Abstract
The relative growth rate of plant cells in vitro is considerably affected by initial cell density. This troublesome effect has interfered with the establishment of efficient plant cell culture systems, especially when only a small number of cells are expected to survive, such as in the genetic transformation of cells under antibiotic selection. To improve the recovery of antibiotic-resistant cells, we examined the use of the peptide plant hormone phytosulfokine (PSK), which has been shown to promote cellular growth and development in vitro. The addition of PSK to selective media increased the recovery of transformed callus from Agrobacterium-infected carrot hypocotyl explants from 7% to 39%, which is more than a fivefold improvement over the control. Most calluses developed into normal plantlets with cotyledons and primary roots and, eventually, formed foliage leaves. Thus, chemical nursing using PSK shows promise as a tool for basic research in plant biology and biotechnological applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic transformation of plants has become an important tool in functional genomics as well as conventional breeding. The establishment of stable transformants generally involves two experimental steps: T-DNA transfer via Agrobacterium, and selective culture using antibiotic-resistant marker genes and corresponding antibiotics. Because only a few cells of a plant organ are usually transformed by infection with Agrobacterium, thereby forming a chimera-like structure of transformed/untransformed cells, a selection procedure that favors transformed cells over untransformed cells is a critical step. However, antibiotic selection significantly decreases the relative density of viable cells by killing untransformed cells, resulting in severe growth inhibition of the surviving transgenic cells. The relative growth rate of plant cells in vitro is considerably affected by initial cell density (Lescure 1966). To promote proliferation of low-density cells, some workers have used nurse culture systems such as a feeder layer in which the feeder cells produce conditioning factor(s) that promote the proliferation of target cells (Muir et al. 1954; Torrey 1957; Raveh et al. 1973; Horsh and Jones 1980). In 1996, Matsubayashi and Sakagami (1996) identified the active factor in the nurse culture systems as PSK, a small sulfated peptide hormone secreted by cells. PSK binds a membrane-localized 120-kDa leucine-rich repeat receptor kinase (Matsubayashi et al. 2002) and induces proliferation of plant cells in vitro at low nanomolar concentrations, even at initial cell densities as low as approximately 300 cells per milliliter culture medium (Matsubayashi and Sakagami 1998). Genes encoding PSK precursors (Yang et al. 2001) are found in many plant species, and a five-amino acid sequence in PSK is uniformly conserved (Lorbiecke and Sauter 2002). In the investigation reported here, we examined the effects of PSK as a chemical nursing agent in selection culture, using carrot hypocotyl as a model case, and found that PSK significantly improves genetic transformation efficiency by promoting the proliferation of surviving transgenic cells under antibiotic selection.
Materials and methods
Plant material
Seeds of carrot (Daucus carota L.) cv. US-Harumaki-gosun were sterilized for 15 min first in 70% ethanol then in a 10% sodium hypochlorite solution. After extensive washing in sterile water, the seeds were germinated on 0.8% agar at 25°C for 7 days in the dark.
Agrobacterium tumefaciens strain
Agrobacterium tumefaciens strain C58C1 harboring the helper vir plasmid pGV2260 (Deblaere et al. 1985) and the binary plasmid pIG121 was used in the present study. The T-DNA region of binary vector pIG121 contained the nptII gene under the control of the nos (nopaline synthase) promoter and the GUS gene with an intron (intron-GUS) under the control of the CaMV (cauliflower mosaic virus) 35S promoter (Ohta et al. 1990). This bacterial strain was inoculated into liquid Luria Bertoni medium containing 50 mg/l kanamycin and 50 mg/l carbenicillin and was incubated for 24 h at 30°C with reciprocal shaking (200 cycles/min).
Transformation
Agrobacterium-mediated genetic transformation was performed using the method of Hardegger and Sturm (1998) with minor modifications. Hypocotyls of 7-day-old seedlings were cut into approximately 1-cm-long segments and immersed in an overnight culture of Agrobacterium for a few minutes. The seedling segments were then removed from the culture, briefly dried on filter paper and placed on semi-solid B5 medium (Gamborg et al. 1968) containing 1 mg/l NAA, 0.5 mg/l BAP, 3% sucrose and 0.8% agar for cocultivation. After 2 days of cocultivation, the segments were washed with sterile water and cultured for 4 weeks on semi-solid B5 medium (50 hypocotyl segments per 9-cm plate) containing 1 mg/l NAA, 0.5 mg/l BAP, 3% sucrose, 200 mg/l cefotaxime, 200 mg/l vancomycin, 10 mg/l geneticin, 0.8% agar and various concentrations of PSK.
GUS histochemical assay
Histochemical analysis of GUS gene expression in the transformed cells was performed using the method of Kosugi et al. (1990). Hypocotyl segments containing resistant calluses were incubated for 16 h at 37°C in 100 mM sodium phosphate buffer (pH 7.0) containing 2 mM 5-bromo-4-chloro-3-indoyl glucuronide (X-gluc), 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6, 0.3% Triton X-100, and 20% methanol. The transformation efficiency (%) was defined as the number of blue calluses counted under the microscope relative to the total number of cocultivated hypocotyls.
Plant regeneration
Geneticin-resistant, GUS-positive calluses were selected manually and transferred onto plates containing semi-solid hormone-free B5 medium with 1.5% sucrose and 200 mg/l cefotaxime. The plates were sealed with Parafilm. After 4 weeks in a growth chamber at 25°C and a 16/8-h (day/night) photoperiod, the plantlets were transferred to the same medium in 15-cm-deep culture tubes.
Polymerase chain reaction and DNA gel blot analysis
Genomic DNA was extracted from leaves of primary transformants using the DNeasy plant kit (Qiagen, Valencia, Calif.) according to the manufacturer’s protocol. The GUS sequence was amplified using a PCR profile of 30 cycles of 30 s at 94°C, 30 s at 55°C, and 2 min at 72°C and the primers 5′-GTCCGTCCTGTAGAAACCCCAAC-3′ and 5′-CCTTTCTTGTTACCGCCAACG-3′. For Southern blot analysis, genomic DNA was digested with EcoRV, separated on the agarose gel (12 μg per lane) and transferred onto a nylon membrane (Hybond-N+, Amersham, UK). The blots were hybridized with a alkaline phosphatase-labeled 3′-fragment of the GUS gene (1.0 kb) using the AlkPhos Direct system (Amersham). Chemiluminescence was detected by LumiVision PRO (Taitec, Japan).
Results and discussion
The recovery efficiency of the transformed calluses was determined with Agrobacterium C58C1 harboring the helper vir plasmid pGV2260 (Deblaere et al. 1985) and the binary plasmid pIG121 with an intron in the coding region of the GUS marker gene (Ohta et al. 1990), which prevents GUS expression in bacteria. The T-DNA of the binary vector also contained the nptII gene, which confers kanamycin resistance to transformed plant cells. Recovery of the transformed cells was confirmed by a histochemical GUS assay. No blue staining was observed when only bacteria with the intron-GUS gene were incubated in a solution of X-gluc (data not shown).
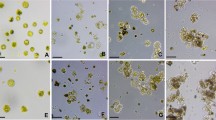
About 120 hypocotyl pieces were cocultivated for 2 days with the Agrobacterium and then transferred to selection medium containing geneticin, a potent kanamycin analog, and various concentrations of PSK. The transformation efficiency (%) was defined as the number of blue calluses counted under the microscope relative to the total number of cocultivated hypocotyls. In three independent experiments, hypocotyls cultured without PSK produced GUS-positive (GUS+) callus at a transformation efficiency of only 7.2±3.4% after 4 weeks of culture. In contrast, when the hypocotyls were cultured with 10−8 M of PSK the transformation efficiency was 31.3±2.1%, which is approximately a fourfold increase over the control (Table 1). At a higher concentration of PSK—10−6 M—the genetic transformation efficiency was 39.3±1.8%, a more than fivefold increase over the control. In those hypocotyls cultured in the presence of PSK, the GUS+ calluses formed predominantly at one side of the explants and they were significantly larger than those of the control (Fig. 1). Even when PSK was added to the coculture medium together with the selection medium, no further improvement in recovery of GUS+ calluses was observed (data not shown). These results indicate that PSK promotes the proliferation of the surviving transformed cells, resulting in a significant improvement of the genetic transformation efficiency. Plant regeneration was further induced in rapidly growing, geneticin-resistant GUS+ calluses by omitting the hormones. Most calluses (12/14) developed into small plantlets with cotyledons and primary roots within 4 weeks of induction and formed foliage leaves within 6 weeks. The genetic transformation of carrot plants derived from geneticin-resistant GUS+ calluses was confirmed by the detection of the GUS gene in isolated genomic DNA. PCR performed using primers specific for sequences in GUS resulted in the formation of a single band with the expected size of 1.7 kb in all regenerants tested (Fig. 2a). Integration of the GUS gene into the plant genome was further confirmed by Southern blot analysis (Fig. 2b).
Histochemical detection of GUS activity in carrot hypocotyls. Hypocotyl segments of 7-day-old seedlings were cocultivated for 2 days with Agrobacterium, then cultured on selective media containing antibiotics and various concentrations of PSK for 4 weeks. The number of GUS+ calluses significantly increased on explants cultured in the presence of 10−8 M (b) or 10−6 M (c) PSK compared to the control (a). Bar: 5 mm
Detection of the GUS gene in transformed carrot plants. a Agarose gel electrophoresis of the PCR amplification of the GUS-specific fragment of DNA isolated from transformed carrot plants. Lanes: 1 DNA size marker (M), 2 positive control (P) (plasmid DNA), 3 negative control (N) (untransformed plant), 4, 5 transgenic plants derived from the GUS+ callus cultured in the absence of PSK, 6–10 transgenic plants derived from the GUS+ callus cultured with 10−8 M PSK, 11–15 transgenic plants derived from the GUS+ callus cultured with 10−6 M PSK. b Southern blot analysis of transformed carrot plants. Genomic DNA (12 μg) was digested with EcoRV to show integration of the DNA into the plant genome. The blots were hybridized with a alkaline phosphatase-labeled GUS gene fragment. Lanes: 1 Negative control (N) (untransformed plant), 2–4 transgenic plants derived from the GUS+ callus cultured with 10−8 M PSK
Nurse culture systems have often been tested and used to promote the growth and regeneration of small numbers of cells such as transformed cells (Jin et al. 1999) and protoplasts (Karamian and Ebrahimzadeh 2001; Rakosy-Tican et al. 2001). In 1996, the active factor in the nursing effect was identified as PSK, a small sulfated pentapeptide secreted by cells (Matsubayashi and Sakagami 1996). Five paralogous genes encoding the approximately 80-amino acid precursors of PSK have been identified in Arabidopsis (Yang et al. 2001). Each predicted protein has a probable secretion signal at the N-terminus and a single PSK sequence close to the C-terminus. Genes encoding possible PSK precursors are present in many plant species; in each precursor protein, a five-amino acid PSK sequence is uniformly conserved (Lorbiecke and Sauter 2002), suggesting that PSK universally functions as a hormone in plants. In addition to its promotion of proliferation of dispersed cells (Matsubayashi and Sakagami 1996) and protoplasts (Matsubayashi et al. 1997), PSK stimulates cellular differentiation such as tracheary element formation (Matsubayashi et al. 1999) and somatic embryogenesis (Kobayashi et al. 1999; Hanai et al. 2000), depending on the ratios and concentrations of auxin and cytokinin. It has recently been reported that highly reproducible regeneration of Cryptomeria japonica, one of the most commercially important conifers in Japan, has been achieved via somatic embryogenesis using PSK as a nursing agent (Igasaki et al. 2003). This indicates that PSK activates cellular competence, which is defined as the ability to respond to induction signals such as auxin/cytokinin and that auxin/cytokinin then determines cell fate.
Our results clearly indicate that PSK significantly improves genetic transformation efficiency by promoting the proliferation of surviving transgenic cells under antibiotic selection. This system should be applicable to plant species in which genetic transformation has previously been unsuccessful due to a low mitotic activity of surviving transgenic cells. PSK can easily be prepared by chemical synthesis (Matsubayashi et al. 1996) and is relatively stable in an aqueous solution. It has the potential to be used as a chemical nursing agent in many phases of plant cell culture in basic research and practical applications.
Abbreviations
- BAP:
-
6-Benzylaminopurine
- GUS:
-
β-Glucuronidase
- NAA:
-
α-Naphthaleneacetic acid
- nptII:
-
Neomycin phosphotransferase II
- PSK:
-
Phytosulfokine
- X-gluc:
-
5-Bromo-4-chloro-3-indoyl glucuronide
References
Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemans J (1985) Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res 13:4777–4788
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Hanai H, Matsuno T, Yamamoto M, Matsubayashi Y, Kobayashi T, Kamada H, Sakagami Y (2000) A secreted peptide growth factor, phytosulfokine, acting as a stimulatory factor of carrot somatic embryo formation. Plant Cell Physiol 41:27–32
Hardegger M, Sturm A (1998) Transformation and regeneration of carrot (Daucus carota L.). Mol Breed 4:119–127
Horsh RB, Jones GE (1980) A double filter paper technique for plating cultured plant cells. In Vitro 16:103–108
Igasaki T, Akashi N, Ujino-Ihara T, Matsubayashi Y, Sakagami Y, Shinohara K (2003) Phytosulfokine stimulates somatic embryogenesis in Cryptomeria japonica. Plant Cell Physiol 44:1412–1416
Jin R-G, Liu Y-B, Tabashnik BE, Borthakur D (1999) Tissue culture and Agrobacterium-mediated transformation of watercress. Plant Cell Tissue Organ Cult 58:171–176
Karamian R, Ebrahimzadeh H (2001) Plantlet regeneration from protoplast-derived embryogenic calli of Crocus cancellatus. Plant Cell Tissue Organ Cult 65:115–121
Kobayashi T, Eun C-H, Hanai H, Matsubayashi Y, Sakagami Y, Kamada H (1999) Phytosulfokine-α, a peptidyl plant growth factor, stimulates somatic embryogenesis in carrot. J Exp Bot 50:1123–1128
Kosugi S, Ohashi Y, Nakajima K, Arai Y (1990) An improved assay for β-glucuronidase in transformed cells: methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant Sci 70:133–140
Lescure AM (1966) Relationships between the density of plant cells and their growth kinetics in submerged medium. Exp Cell Res 44:620–623
Lorbiecke R, Sauter M (2002) Comparative analysis of PSK peptide growth factor precursor homologs. Plant Sci 163:321–332
Matsubayashi Y, Sakagami Y (1996) Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L. Proc Natl Acad Sci USA 93:7623–7627
Matsubayashi Y, Sakagami Y (1998) Effects of the medium ammonium-nitrate ratio on competence for asparagus cell division induced by phytosulfokine-α. Plant Cell Rep 17:368–372
Matsubayashi Y, Hanai H, Hara O, Sakagami Y (1996) Active fragments and analogs of the plant growth factor, phytosulfokine: structure-activity relationships. Biochem Biophys Res Commun 225:209–214
Matsubayashi Y, Takagi L, Sakagami Y (1997) Phytosulfokine-α, a sulfated pentapeptide, stimulates the proliferation of rice cells by means of specific high- and low-affinity binding sites. Proc Natl Acad Sci USA 94:13357–13362
Matsubayashi Y, Takagi L, Omura N, Morita A, Sakagami Y (1999) The endogenous sulfated pentapeptide phytosulfokine-α stimulates tracheary element differentiation of isolated mesophyll cells of Zinnia. Plant Physiol 120:1043–1048
Matsubayashi Y, Ogawa M, Morita A, Sakagami Y (2002) An LRR receptor kinase involved in perception of a peptide plant hormone, phytosulfokine. Science 296:1470–1472
Muir WH, Hildebrandt AC, Riker AJ (1954) Plant tissue cultures produced from single isolated cells. Science 119:877–878
Ohta S, Mita S, Hattori T, Nakamura K (1990) Construction and expression in tobacco of a β-glucronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol 31:805–813
Rakosy-Tican L, Hornok M, Menczel L (2001) An improved procedure for protoplast microelectrofusion and culture of Nicotiana tabacum intraspecific somatic hybrids: plant regeneration and initial proofs on organelle segregation. Plant Cell Tissue Organ Cult 67:153–158
Raveh D, Hubermann E, Galun E (1973) In vitro culture of tobacco protoplasts: use of feeder techniques to support division of cells plated at low densities. In Vitro 9:216–222
Torrey JG (1957) Cell division in single isolated cells in vitro. Proc Natl Acad Sci USA 43:887–891
Yang H, Matsubayashi Y, Nakamura K, Sakagami Y (2001) Diversity of Arabidopsis genes encoding precursors for phytosulfokine, a peptide growth factor. Plant Physiol 127:842–851
Acknowledgements
We thank Dr. K. Nakamura for providing the binary vector pIG121. This work was supported in part by a Grant-in-Aid for COE research (13CE2005 and 14COEA02) and Scientific Research for Priority Area (14036214).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Sato
Rights and permissions
About this article
Cite this article
Matsubayashi, Y., Goto, T. & Sakagami, Y. Chemical nursing: phytosulfokine improves genetic transformation efficiency by promoting the proliferation of surviving cells on selective media. Plant Cell Rep 23, 155–158 (2004). https://doi.org/10.1007/s00299-004-0816-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0816-9