of sucrose metabolism: evidence from maize invertase gene family. Plant Cell 8:1209–1220
Ylstra B, Garrido D, Busscher J, Van-Tunen AJ (1998) Hexose transport in growing Petunia pollen tubes and characterization of a pollen-specific, putative monosaccharide transporter. Plant Physiol 118:297–304
Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, Cao M, Liu J, Sun J, Tang J, Chen Y, Huang X, Lin W, Ye C, Tong W, Cong L, Geng J, Han Y, Li L, Li W, Hu G, Huang X, Li W, Li J, Liu Z, Li L, Liu J, Qi Q, Liu J, Li L, Li T, Wang X, Lu H, Wu T, Zhu M, Ni P, Han H, Dong W, Ren X, Feng X, Cui P, Li X, Wang H, Xu X, Zhai W, Xu Z, Zhang J, He S, Zhang J, Xu J, Zhang K, Zheng X, Dong J, Zeng W, Tao L, Ye J, Tan J, Ren X, Chen X, He J, Liu D, Tian W, Tian C, Xia H, Bao Q, Li G, Gao H, Cao T, Wang J, Zhao W, Li P, Chen W, Wang X, Zhang Y, Hu J, Wang J, Liu S, Yang J, Zhang G, Xiong Y, Li Z, Mao L, Zhou C, Zhu Z, Chen R, Hao B, Zheng W, Chen S, Guo W, Li G, Liu S, Tao M, Wang J, Zhu L, Yuan L, Yang H (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica) . Science 296:79–92
Acknowledgements
We thank the members of the Plant Metabolism Research Centre (PMRC) for helpful discussions, Junok Lee for technical assistance, and Priscilla Licht for the critical reading of the manuscript. This work was supported, in part, by grants from SRC for the PMRC, Korea Science and Engineering Foundation (KOSEF) Program; from the Biogreen 21 Program, Rural Development Administration; from the Crop Functional Genomic Centre, the 21 Century Frontier Program; and from the BK21 program, Ministry of Education.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by I.S. Chung
Rights and permissions
About this article
Cite this article
Cho, JI., Lee, SK., Ko, S. et al. Molecular cloning and expression analysis of the cell-wall invertase gene family in rice (Oryza sativa L.). Plant Cell Rep 24, 225–236 (2005). https://doi.org/10.1007/s00299-004-0910-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0910-z