Abstract
We have evaluated the expression of the reporter β-glucuronidase (GUS) gene driven by the cauliflower mosaic virus 35S (CaMV 35S) promoter in flowers and pollen from 14 independent transgenic strawberry lines. Of the 14 lines evaluated, 13 (92.8%) showed GUS activity—as estimated by the histochemical GUS assay—in some floral organs, with expression being most common in the flower stem, sepals, petals, ovary and stigma. Ten of these thirteen transgenic lines (77%) showed GUS activity in pollen, although the percentages of positive pollen per flower varied greatly among the different lines. A study of the GUS expression during pollen maturation showed that the (CaMV 35S) promoter showed low expression in pollen from flower buds before anthesis but was activated in mature pollen following anther dehiscence. The percentages of pollen grains that showed GUS activity ranged from 2.1% to 46.3%. These percentages were similar or even higher when mature pollen was stored dry at room temperature for 2 weeks. After 5 weeks of storage, the percentages of GUS-positive pollen decreased in two of the six lines analysed but remained at similar values in the other four lines. GUS activity was also measured in protein extracts of mature pollen by means of the fluorometric GUS assay, with the values obtained ranging from 3.8 μmol MU mg protein−1 h−1 to 0.26 μmol MU mg protein−1 h−1. Contrary to the generally held view that the CaMV 35S promoter is virtually silent in pollen, we conclude that it is highly expressed in transgenic strawberry pollen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The environmental spread of transgenes and their products through pollen dispersal is one of the main stumbling blocks to the commercial release of transgenic plants. In addition to gene flow through cross-pollination with wild relative species, pollen can be a vehicle to the spread of transgenic proteins that are either expressed or deposited on the grain. The dispersal of transgene products via pollen poses potential risks, such as ecological effects on non-target beneficial insects in the case of insecticidal proteins (Poppy 2000), the inhalation of airborne allergens or the introduction of allergenic proteins into the food chain by bee contamination of honey (Eady et al. 1995; Rogers and Parkes 1995). The likelihood of transgenic protein dispersal via pollen will initially depend on the activity of the promoter driving the transgene in the male gametophyte. In this respect, information concerning the activity of promoters commonly used to drive gene expression in transgenic plants, like the cauliflower mosaic virus promoter (CaMV 35S) or nopaline synthase promoter (nos), is scarce and limited to a few species.
TheCaMV 35S promoter is widely used in transformation experiments due to its high activity in most tissues of a broad range of species. Based on its wide expression, CaMV 35S is generally considered to be a constitutive promoter, although physiological and/or environmental factors, such as developmental stage (Pret’ová et al. 2001), photoperiod and temperature (Schnurr and Guerra 2000), can modulate its expression. Despite its widespread use in biotechnological plant improvement programs, our knowledge of CaMV 35S activity on reproductive tissues and, particularly, in pollen grains is scant and confusing. To date transient transformation experiments of pollen with the GUS reporter gene under the control of CaMV 35S have yielded no conclusive results. Nishihara et al. (1993) detected GUS activity in pollen of five species transformed by particle bombardment. Similarly, Twell et al. (1989) found low but consistent levels of GUS activity in bombarded pollen of tobacco and tomato. In contrast, GUS expression was not observed in either bombarded pollen of Nicotiana glutinosa and Lilium longiflorum (Van der Leede-Plegt et al. 1992) or in electroporated maize microspores (Fennell and Hauptmann 1992). Reports on CaMV 35S expression in pollen from stably transformed plants are also inconclusive; for example, no promoter expression has been detected in mature pollen of tobacco, tomato (Van der Leede-Plegt et al. 1992), petunia (Mascarenhas and Hamilton 1992) and Arabidopsis (Wilkinson et al. 1997). However, Twell et al. (1989) and Wilkinson et al. (1997) reported low levels of GUS activity driven by CaMV 35S in tobacco pollen. In any case, the expression of this promoter is several fold lower than the expression of such pollen-specific promoters as lat52 from tomato (Twell et al. 1989).
Similar to these Solanaceae crops, strawberry plants are also included in several biotechnological breeding programmes (Faedi et al. 2002). During the 1990s, reliable protocols for the genetic transformation of strawberry were established for several commercial cultivars (Barceló et al. 1998; James et al. 1990; Mathews et al. 1995). Recently, these have been successfully employed to modify selected traits like insect resistance (Watt et al. 1999), herbicide tolerance (Morgan et al. 2002) and fruit softening (Jiménez-Bermúdez et al. 2002). While most of these transformations used the CaMV 35S promoter to control transgene expression, there is no information available on the expression of this promoter in strawberry reproductive tissues and pollen. The aim of the investigation reported here was to evaluate the activity of the CaMV 35S promoter in the different organs of the strawberry flower and in the pollen grains.
Materials and methods
Plant material
Transgenic Fragaria × ananassa Duch. plants, cv. Chandler, had been obtained previously by the Agrobacterium-biolistic combined method developed for this species (Cordero de Mesa et al. 2000). These plants were transformed with the pGUSINT plasmid (Vancanneyt et al. 1990) containing the nptII gene for kanamycin resistance and the uidA reporter gene (coding for GUS) under the control of a single (CaMV 35S) promoter. Primary transgenics were grown in a greenhouse and propagated vegetatively via runners (cultivated strawberry is not reproduced by sexual crosses). Plants used in this work correspond to the second and third vegetative generations and were evaluated in consecutive years. Fourteen independent transgenic lines, eight copies per line, as well as non-transformed plants were used. The plants were grown in a greenhouse under natural temperature and photoperiod, and flower and pollen samples were obtained at the middle of the fruiting season (April-May).
Histochemical detection of GUS activity in flowers and pollen
Expression of the GUS gene in whole strawberry flowers and isolated pollen samples was detected histochemically following the procedure of Jefferson et al. (1987). Samples were vacuum infiltrated for 5 min in the staining solution (1 mM X-gluc in 50 mM phosphate buffer pH 7) and incubated for 24 h at 37°C. The flowers were then washed in 95% ethanol to extract the chlorophylls. Flowers were collected at anthesis, and pollen samples were obtained by crushing isolated anthers in a small volume of GUS staining solution. Six flowers per line from different plants were analysed in two consecutive years.
To analyse the expression of the CaMV 35S promoter during pollen maturation and storage, we collected pollen at different developmental stages: flower buds before anthesis, flowers at anthesis without visible dehiscent pollen and flowers with dehiscent pollen. In the first two cases, pollen was released by crushing isolated anthers in GUS staining solution. Dehiscent pollen from each individual flower was collected by gently shaking the flowers and subsequently fractionated into three samples. One of these samples was immediately assayed for GUS activity, and the others were stored in crystal vials at 25°C. After 2 weeks and 5 weeks of storage, the pollen was stained for GUS activity. Four to six independent samples for each line, obtained from different plants, were analysed. A minimum of 100 pollen grains per sample was recorded, and the percentage of pollen grains showing GUS activity was estimated from all of the grains recorded, both viable and non-viable grains.
Fluorometric assay for GUS activity
GUS enzyme activity was measured in the pollen grains by the fluorometric assay, as described by Jefferson et al. (1987). Samples of dehiscent pollen from six to ten flowers, collected by shaking the flowers on a petri dish, were homogenized in 2 ml of extraction buffer [50 mM phosphate buffer, pH 7, 10 mM EDTA, 0.1% (v/v) Triton-X 100, 10 mM β-mercaptoethanol]. Aliquots of the extracts (100 μl) were added to 1 ml of assay buffer (extraction buffer supplemented with 1 mM MU), prewarmed and incubated at 37°C. After 0, 5 and 20 min of incubation, 100-μl samples were removed and placed in 1.9 ml stop buffer (200 mM sodium carbonate). Fluorescence was measured using a DyNA Quant 200 fluorometer (Hoefer Pharmacia Biotech). Protein concentrations in the extracts were determined by the Bradford assay (Bradford 1976), and GUS activity was expressed as micromole MU per hour per milligram protein. Three independent extractions were performed for each line.
Southern analysis
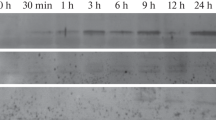
Genomic DNA was extracted from young strawberry leaves using the DNeasy plant mini kit (Qiagen, Valencia, Calif. ). Previous to this, the plant material had been washed several times with washing buffer to eliminate DNA contaminants (Mercado et al. 1999). Ten micrograms of DNA was digested overnight with EcoRI in the presence of 1 mM spermidine, electrophoresed in a 0.8% agarose gel and then transferred to Hybond N+ membranes. A 900-bp PCR fragment containing the nos-nptII gene was used as probe. This probe was labelled with digoxigenin using the DIG High Prime DNA labelling and detection starter kit (Roche, Indianapolis, Ind.). The filter was prehybridised at 42°C in DIG Easy Hyb buffer and hybridised overnight in the same buffer containing the probe. The filter was then washed and the probe detected following the manufacturer’s instructions.
Results
GUS activity driven by the CaMV 35S promoter in strawberry flowers
Fourteen transgenic lines transformed with the pGUSINT plasmid were used in this investigation. The transgenic nature of these plants was previously assessed by PCR analysis and confirmed in several lines based on kanamycin segregation of seeds (Cordero de Mesa et al. 2000). Despite the presence of large amounts of polyphenols and polymeric carbohydrates in strawberry tissue, which made it difficult to extract pure DNA suitable for molecular analysis, we successfully obtained purified DNA by combining a previous protocol (Mercado et al. 1999) with a commercial DNA extraction kit. The presence of T-DNA was confirmed in these samples by Southern hybridisation with a nos-nptII probe (Fig. 1).
GUS activity was absent in all of the tissues from wild-type flowers; that is, under the staining conditions employed no blue coloration was observed. However, 13 of the 14 independent transgenic lines containing the pGUSINT plasmid (92.8%) showed GUS activity in their floral organs. An example of GUS staining in strawberry flowers is shown in Fig. 2. The transgenic plants were analysed during two consecutive years, and very minor differences in GUS activity were observed between the two cycles of vegetative propagation studied. No statistically significant differences were found using an analysis of frequencies at P=0.05. Not all of the transgenic lines displayed the same pattern of GUS expression (Fig. 3). GUS activity was generally found in the flower stem, sepals, petals, ovary, stigma and pollen; this pattern was exhibited by 8 of the 13 lines (61.5%) that showed GUS staining in some floral organs. The presence of GUS activity in the anther and filament was detected in 50% of the transgenic lines. In contrast, GUS staining in the style and receptacle was only observed in two (15.4%) and one (7.7%) line, respectively. It is noteworthy that 10 of the 13 transgenic lines (77%) showed GUS activity in the pollen grains.
Histochemical staining for GUS activity driven by the CaMV 35S promoter in strawberry flowers and pollen. A, C Control non-transformed flower showing the absence of staining. B Transgenic flower from line GUS7 showing GUS activity in most of its tissues. D Transgenic flower from line GUS1 showing GUS activity in floral pistils. E Mature pollen from control non-transformed plants showing the absence of GUS activity. F Mature transgenic pollen from line GUS12 stained for GUS activity. In both E and F, pollen was obtained by gently shaking dehiscent anthers. Bars (E, F): 100 μm
Percentages of independent transgenic strawberry lines that displayed GUS activity driven by the CaMV 35S promoter in the different floral organs and in pollen grains. Percentages were calculated from the 13 lines that showed GUS activity in some floral organs. Empty bars correspond to plants from the second round of vegetative propagation, diagonally stripped bars correspond to plants belonging to the third cycle of propagation
CaMV 35S promoter expression in strawberry pollen
Initially our study was focused at the time of flower anthesis. Pollen grains from both the control plants and the different transgenic lines were collected by crushing the anthers of recently opened flowers and then stained with X-gluc to detect GUS activity. These samples contained a mixture of pollen from dehiscent and non-dehiscent anthers. No blue staining was observed in any of the pollen samples obtained from wild-type strawberry plants. In the transgenic plants, the percentages of stained pollen grains ranged from 0.4% in the GUS1 line to 25.6% in the GUS8 line (Fig. 4). In five of the ten pollen-positive lines (50%), stained pollen was observed in all of the flowers assayed. These lines (GUS2, -8, -10, -11 and -12) together with GUS15 that also showed a high percentage of GUS-stained pollen were selected for further analysis.
Percentages of pollen grains that showed GUS activity driven by the CaMV 35S promoter in the different transgenic strawberry lines. Pollen was obtained by crushing the anthers of recently opened flowers. A minimum of 100 pollen grains from each flower was analysed. Values are means ± standard deviation of six flowers from different plants per line
As a second step, we evaluated the activity of the CaMV 35S promoter during transgenic strawberry pollen maturation and after 2 weeks and 5 weeks of storage (Table 1). In the six selected lines analysed, the percentages of stained grains were low when pollen was obtained from the anthers of closed flower buds or recently opened flowers with non-dehiscent anthers (without visible pollen on the anther surface). In contrast, the percentages of positive grains increased significantly during the later stages of pollen development. In mature pollen grains, which we obtained by gently shaking flowers with dehiscent anthers, the percentages of stained grains ranged from 2.1% to 46.3%, corresponding to lines 15 and 12, respectively. After 2 weeks of storage at room temperature, pollen grains displayed similar (GUS10 and -11) or higher (GUS2, -8, -12 and -15) percentages of GUS staining than freshly assayed dehiscent pollen. The highest percentage of stained pollen, 75%, was observed in the GUS12 transgenic line. After 5 weeks of storage, the pattern of expression was variable, depending on the transgenic line: the percentage of pollen grains showing GUS activity slightly increased in GUS2, remained at similar values in GUS8, -10 and -11 and decreased significantly in GUS12 and, specially, in GUS15, where no stained pollen was detected in any of the sample analysed. This trend was observed in two different growing seasons with plants belonging to two cycles of vegetative propagation. Segregation of GUS activity in strawberry pollen did not follow simple Mendelian ratios, probably due to the complex nature of the octoploid strawberry genome. Therefore, it was not possible to establish a correlation between GUS expression in the pollen grains and the number of T-DNA copies.
The presence of GUS activity in mature pollen was further verified by measuring GUS activity quantitatively by the fluorometric assay. No activity was detected in the protein extracts from control pollen. In contrast, transgenic lines showed significant levels of GUS activity, ranging from 3.8 μmol MU mg protein−1 h−1 in GUS8 to 0.26 μmol MU mg protein−1 h−1 in GUS12 and GUS15 (Fig. 5). These values fall within the range of those obtained in leaf petioles or fruit protein extracts from the same transgenic lines.
Discussion
The purpose of the present investigation was to evaluate the activity of the CaMV 35S promoter in reproductive tissue of strawberry plants and, especially, in pollen grains. With respect to the first-mentioned, GUS activity driven by the CaMV 35S promoter in floral tissue was detected in 13 of the 14 independent transgenic lines analysed. The sole line that did not show any blue coloration in its flowers also showed a negative reaction when its vegetative tissue was analysed for GUS activity. This positive correspondence between expression in vegetative and reproductive tissues occurred in all of the plants analysed in the present study. Consequently, we consider the CaMV 35S promoter to be constitutively active in strawberry plants. Contrary to this result, in transgenic Arabidopsis plants, Wilkinson et al. (1997) found that only 57% of the lines analysed displayed GUS activity in flowers driven by CaMV 35S, although all lines exhibited GUS activity in the leaves. The most common pattern of GUS expression observed in strawberry was also different from the one reported in Arabidopsis. Both patterns consist of GUS expression in the flower stem and sepals and low expression in the style. However, while more than 80% of the transgenic strawberry lines showed GUS activity in petals, stigma and pollen, in Arabidopsis flowers expression in these tissues was either restricted to a few lines or was absent, as in the case of pollen. As concluded by Wilkinson et al. (1997), it is clear that the activity of the CaMV 35S promoter varies greatly between different plant species and that its expression cannot be predicted with certainty. Therefore, more analysis of expression in different species would be required to obtain a clearer picture.
With respect to the GUS activity driven by the CaM V35S promoter in pollen, we found that 77% of the transgenic lines analysed displayed GUS activity in pollen grains, with expression being highly variable among the different lines. These results are in contrast to previous results that showed theCaMV 35S promoter to be virtually inactive in pollen and, consequently, to have a low potential to cause harmful effects through pollen expression (Rogers and Parkes 1995; Wilkinson et al. 1997). In both transient transformation experiments and in stably transformed plants, a lack of CaMV 35S promoter activity or only low levels of activity have been reported in the pollen grains of several plant species, such as tobacco, tomato, petunia and Arabidopsis (Mascarenhas and Hamilton 1992; Twell et al. 1989; Van der Leede-Plegt et al. 1992; Wilkinson et al. 1997). However, similar to our observations, the CaMV 35S promoter appears to be active in pollen from different pine species (Fernando et al. 2000). Furthermore, although a single CaMV 35S promoter is ineffective in inducing GUS expression in tobacco pollen, Conner et al. (1999) detected GUS activity in pollen from transgenic tobacco lines containing a double CaMV 35S promoter. It seems clear that while the activity of the CaMV 35S promoter in pollen grains may be dependent on the species studied, some of the discrepancies among the results of the various investigations may also be a result of the developmental stage of the pollen used for analysis. Our results show that the activity of the CaMV 35S promoter in strawberry pollen depends mainly on the maturation stage of the grains. Thus, its expression is low in immature grains, prior to anther dehiscence, but it is activated in fully mature pollen grains, at least during the first 2 weeks after anther dehiscence. The level of the transgene product, namely GUS activity, clearly increased from the stage of anther dehiscence to the period 2 weeks later in five of the six lines analysed. This increment in activity was probably due to an activation of the CaMV 35S promoter and not to an accumulation of GUS protein, since the latter has been shown to have a half-life of 50 h in mesophyll protoplasts (Jefferson et al. 1987). Similarly, the unexpected high expression of the promoter from the potato Lhca3.St.1 gene, which encodes a light-harvesting protein, in transgenic tobacco pollen was only observed in fully mature pollen but it remained active in senesced dried anthers (Conner et al. 1999). It is noteworthy that the CaMV 35S promoter remained active in strawberry pollen even after 5 weeks of pollen storage under dry conditions. The activity of the transgene promoters after a long time of pollen storage is not a novel result. Eady et al. (1995) found high levels of GUS activity driven by the pollen-specific promoter lat52 in tobacco pollen stored up to 6 weeks in honey. Mascarenhas (1990) separated pollen genes into two classes—one group comprises genes that are expressed soon after meiosis and are undetectable in mature pollen, and a second group includes genes that are first detected around the time of microspore mitosis and that continue to accumulate as the pollen grains mature. The expression pattern of CaMV 35S detected in strawberry pollen may indicate that this promoter shares some cis-regulatory sequences with promoters from genes of this latter group. However, although several putative regulatory motifs have been identified, the molecular basis of the specificity of pollen gene expression is still unknown (McCormick 1993). More probably, due to the complex structure of the CaMV 35S promoter, which contains two major enhancer domains and at least five subdomains that synergistically confer developmental and tissue-specific expression (Benfey et al. 1989, 1990), it may be able to interact with a wide range of native gene enhancer sequences expressed in mature pollen and/or during pollen germination.
Gametophytic segregation of GUS activity in strawberry did not follow the classic Mendelian segregation ratios as expected in diploid species. Fragaria × ananassa is an octoploid hybrid plant that arose as an interspecific cross between F. virginiana and F. chiloensis. It is assumed that the octoploid strawberry has mixed polyploidy—disomic and polysomic (Senanayake and Bringhurst 1967). The inheritance pattern in this species is therefore complex, which makes it difficult to establish the correlation between GUS expression in pollen and T-DNA copy number.
In conclusion, our analysis of the CaMV 35S promoter in pollen from different transgenic strawberry lines showed a consistent expression pattern—the CaMV 35S promoter was either silent or had very little activity in immature pollen, but it was active in later stages, especially following anther dehiscence. These results indicate that, contrary to the general view, the CaMV 35S promoter is active in pollen and, therefore, is a factor to be taken into consideration in the biosafety assessment of transgenic plants carrying this promoter.
Abbreviations
- CaMV 35S :
-
Cauliflower mosaic virus promoter
- GUS :
-
β-Glucuronidase (EC 3.2.1.31)
- MU:
-
4-Methyl umbelliferone
- nos :
-
Nopaline synthase promoter
- nptII :
-
Neomycin phosphotransferase
- X-Gluc:
-
5-Bromo-4-chloro-3-indolyl-β-d-glucuronic acid
References
Barceló M, El Mansouri I, Mercado JA, Quesada MA, Pliego-Alfaro F (1998) Regeneration and transformation via Agrobacterium tumefaciens of the strawberry cultivar Chandler. Plant Cell Tissue Organ Cult 54:29–36
Benfey PN, Ren L, Chua N-H (1989) The CaMV 35S enhancer contains at least two domains which can confer different developmental and tissue-specific expression patterns. EMBO J 8:2195–2202
Benfey PN, Ren L, Chua N-H (1990) Combinatorial and synergistic properties of CaMV 35S enhancer subdomains. EMBO J 9:1685–1696
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Conner AJ, Mlynarova L, Stiekema WJ, Nap J-P (1999) Gametophytic expression of GUS activity controlled by the potato Lhca3.St.1 promoter in tobacco pollen. J Exp Bot 50:1471–1479
Cordero de Mesa M, Jiménez-Bermúdez S, Pliego-Alfaro F, Quesada MA, Mercado JA (2000) Agrobacterium cells as microprojectile coating: a novel approach to enhance stable transformation rates in strawberry. Aust J Plant Physiol 27:1093–1100
Eady C, Twell D, Lindsey K (1995) Pollen viability and transgene expression following storage in honey. Transgenic Res 4:226–231
Faedi W, Mourgues F, Rosati C (2002) Strawberry breeding and varieties: situation and perspectives. Acta Hortic 567:51–59
Fennell A, Hauptmann R (1992) Electroporation and PEG delivery of DNA into maize microspores. Plant Cell Rep 11:567–570
Fernando DD, Owens JN, Misra S (2000) Transient gene expression in pine pollen tubes following particle bombardment. Plant Cell Rep 19:224–228
James DJ, Passey AJ, Barbara DJ (1990) Agrobacterium-mediated transformation of the cultivated strawberry (Fragaria × ananassa Duch.) using disarmed binary vectors. Plant Sci 69:79–94
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jiménez-Bermúdez S, Redondo-Nevado J, Muñoz-Blanco J, Caballero JL, López-Aranda JM, Valpuesta V, Pliego-Alfaro F, Quesada MA, Mercado JA (2002) Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol 128:751–759
Mascarenhas JP (1990) Gene activity during pollen development. Annu Rev Plant Physiol Plant Mol Biol 41:317–338
Mascarenhas JP, Hamilton DA (1992) Artifacts in the localization of GUS activity in anthers of petunia transformed with a CaMV35S-GUS construct. Plant J 2:405–408
Mathews H, Wagoner W, Kellogg J, Bestwick R (1995) Genetic transformation of strawberry: stable integration of a gene to control biosynthesis of ethylene. In Vitro Cell Dev Biol Plant 31:36–43
McCormick S (1993) Male gametophyte development. Plant Cell 5:1265–1275
Mercado JA, El Mansouri I, Jiménez-Bermúdez S, Pliego-Alfaro F, Quesada MA (1999) A convenient protocol for extraction and purification of DNA from Fragaria. In Vitro Cell Dev Biol Plant 35:152–153
Morgan A, Baker CM, Chu JSF, Lee K, Crandall BA, Jose L (2002) Production of herbicide tolerant strawberry through genetic engineering. Acta Hortic 567:113–115
Nishihara M, Ito M, Tanaka I, Kyo M, Ono K, Irifune K, Morikawa H (1993) Expression of the β-glucuronidase gene in pollen of lily (Lilium longiflorum), tobacco (Nicotiana tabacum), Nicotiana rustica, and peony (Paeonia lactiflora) by particle bombardment. Plant Physiol 102:357–361
Poppy G (2000) GM crops: environmental risks and non-target effects. Trends Plant Sci 5:4–6
Pret’ová A, Obert B, Wetzstein HY (2001) Leaf developmental stage and tissue location affect the detection of β-glucuronidase in transgenic tobacco plants. Biotechnol Lett 23:555–558
Rogers HJ, Parkes HC (1995) Transgenic plants and the environment. J Exp Bot 46:467–488
Schnurr JA, Guerra DJ (2000) The CaMV-35S promoter is sensitive to shortened photoperiod in transgenic tobacco. Plant Cell Rep 19:279–282
Senanayake YDA, Bringhurst RS (1967) Origin of Fragaria polyploids. I. Cytological analysis. Am J Bot 54:221–228
Twell D, Klein TM, Fromm ME, McCormick S (1989) Transient expression of chimeric genes delivered into pollen by microprojectile bombardment. Plant Physiol 91:1270–1274
Vancanneyt G, Schmidt R, O’Connor-Sanchez A, Willmitzer L, Rocha-Sosa M (1990) Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220:245–250
Van der Leede-Plegt LM, van de Ven BCE, Bino RJ, van der Salm TPM, van Tunen AJ (1992) Introduction and differential use of various promoters in pollen grains of Nicotiana glutinosa and Lilium longiflorum. Plant Cell Rep 11:20–24
Watt K, Graham J, Gordon SC, Woodhead M, McNicol RJ (1999) Current and future transgenic control strategies to vine weevil and other insect resistance in strawberry. J Hortic Sci Biotechnol 74:409–421
Wilkinson JE, Twell D, Lindsey K (1997) Activities of CaMV 35S and nos promoters in pollen: implications for field release of transgenic plants. J Exp Bot 48:265–275
Acknowledgements
This work was supported by the Ministerio de Ciencia y Tecnología, Spain and EU FEDER funds (grant nos. IFD97/0843C0504 and AGL 2001-1897).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Peña
Rights and permissions
About this article
Cite this article
de Mesa, M.C., Santiago-Doménech, N., Pliego-Alfaro, F. et al. TheCaMV 35S promoter is highly active on floral organs and pollen of transgenic strawberry plants. Plant Cell Rep 23, 32–38 (2004). https://doi.org/10.1007/s00299-004-0776-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0776-0