Abstract
Based on a protocol for microspore culture in apple (Malus domestica Borkh.), the embryo induction phase has been improved with regard to pretreatment of microspores for initiation of microspore embryogenesis, the concentration of carbon source in the induction medium and the microspore density in the suspension. Furthermore, the effect of the genotype was studied. To determine the efficiency of in vitro androgenesis, both methods, via anther and microspore culture, were investigated using the same bud material. A comparison of the efficiency of embryo induction in anther and microspore cultures showed that microspore culture resulted in an increase up to 10 times, depending on the genotype. The regeneration route in microspore culture is similar to that of androgenic embryos via anther culture and showed adventitious shoot formation in most cases after a long period of secondary embryogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the first report on embryogenesis from pollen of Datura innoxia by Guha and Maheshwari (1964, 1966), isolated anther/microspore culture has become an important tool for the production of homozygous doubled haploid plants in many important crops (Jain et al. 1996). Until now, anther culture has been mainly used in practical breeding, but recent progress in gametic embryogenesis of isolated microspores in Brassica (Ferrie et al. 1999; Hansen 2000), in Hordeum (van Bergen et al. 1999; Ryan et al. 1999), in Oryza (Chowdhury and Mandal 2001) and in Triticum (Kunz et al. 2000) have made isolated microspore culture a practical approach for doubled haploid production. Moreover, isolated microspore culture provides an accessible haploid system for biochemical and molecular analyses and for in vitro selection for desirable traits (Jähne and Lörz 1995) without the interference of the anther wall; it also supplies a target for genetic transformation (Touraev et al. 1997; Carlson et al. 2001).
Although anther culture is much simpler in handling, microspore culture shows several important advantages, notably the formation of calli and embryos from somatic tissues of the anther is avoided. Secondly, there is direct access to the microspores which speeds up the optimization of culture conditions. Furthermore, in certain plant species, the potential number of embryos per anther obtained in isolated microspore culture is higher than in anther culture.
The production of doubled haploids can offer new possibilities for genetic studies and breeding, especially in perennial fruit species, which are characterized by a long juvenile phase, a tendency to allogamy and a high degree of heterozygosity. However, in vitro approaches to inducing haploids in apple have had rather limited success until recently, in comparison with other plant species (Höfer and Lespinasse 1996). Induction of embryogenesis and limited plant formation has been reported from anther cultures in apple (Fei and Xue 1981; Xue and Niu 1984; Höfer 1995). Although regeneration from embryos is reproducible via adventitious shoot formation, the induction rate of embryogenesis from cultured apple anthers is still low and highly genotype-dependent (Höfer 1995, 1997). Recently, a protocol for isolated microspore culture was developed in apple (Malus domestica Borkh.) for one genotype, and successful plant regeneration has been obtained from isolated microspores (Höfer et al. 1999).
In the present paper, the embryo induction phase was improved studying the effects of the pretreatment applied to microspores for initiation of microspore embryogenesis, of Percoll gradient centrifugation, of the maltose concentration in the induction medium and of the microspore density in the suspension. Furthermore, the effect of the genotype was evaluated and the efficiency of in vitro androgenesis using both methods, anther and isolated microspore culture, was compared.
Materials and methods
Plant material
Experiments were carried out with the apple cultivars 'Alkmene', 'Rene', 'Remo' and 'Realka' obtained from the conventional apple-breeding programme at Dresden-Pillnitz. Flower buds were taken from cut dormant bud wood and forced at different temperature/light regimes (12 h at 16°C in the light and 12 h at 12°C in the dark, and also at room temperature) and also directly from trees growing in the orchard. Flower buds were collected when microspores were at the mid unicelluar stage for anther culture and late unicellular stage of development for microspore culture (Höfer and Hanke 1994), surface-sterilized with 0.1% mercuric hypochloride and washed twice in sterile water.
Isolation and culture of microspores and regeneration
Microspores were isolated from anthers using the following protocol: anthers were stirred in 2 ml medium B (see "Media composition", below) with a magnetic stirrer in a small tube for 4 min at 250 rpm; the crude microspore population was then filtered through a 30-μm filter; and the resultant filtrate was washed three times with the same medium by centrifugation at 220 g for 5 min. The microspore pellet obtained after a final centrifugation step was resuspended in medium B (in Petri dishes of 3.5-cm diameter) or induction medium (in 4-well plates).
During the induction phase, the cultures were kept at 27°C in the dark. Microspore viability was determined by fluorecein diacetate staining (Heslop-Harrison and Heslop-Harrison 1970) immediately after isolation of microspores, starvation and Percoll gradient centrifugation. Well-developed embryos were transferred to regeneration medium and cultured at 23°C under a light intensity of 35 μmol m−2 s−1 with a 16 h photoperiod.
Media composition
Medium B for isolation and starvation was according to Kyo and Harada (1986). For the induction of apple microspore embryogenesis, the basal medium of Touraev et al. (1996) was used with four different maltose concentrations (150, 250, 350 and 500 mM). The regeneration medium was MS-medium (Murashige and Skoog 1962; 30 g/l sucrose) supplemented with 0.1 mg/l thidiazuron (Höfer 1995).
Starvation treatment
Isolated microspores were incubated in medium B at 4, 27 or 33°C for 1, 2, 3 or 4 days under dark conditions. Constant humidity was maintained by placing small Petri dishes with sterile water together with the microspore cultures in a larger dish.
Percoll gradient
For Percoll gradient centrifugation, 1 ml microspore suspension collected directly after isolation or starvation was layered onto a Percoll gradient (70% Percoll in 3.3×medium B) for 5 min at 437 g (Kyo and Harada 1986). Each band of microspores obtained after separation in the Percoll gradient characterized by microspore density and viability of microspores was carefully collected, resuspended in medium B, washed twice by centrifugation (5 min at 220 g) and cultured in induction medium.
Microspore density
The microspore density was determined by using a counting chamber. For the experiments carried out only in the first year (variation of pretreatment and maltose concentration, and application of Percoll centrifugation) the microspore density was adjusted to 7×105–10×105 microspores/ml. For the experiments investigating the effect of genotype and microspore density, different numbers of buds were used to obtain suspensions with a defined range of microspore densities for both experimental years (AT3 with 250 mM or 350 mM maltose and 2–3 days starvation at 4°C).
The efficiency of microspore induction was calculated by comparing the number of embryos per isolated anther in anther culture with the corresponding number from microspore culture for each variant of microspore culture. The number of replications and the factors tested and fixed in each experiment are mentioned in the relevant Figure.
Calculating the standard error by using experiments from different years was not considered scientifically valid because the flower buds of the apple tree developed in the year before flowering under different weather conditions.
Anther culture
For comparison of the efficiency of anther and microspore culture, experiments were carried out by using the same donor material for both methods in two experimental years. The detailed method of anther culture was described in Höfer (1995). Out of all tested variants for optimization of the induction medium, three combinations have been identified: 0.2 mg/l kinetin and indole-3-butyric acid with 0, 10 or 50 μM sodium fluoride. The average of these three media were used for the comparison with microspore culture.
Results and discussion
Androgenic pathway in microspore culture in apple
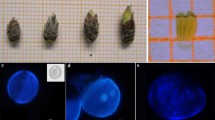
The microspore population used to initiate the cultures was a mixture of different developmental stages, the majority of which were at the late unicellular stage (Fig. 1). The first torpedo-shaped embryos could be observed after 12-weeks culture. Evaluations carried out monthly demonstrated embryo induction until 9 months after isolation. The regeneration process is similar to that for androgenic embryos via anther culture and demonstrates adventitious shoot formation, in most cases after a long period of secondary embryogenesis, and takes between 3 and 18 months. At present, the first androgenic lines induced via microspore culture exist as grafts in the orchard and are available for comprehensive evaluation in future studies.
Effect of starvation and Percoll gradient centrifugation
The first set of experiments was carried out with isolated microspores cultured under different starvation conditions to test further variants compared to the former results (Höfer et al. 1999). The new data (Fig. 2) demonstrated that pretreatment can improve the embryo induction rate but is not necessary for the induction of embryos and their later development. The most effective treatment for the induction of microspore embryos was found to be starvation of microspores for 2 or 3 days at 4°C. Higher temperatures and longer starvation treatments decreased the efficiency of embryo induction considerably. The main reason appears to be the strong decrease of microspore viability during the starvation process. This decrease is much faster at higher temperatures where secretion of phenolic compounds has also been observed (data not shown).
A parallel set of experiments was performed using the microspore suspensions, after the starvation treatment and immediately after isolation, for Percoll gradient centrifugation. The optimal Percoll concentration of 70% was determined in pilot tests (data not shown). Regarding the determination of the viability and the visible characterization, the population of embryogenic microspores was obtained in the interface between the medium and the 70% Percoll solution. The purification of the suspension by Percoll gradient centrifugation only resulted in an enhancing effect on embryo induction for the variants control and 1-day starvation. The optimal pretreatment time (2 or 3 days) caused a higher embryo induction rate without purification of the suspension. These variants were used in all subsequent experiments. Compared to the literature (Touraev et al. 1996), we did not observe the expected two distinct populations of microspores after starvation, but we found all sizes of microspores. This finding made further sample separation very difficult.
Our experiments with apple demonstrated that heat shock pretreatment is deleterious for apple microspores, while cold temperatures induced a higher frequency of viable embryogenic microspores compared to the variant without pretreatment. In general, starvation is an effective stress treatment for embryogenic induction but the requirement of a heat or cold pretreatment has been shown to be dependent on the plant species. For woody species different pretreatments have been identified, such as 10 days at 4°C for Citrus floral buds (Germana et al. 2000) and 5 days at 33°C for cork oak anthers (Bueno et al. 1997).
Effect of maltose concentration, pH, supplement of spermidine and sodium fluoride
The induction medium is a key factor in the induction and progression of microspore embryogenesis. Previous experiments have demonstrated that embryogenesis from apple microspores was only obtained on the maltose-containing AT3 medium, which is a derivate of N6 medium (Höfer et al. 1999). In the new set of experiments, four maltose concentrations in AT3 medium were evaluated. The results indicated an optimum maltose concentration between 250 and 350 mM (Fig. 3). In subsequent experiments, other apple genotypes were tested and showed the same tendency (data not shown). Compared to our results in anther culture, the optimum was 146 mM and the most active sugar was sucrose (Höfer and Hanke 1990). Data from the literature demonstrated a species-dependent effect in microspore culture; the sugar components and the optimal concentrations are varied (Pescitelli et al. 1990; Ferrie et al. 1999; Guo and Pulli 2000).
Further experiments optimizing the induction medium for microspore embryogenesis were carried out. Neither variation of pH between 6.0 and 6.5 nor a supplement of 2.4 mM spermidine or 5 μM–10 μM sodium fluoride as phosphate inhibitors were fully reproducible.
Effect of microspore density
Although the density at which microspores are plated can influence the success of embryogenesis, few experiments have evaluated microspore density. In previous experiments we have investigated the effect of culture vessels. Small Petri dishes, small Petri dishes coated with collagen and 4-well plates were tested, but embryo induction was obtained only using the last of these. Looking for an explanation, it was obvious that, in contrast to all other dishes used, the 4-well plates cause migration to the center of the plate and therefore give a very high concentration of microspores there (data not shown). This finding was the reason for testing higher microspore densities in the induction medium. Figure 4 shows the average results of two experimental years for the four tested genotypes. In general the values for the single genotypes demonstrated the same tendencies. However, for calculating the polynomic trendlines, to determine the optimal microspore density, the average of all suspensions per year were used. The optimal density was determined to be 14×105 microspores per ml. Therefore we plan further experiments in the interval between 10×105 and 15×105 microspores per ml. Compared to other results from the literature this value is very high. The optimal microspore density was cited as 1×105–1.5×105 microspores per ml in Brassica (Huang et al. 1990; Barro and Martin 1999; Ferrie et al. 1999) and 2×104 microspores per ml in Hordeum (Hoekstra et al. 1993).
Effect of genotype—comparison of efficiency between anther and microspore culture
The four genotypes tested have already demonstrated androgenic response in anther culture (Höfer 1997). Differences in microspore embryogenesis were observed among these cultivars (Fig. 5) when using the same material in the two experimental years. In many species, for example Brassica carinata (Barro and Martin 1999), B. oleracea (Ferrie et al. 1999) and Phleum pratense L. (Guo and Pulli 2000), genotypic differences in microspore embryogenesis occur, and our results in M. domestica concur.
Comparing the efficiency of anther and microspore culture, only the results of the best variants regarding the induction medium and the pretreatment were considered. The data reported in Fig. 5 illustrates big differences between the androgenic pathways depending on the genotype. While the cultivar 'Alkmene' demonstrated a higher embryo induction via anther culture, all other cultivars tested gave better results from microspore culture. An increase of up to 10 times, depending on the genotype is possible. Studies in B. napus and Hordeum vulgare (Siebel and Pauls 1989; Hoekstra et al. 1992) showed that microspore culture can be 5–10 times more efficient than anther culture for embryo production.
In conclusion, the protocol for the induction of isolated microspore embryogenesis have been improved and was made reproducible for other genotypes in apple. Starvation treatment, induction medium, maltose concentration, type of culture vessel, microspore density and genotype influenced embryo induction in M. domestica Borkh. Compared to anther culture, isolated microspore culture can be an effective alternative method of androgenesis in apple. A further comparison of the efficiency of anther and microspore culture, taking into consideration later regeneration processes, is in progress.
References
Barro F, Martin A (1999) Response of different genotypes of Brassica carinata to microspore culture. Plant Breed 118:79–81
Bergen S van, Kottenhagen MJ, van der Meulen RM, Wang M (1999) The role of abscisic acid in induction of androgenesis: a comparative study between Hordeum vulgare L. Cvs. Igri and Digger. J. Plant Growth Regul 18:135–143
Bueno MA, Gomez A, Boscaiu M, Manzanera JA, Vincente O (1997) Stress-induced formation of haploid plants through anther culture in cork oak (Quercus suber). Physiol Plant 99:335–341
Carlson AR, Letarte J, Chen J, Kasha KJ (2001) Visual screening of microspore-derived transgenic barley (Hordeum vulgare L.) with green fluorescent protein. Plant Cell Rep 20:331–337
Chowdhury B, Mandal AB (2001) Microspore embryogenesis and fertile plantlet regeneration in a salt susceptible x salt tolerant rice hybrid. Plant Cell Tissue Organ Cult 65: 141–147
Fei KW, Xue GR (1981) Induction of haploid plantlets by anther culture in vitro in apple cv. 'Delicious'. Sci Agric Sin 4:41–44
Ferrie AMR, Taylor DC, MacKenzie SL, Keller WA (1999) Microspore embryogenesis of high sn-2 erucic acid Brassica oleracea germplasm. Plant Cell Tissue Organ Cult 57:79–84
Germana MA, Crescimanno FG, Motisi A (2000) Factors affecting androgenesis in Citrus clementina Hort. ex Tan. Adv Hortic Sci 14:43–51
Guha S, Maheshwari SC (1964) In vitro production of embryos from anthers of Datura. Nature 4957:497
Guha S, Maheshwari SC (1966) Cell division and differentiation of embryos in the pollen grains of Datura in vitro. Nature 5057:97–98
Guo Y-D, Pulli S (2000) An efficient androgenic embryogenesis and plant regeneration method through isolated microspore culture in timothy (Phleum pratense L.). Plant Cell Rep 19:761–767
Hansen M (2000) ABA Treatment and desiccation of microspore-derived embryos of cabbage (Brassica oleracea ssp. capitata L.) Improves plant development. J Plant Physiol 156:164–167
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence: Intracellular hydrolysis of fluorecein diacetate. Stain Technol 45:115–120
Höfer M (1995) In-vitro-Androgenese bei Apfel. Gartenbauwissenschaft 60:12–15
Höfer M (1997) In vitro androgenesis in apple: optimization of the anther culture. Acta Hortic 447:341–344
Höfer M, Hanke V (1990) Induction of androgenesis in vitro in apple and sweet cherry. Acta Hortic 280:333–336
Höfer M, Hanke V (1994) Antherenkultur bei Apfel und Kirsche: Einfluß des Pollenentwicklungsstadiums—Korrelative Beziehungen zu morphologischen Blütenmerkmalen. Gartenbauwissenschaft 59:225–228
Höfer M, Lespinasse Y (1996) Haploidy in apple. In: Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants, vol 3. Kluwer, Dordrecht, pp 259–274
Höfer M, Touraev A, Heberle-Bors E (1999) Induction of embryogenesis from isolated apple microspores. Plant Cell Rep 18:1012–1017
Hoekstra S, van Zijderveld MH, Louwerse JD, Heidekamp F, van der Mark F (1992) Anther and microspore culture of Hordeum vulgare L. cv. Igri. Plant Sci 86:89–96
Hoekstra S, van Zijderveld MN, Heidekamp F, van der Mark F (1993) Microspore culture of Hordeum vulgare L.: the influence of density and osmolality. Plant Cell Rep 12: 661–665
Huang B, Bird S, Kemble R, Simmonds D, Keller W, Miki B (1990) Effects of culture density, conditioned medium and feeder cultures on microspore embryogenesis in Brassica napus L. cv. Topas. Plant Cell Rep 8:594–597
Jähne A, Lörz H (1995) Cereal microspore culture. Plant Sci 109:1–12
Jain SM, Sopory SK, Veilleux RE (1996) In vitro haploid production in higher plants, vol 2. Applications. Kluwer, Dordrecht
Kunz C, Islam SMS, Berberat J, Peter SO, Büter B, Stamp P, Schmid JE (2000) Assessment and Improvement of wheat microspore derived embryo induction and regeneration. J Plant Physiol 156:190–196
Kyo M, Harada H (1986) Control of the developmental pathway of tobacco pollen in vitro. Planta 168:427–432
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pescitelli SM, Johanson CD, Petolino JF (1990) Isolated microspore culture of maize: effects of isolation technique, reduced temperature, and sucrose level. Plant Cell Rep 8: 628–631
Ryan AB, Castillo AM, Valles MP, Sanz JM, Cistue L (1999) Desiccated doubled-haploid embryos obtained from microspore culture of barley cv. Igri. Plant Cell Rep 18: 924–928
Siebel J, Pauls KP (1989) A comparison of anther and microspore culture as a breeding tool in Brassica napus. Theor Appl Genet 78:473–479
Touraev A, Indrianto A, Wratschko I, Vicente O, Heberle-Bors E (1996) Efficient microspore embryogenesis in wheat (Triticum aestivum L.) induced by starvation at high temperatures. Sex Plant Reprod 9:209–215
Touraev A, Stöger E, Voronin V, Heberle-Bors E (1997) Plant male germ line transformation. Plant J 12:949–956
Xue GR, Niu JZ (1984) A study on the induction of apple pollen plants. Acta Hortic Sin 11:161–164
Acknowledgements
I am grateful to Prof. E. Heberle-Bors and to Dr. A. Touraev for the opportunity to learn the method of microspore culture in tobacco and wheat in their laboratory during a short-term scientific mission of COST Action 824. I thank S. Schöber and R. Gläß for technical assistance in microspore culture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Lörz
Rights and permissions
About this article
Cite this article
Höfer, M. In vitro androgenesis in apple—improvement of the induction phase. Plant Cell Rep 22, 365–370 (2004). https://doi.org/10.1007/s00299-003-0701-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0701-y