Abstract
An improvement of the protocol for haploid induction through anther culture of Citrus clementina Hort. ex Tan. cv. Nules was achieved following the evaluation of a number of the factors affecting androgenesis. The influence of thidiazuron (TDZ) and three temperature pre-treatments (4°C, 25°C, 32°C) on the floral buds with respect to anther culture of C. clementina Hort. ex Tan., cv. Nules was investigated. An increased embryoid production was induced in the medium supplemented with TDZ. Pre-treatment temperatures of 4°C and 25°C were more favorable for embryo production than 32°C. Regeneration of androgenic haploid plantlets from cv. SRA 63 of C. clementina is reported here for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vitro androgenesis has been reported for over 200 plant species (Dunwell 1986; Sangwan-Norreel et al. 1986; Bajaj 1990; Raghavan 1990; Wenzel et al. 1995). Despite considerable efforts, microspore embryogenesis protocols have been less successful in woody plants, which are generally characterized by a long reproductive cycle, a high degree of heterozygosity and, sometimes, by self-incompatibility. Several methods have been used to obtain haploids, such as anther culture, isolated microspore culture, and wide hybridization (chromosome elimination; bulbosum method; Kasha and Kao 1970). In situ parthenogenesis following pollination with irradiated pollen (Ollitrault et al. 1996) or with triploid pollen (Oiyama and Kobayashi 1993) and in vitro parthenogenesis following pollination with triploid pollen (Germanà and Chiancone 2001) have also been successfully used to obtain haploids in Citrus.
Androgenesis through in vitro anther and isolated microspore culture is the most widely used system for haploid production. Although extensive research has been carried out on androgenesis in Citrus spp. and their relatives, very few successful results have been published. Haploid calli and plantlets have been recovered from Poncirus trifoliata L. Raf. (Hidaka et al. 1979), Citrus madurensis Lour. (Chen et al. 1980), and C. clementina Hort. ex Tan. (Germanà et al. 1994). Haploid but albino embryoids of Mapo tangelo (C. deliciosa × C. paradisi) (Germanà and Reforgiato 1997) have been reported as well as the recovery of embryoids and leafy structures of C. limon L. Burm. f. (Germanà et al. 1991). Haploid embryoids of Clausena excavata (Froelicher and Ollitrault 2000) have also been obtained.
Since the first production of haploid embryogenic calli and subsequent plantlet regeneration by anther culture of Citrus clementina cv. Nules was reported (Germanà et al. 1994), several studies have been carried out to improve both the frequency of microspore embryogenesis and the percentage of plantlet regeneration (Germanà and Reforgiato 1997; Germanà et al. 2000a, 2000b). In fact, before practical advantage of the haploid potential of Citrus breeding can be applied, fundamental research goals, such as an increase in the number of respondent genotypes, an improvement in the induction rate (the frequency of pollen grains which form embryoids), and an increase in the survival rate [the percentage of regenerated haploid and doubled haploid (DH) plants successfully transferred from in vitro to in vivo conditions], have to be met.
The successful recovery of haploids depends on several factors, such as genotype, pre-treatment, pollen development stage, media composition, and culture conditions. The research reported here has been carried out to improve the induction rate of androgenesis in Citrus clementina cv. Nules. For this purpose, we investigated: (1) the effect of thidiazuron (TDZ) in the induction medium, (2) the effect on anther culture at three temperature pre-treatments (4°C, 25°C, 32°C) applied to the flower buds. Improvement in the induction rate has also allowed us to increase the number of C. clementina cultivars respondent to androgenesis.
Materials and methods
Plant material
Flower buds were harvested in March from 25-year-old (approximately) trees of Citrus clementina cvs. Nules and SRA 63 that grow at the collection orchard of the Istituto di Ricerca per la Genetica degli Agrumi, National Research Council in Lascari (Palermo, 38°N). Cultivar Nules, discovered as a bud mutation on a Fina tree, is one of the most extensively grown clementine varieties in Spain. Cultivar SRA 63 was selected at the Citrus Research Station (Station de Recherches Agrumicoles) of San Giuliano (Corsica) from a collection of Moroccan clementines (Saunt 1990).
The experiments were carried out in two consecutive years. In the first year, the experiments on the effect of TDZ and temperature pre-treatments were carried out on cv. Nules. In the second year, the trials dealt with both cultivars, Nules and SRA 63.
Pollen developmental stage
The stage of pollen development was tested in one anther per bud size using the acetic-carmine method (Sharma and Sharma 1972). The anthers were collected from flower buds at different stages of development and squashed in 1% acetocarmine in 45% acetic acid for observation under an optic microscope to identify the uninucleate stage of pollen development. This stage had previously been determined to be the most responsive for clementine androgenesis (our unpublished results). For subsequent experiments, only flower buds of the same size as those with anthers (3.5–4.0 mm in length) with microspores at the uninucleate stage were selected for culture.
Anther culture
Following the pre-treatments, the flower buds were surface-sterilized by immersion for 3 min in 70% (v/v) ethyl alcohol, followed by immersion in a sodium hypochlorite solution (about 0.5% active chlorine in water) for 20 min, and finally rinsed three times for 5 min each time with sterile distilled water. Petals were aseptically removed with small forceps, and the anthers were carefully dissected and placed in 6-cm-diameter Petri dishes containing 10 ml of solid medium. Between 60 and 65 anthers were placed in each dish. The subculture period was 40 days.
Culture conditions
The Petri dishes with anthers were sealed with Parafilm, incubated initially at 27±1°C for 15 days in the dark, followed by incubation under a 16/8-h photoperiod with light supplied by cool-white fluorescent lamps (Philips TLM 30 W/84) at a photosynthetic photon flux density of 35 μmol m−2 s−1.
Media composition
Induction media
The basal medium used was N6 Chu (1978) supplemented with Nitsch and Nitsch vitamins (1969), 18 g/l lactose, 9 g/l galactose, 5% coconut water (Sigma, St. Louis, Mo.), 500 mg/l casein, 200 mg/l l-glutamine, 0.5 mg/l biotin, and 500 mg/l ascorbic acid. The following growth regulators were added to the culture medium before autoclaving (in milligrams per liter): NAA, 0.02; 2,4-D, 0.02; KI, 1.0; 6-BA, 0.5; ZEA, 0.5; TDZ, 0.1; GA3, 0.5. The pH was adjusted to 5.8 with 1 N KOH before autoclaving (20 min, 120°C). Agar (0.8% of washed agar; Sigma) was added as the gelling agent. The embryogenic haploid calli were multiplied on MS (Murashige and Skoog 1962) medium supplemented with 5% sucrose, 0.02 mg/l NAA and 0.8% agar.
Germination medium
The embryoids were germinated as soon as they reached 2–3 mm in size, either in Magenta boxes (Sigma V8505) or in test tubes with MS medium containing 3% sucrose, 1 mg/l GA3, 0.01 mg/l NAA, and 0.75% agar.
Experiments
In the first year, floral buds were harvested, pre-treated at 4°C, 25°C or 32°C in darkness for 48 h and the anthers, were then cultured as described. Anthers pre-treated at 4°C were placed in the medium above described and in the same medium without TDZ to test the effect of this growth regulator. A randomized complete block design was used for the experiments. Fifteen Petri dishes with 60 explants each (anthers/Petri dish) were used per each experimental treatment (900 anthers for treatment for a total of 3,600 anthers).
In the second year, anthers of cvs. Nules and SRA 63 were cultivated in the medium supplemented with TDZ (at 0.1 mg/l) after a pre-treatment at 4°C for 48 h. Thirty Petri dishes with 65 explants each (anthers/Petri dish) were prepared for each genotype (1,950 anthers per genotype for a total of 3,900 anthers).
Data collection
Every month up to 10 months after induction, the number of anthers that did not develop, those that became swollen, and/or those that produced embryoids and calli were observed in each Petri dish. This interval was chosen because it had been observed previously that after 10 months Citrus anthers do not undergo further significant development.
The percentages of anthers with callus and with embryoids or embryogenic calli were calculated within each Petri dish. These values were used to calculate means. The effects of temperature pre-treatment, TDZ, and genotype on the data collected were tested by analysis of variance at the P≤0.05 level. Differences among means were tested by Student-Newman-Keuls' (SNK) Multiple Comparison test.
Plant recovery and morphological observations
Because of previously observed slow growth of plantlets in soil— presumably due to the expression of harmful recessive genes expressed in homozygosity (inbreeding depression)—small apexes (2–3 mm) of regenerated plantlets were grafted in vitro onto 20-day-old, etiolated Troyer citrange [Citrus sinensis (L.) Osbeck × Poncirus trifoliata (L.) Raf.] seedlings. The plantlets obtained were subsequently transferred to the greenhouse in pots containing sterilized peat moss, sand and soil in a 1:1:1 ration for the hardening phase.
Cytological observations
Cytological observations based on the acetocarmine method were performed weekly during the first month of culture and then every 2 weeks on the anthers in culture to observe the nuclear divisions of the microspores. Chromosome number was counted in calli and root-tip cells from regenerated embryoids and plantlets using the standard Feulgen technique (Lillie 1951). The explants were pre-treated with a 0.05% (w/v) aqueous solution of colchicine for 2 h at room temperature, fixed overnight in ethanol:glacial acetic acid (3:1, v/v), and stored in 70% ethanol until they could be observed under the microscope.
Microsatellite analyses
Three microsatellites (TAA27, CAC23 and TAA41) described in Kijas et al. (1997) were analyzed to study homozygosity of the regenerated plantlets. PCR amplifications were performed as described in Kijas et al. (1997) with minor modifications using 300 ng of genomic DNA per 25 μl of reaction. Protocols for DNA extraction, polyacrylamide gel electrophoresis and silver staining of amplification products are described in Ruiz et al. (2000).
Results and discussion
Embryogenesis and regeneration of plantlets
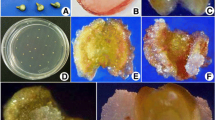
After 1 week of culture, most of the anthers were swollen, and after 2–3 months they started to produce calli. Most calli were not morphogenic, but some of them (20 in the first year, 100 in the second one) appeared to be highly embryogenic. Morphogenic calli were friable (Fig. 1A) and white. Occasionally calli developed from two different lobes of an anther (Fig. 1B). Embryogenic calli differentiated into clumps of embryoids (Fig. 1C). Well-structured embryoids showed normal developmental patterns: globular, heart, torpedo and cotyledonary stages. Secondary embryoids developed as well.
A Morphogenic, friable, white callus emerging after 3 months of culture from the inside of an anther, cv. Nules. B Calli emerging from two different lobes of an anther of cv. Nules. C Groups of haploid embryoids at different stages developing from a single SRA 63 anther in culture. D Embryoids without callus formation from Citrus anther culture. E Shoot regeneration from a compact structure produced by Citrus anther culture. F Doubled-haploid cv. Nules in vitro grafted onto Troyer citrange seedlings to enhance plant recovery
Pluri-cotyledonary embryoids, green and spherical pseudobulbils, with or without callus, were often found. Sometimes direct gametic embryogenesis without callus formation was obtained (Fig. 1D). This was observed in 7% of the respondent anthers of Nules and in 45% of the respondent anthers of SRA 63. Plant regeneration was also obtained via direct organogenesis in 2% of the cases from compact structures produced by anthers when these structures were transferred to germination medium (Fig. 1E).
Most calli were able to regenerate for a long period by secondary embryogenesis. Counting performed after the second subculture on homozygous embryoids obtained in the second year showed that Nules and SRA 63 anthers had produced by this time a total of approximately 1,000 and 570 embryoids, respectively. That this is an improvement in haploid induction is evident when we compare these results with previous ones obtained with other androgenic and gynogenic protocols. Germanà et al. (1994) reported the production of only six haploid plantlets by anther culture of C. clementina cv. Nules. The production of nine haploid plantlets and two embryogenic callus lines has also been reported in clementine (C. clementina Hort. ex Tan.) cv. SRA 63 after in situ parthenogenesis induced by irradiated pollen of Meyer lemon (C. meyeri Y. Tan.) (Ollitrault et al. 1996). However, the SRA plantlets did not develop further and ultimately died. Moreover, three haploid plants were obtained from two mono-embryonic diploid (clementine and Lee)×triploid hybrid of Kawano natsudaidai (C. natsudaidai) in vivo crosses (Oiyama and Kobayashi 1993). The recovery of 14 haploid plantlets regenerated through gynogenesis in C. clementina Hort. ex Tan. cv. Nules after in vitro pollination with pollen grains of Oroblanco, a triploid cultivar of grapefruit, has been recently reported (Germanà and Chiancone 2001).
The well-structured embryoids were isolated and transferred into test tubes or in Magenta GA-7 vessels containing germination medium. The haploid embryoids germinated, and the resulting plantlets were highly vigorous as were the somatic embryoids. Because of the very high conversion rate of well-structured embryoids (96%, independentl of the cultivar used), the improvement was clear when compared with the previous results of clementine anther culture (Germanà et al. 1994). Plantlet losses were mainly due to contamination during the in vitro culture phases and during the acclimatization in vivo. The grafting in vitro of DH material onto etiolated Troyer citrange seedlings represents a successful method by which to transfer the regenerants from in vitro to in vivo (Fig. 1F). To date, 54 plants have been established in soil.
Cytological observation
Cytological observations showed the presence of multinucleated pollen grains after 1 week of culture. Chromosome counts carried out on root apexes of embryoids obtained from clementine anther culture showed a haploid set of chromosomes (n=x=9) (results not shown). During culture, most haploid calli diploidized spontaneously, producing DH embryoids and plantlets (Germanà 1997), and sometimes triploidized.
Microsatellite analyses
Because of the spontaneous diploidization of the haploid calli, cytological analyses could not always be used to determine androgenic plants. Microsatellites are short, tandemly repeated DNA sequences that are suitable markers for several applications in genetic analyses because of their positive features, such as co-dominant inheritance and high polymorphism. The mother plants and the regenerants of C. clementina cv. Nules and SRA 63 were analyzed using three microsatellites (TAA27, CAC23, and TAA41) described in Kijas et al. (1997). All of the mother plants showed two small amplification bands (indicated with an arrow in Fig. 2), while those that originated from them through anther culture had a single band at the same level, demonstrating that they retained the homozygous state (Fig. 2). Microsatellite analysis confirmed the androgenic nature of the regenerants in most of the samples examined (25 Nules from the first year, 30 Nules and 35 SRA 63 from the second year), with one exception in the first year. This heterozygous anther-derived plantlet may have arisen from: (1) somatic tissues of anther walls, (2) fusion of nuclei, (3) endomitosis, or (4) irregular microspores formed by meiotic irregularities (D'Amato 1977; Sunderland and Dunwell 1977; Narayanaswamy and George 1982; Sangwan-Norreel1983).
Polyacrylamide gel electrophoresis of microsatellites TAA27, CAC23 and TAA41 showing the homozygous state of anther culture-derived plants. Genomic DNA was extracted from leaves of mother plants (lanes 1, 6 and 11) and plants derived from them through anther culture (lanes 2–5, 7–10 and 12–15). M DNA ladder (Real Escala no. 2, Durviz S.L.)
Influence of temperature pre-treatments
The effect of the temperature pre-treatments on the percentage of anthers with calli and embryoids or embryogenic calli is shown in Table 1. The percentage of anthers that did not develop was similar in all three temperature pre-treatments. The percentage of swollen anthers was higher following the 32°C pre-treatment , while the greatest number of anthers producing callus and embryoids or embryogenic calli was observed when the floral buds were pre-treated at 25°C. There was not significant difference between the percentages of anthers producing embryoids and/or embryogenic callus coming from floral buds pre-treated at 25°C and at 4°C. These results indicate that the highest temperature (32°C) applied to the floral buds before culture is not recommended for androgenesis in Citrus cv. Nules. The 48-h treatment was chosen because a longer period at high temperature (32°C) was even more detrimental for excised Citrus floral buds. The beneficial effect of pre-treatment at an elevated temperature for androgenesis has been reported in Solanum melongena L. (Rotino 1996), in Brassica oleracea (Keller et al. 1983), and Solanum chacoense (Cappadocia et al. 1984).
A number of hypotheses exist to explain the beneficial effect of a cold pre-treatment with respect to embryo induction. It seems that a cold pre-treatment increases the number of pollen grains with two equal nuclei and stimulates embryoid formation by delaying and modifying pollen mitosis or by blocking starch production, thereby dissolving microtubules (Nitsch 1977) or maintaining viability of the cultured P-grains (Heberle-Bors 1985). Cold pre-treatments are routinely used in the anther culture of many crops, and their effects are genotype-dependent (Powell 1988; Osolnik et al. 1993).
In Citrus anther culture, a low temperature pre-treatment (at 4–5°C for either 2 h, overnight, or for 3–6 days) has been previously used (Starrantino 1986; Ling et al. 1988; Geraci and Starrantino 1990; Germanà et al. 1991, 1994; Deng et al. 1992). In a study carried out by Chen (1985), the effect of pre-treatment at 3°C for 5–10 days on C. madurensis Lour. was beneficial for inducing callus and embryoids.
Influence of TDZ
The effects of TDZ on clementine Nules anther culture are shown in Table 2.
The percentage of anthers producing embryoids or embryogenic callus was statistically higher (1.8%, sixfold the control) in anthers cultivated in the medium supplemented with TDZ. Although the use of TDZ had a distinct positive effect, further investigations are necessary to test different concentrations of this cytokinin.
TDZ is one of the most active cytokinins used in woody plant tissue culture and enables micropropagation of the most recalcitrant species (Huetteman and Preece 1993). TDZ has also been employed in the anther culture of several plant species. For instance, in Hordeum vulgare androgenesis, the addition of 0.01 mg/l TDZ increased or decreased the number of embryos per 100 anthers or it had no effect, depending on the cultivar (Ouèdraogo et al. 1998). Conversely, in Paeonia albiflora anther culture, the addition of 0.0002 mg/l TDZ improved embryogenesis (Lee et al. 1992) as in grapevine anther culture, in which the a TDZ supplement (2.0 mg/l) enhanced somatic embryogenesis (Harst-Langenbucher and Alleweldt 1993). TDZ (0.1 mg/l) has also been successfully used in the regeneration medium of androgenetic embryoids of apple (Hofer 1995).
Influence of genotype
The results of the anther culture experiments of clementine Nules and SRA 63 carried out in the second year are shown in Table 3. With respect to the genotype influence, a better performance (a statistically higher percentage of anthers producing embryogenic calli) of Nules (4.3%) was observed when compared to SRA 63 (3.0%). The trees from which the floral buds were collected are close to each other, and they grow under identical cultural conditions. As all other factors influencing androgenesis are the same, it is possible to argue that the Nules genotype responds better than SRA 63 to these tested conditions. It has been shown in many species that genotype differences do exist with respect to androgenesis (Vasil 1980).
Widening the range of genotypes responding to androgenesis is one of the purposes of anther culture protocols of recalcitrant genera like Citrus. Moreover, for C. clementina cvs. Nules and SRA 63, which are characterized by self-incompatibility, the production of highly embryogenic haploid callus and homozygous plantlets is of great interest. Haploids from self-incompatible C. clementina plants would be desirable for fixing quantitative characters in completely homozygous self-incompatible diploid lines (de Nettancourt and Devreux 1977).
The number of anthers of cv. Nules producing embryoids or embryogenic calli was higher in the second year than in the first year. The different androgenic response may be due to the physiological state of the donor plants, one of the factors affecting androgenesis (Vasil 1980). The physiological condition of the donor plant, influenced by climatic and ecological conditions, should be evaluated in further experiments. This may help us to explain the reasons why the response to anther culture is so season-dependent.
The results of these experiments show that the ability to produce haploids of recalcitrant genotypes, such as C. clementina, can be improved investigating the factors affecting gametic embryogenesis. For this reason, more basic studies on the sporophytic pathway of pollen grains should be carried out.
Abbreviations
- 6-BA :
-
6-Benzylaminopurine
- 2,4-D :
-
2,4-Dichlorophenoxyacetic acid
- GA 3 :
-
Gibberellic acid
- KI :
-
Kinetin
- NAA :
-
α-Naphthaleneacetic acid
- TDZ :
-
Thidiazuron (N-phenyl-1,2,3,-thi-diazol-5-ylurea)
- ZEA :
-
Zeatin
References
Bajaj YPS (1990) In vitro production of haploids and their use in cell genetics and plant breeding. In: YPS Bajaj (ed) Haploids in crop improvement. Biotechnology in agriculture and forestry, vol 12. Springer, Berlin Heidelberg New York, pp 1–44
Cappadocia M, Cheng DSK, Ludlum-Simonette R (1984) Plant regeneration from in vitro culture of anthers of Solanum chacoense Bitt. and interspecific diploid hybrids S. tuberosum L.×S. chacoense Bitt. Theor Appl Genet 62:155–159
Chen Z (1985) A study on induction of plants from Citrus pollen. Fruit Var J:44–50
Chen Z, Wang H, Liao H (1980) The induction of Citrus pollen plants in artificial media. Acta Genet Sin 7:189–192
Chu C (1978) The N6 medium and its applications to anther culture of cereal crops. In: Proc Symp Plant Tissue Cult. Science Press, Peking, pp 43–50
D'Amato F (1977) Cytogenetics of differentiation in tissue and cell cultures. In: Reinert J, Bajaj YPS (eds) Applied and fundamental aspects of plant cell, tissue, and organ culture. Springer, Berlin Heidelberg New York, pp 343–356
Deng XX, Deng ZA, Xiao SY, Zhang WC (1992) Pollen derived plantlets from anther culture of Ichang papeda hybrids No.14 and Trifoliate orange. Proc Intl Soc Citricult 1:190–192
Dunwell JM (1986) Pollen, ovule and embryo culture, as tools in plant breeding. In: Withers LA, Alderson PG (eds) Plant tissue culture and its agricultural applications. Butterworths, London, pp 375–404
Froelicher Y, Ollitrault P (2000) Effects of the hormonal balance on Clausena excavata androgenesis. In: Goren R, Goldschmidt EE (eds) Proc 1st Int Symp Citrus Biotechnol. Acta Hortic 535:139–146
Geraci G, Starrantino A (1990) Attempts to regenerate haploid plants from in vitro cultures of Citrus anthers. Acta Hortic 280:315–320
Germanà MA (1997) Haploidy in Citrus. In: Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants, vol 5. Kluwer, Dordrecht, pp 195–217
Germanà MA, Chiancone B (2001) Gynogenetic haploids of Citrus after in vitro pollination with triploid pollen grains of a triploid cultivar. Plant Cell Tissue Organ Cult 66:59–66
Germanà MA, Reforgiato G (1997) Haploid embryos regeneration from anther culture of 'Mapo' tangelo (Citrus deliciosa × C. paradisi). Adv Hortic Sci 11:147–152
Germanà MA, Crescimanno FG, De Pasquale F, Wang YY (1991) Androgenesis in 5 cultivars of Citrus limon L. Burm. f. Acta Hortic 300:315–324
Germanà MA, Wang YY, Barbagallo MG, Iannolino G, Crescimanno FG (1994) Recovery of haploid and diploid plantlets from anther culture of Citrus clementina Hort. ex Tan. and Citrus reticulata Blanco. J Hortic Sci 69:473–480
Germanà MA, Crescimanno FG, Motisi A (2000a) Factors affecting androgenesis in Citrus clementina Hort. ex Tan. Adv Hortic Sci 14:49–58
Germanà MA, Crescimanno FG, Reforgiato G, Russo MP (2000b) Preliminary characterization of several doubled haploids of Citrus clementina cv. Nules. In: Goren R, Goldschmidt EE (eds) Proc 1st Int Symp Citrus Biotechnol. Acta Hortic 535:183–190
Harst-Langenbucher M, Alleweldt G (1993) The effect of different pre-treatments on induction of somatic embryogenesis in anthers of grapevine cv. Riesling. Vitis 32:1-7
Heberle-Bors E (1985) In vitro haploid formation from pollen: a critical review. Theor Appl Genet 71:361–374
Hidaka T, Yamada Y, Shichijo T (1979) In vitro differentiation of haploid plants by anther culture in Poncirus trifoliata (L.) Raf. Jpn J Breed 29:248–254
Hofer M (1995) In vitro androgenesis in apple. Gartenbauwissenschaft 60:12–15
Huetteman A, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Kasha KJ, Kao KN (1970) High frequency haploid production in barley (Hordeum vulgare L.). Nature 225:874–876
Keller WA, Armstrong KC, Dela Roche IA (1983) The production and utilization of microspore-derived haploids in Brassica crops. In: Sen SK, Giles KL (eds) Plant cell culture in crop improvement. Plenum, New York, pp 169–183
Kijas JMH, Thomas MR, Fowler JCS, Roose ML (1997) Integration of trinucleotide microsatellites into a linkage map of Citrus. Theor Appl Genet 94:701–706
Lee BK, Ko JA, Kim YS (1992) Studies on the thidiazuron treatment of anther culture in Paeonia albiflora. J Korean Soc Hortic Sci 33:384–395
Lillie RD (1951) Simplification of the manufacture of Schiff reagent for use in histochemical procedures. Stain Technol 26:163–165
Ling J, Iwamasa M, Nito N (1988) Plantlet regeneration by anther culture of Calamondin (C. madurensis Lour.). Proc 6th Intl Soc Citricult 1:251–256
Murashige T, Skoog FA (1962) Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Narayanaswamy S, George L (1982) Anther culture. In: Johri BM (ed) Experimental embryology of vascular plants. Springer, Berlin Heidelberg New York, pp 79–103
Nettancourt D de, Devreux M (1977) Incompatibility and in vitro cultures. In: Reinert J, Bajaj YPS (eds) Applied and fundamental aspects of plant cell, tissue and organ culture. Springer, Berlin Heidelberg New York, pp 426–441
Nitsch C (1977) Culture of isolated microspores. In: Reinert J, Bajaj YPS (eds) Applied and fundamental aspects of plant cell, tissue and organ culture. Springer, Berlin Heidelberg New York, pp 268-278
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163:85–87
Oiyama, II, Kobayashi S (1993) Haploid obtained from diploid × triploid crosses of Citrus. J Jpn Soc Hortic Sci 62:89–93
Ollitrault P, Allent V, Luro F (1996) Production of haploid plants and embryogenic calli of clementine (Citrus reticulata Blanco) after in situ parthenogenesis induced by irradiated pollen. Proc Int Soc Citricult 2:913–917
Osolnik B, Bohanec B, Jelaska S (1993) Stimulation of androgenesis in white cabbage (Brassica oleracea var. capatata) anthers by low temperature and anther dissection. Plant Cell Tissue Organ Cult 32:241–246
Ouédraogo JT, St. Pierre CA, Collin J, Rioux S, Comeau A (1998) Effect of amino acids, growth regulators and genotype on androgenesis in barley. Plant Cell Tissue Organ Cult 531:59–66
Powell W (1988) The influence of genotype and temperature pre-treatment on anther culture response in barley (Hordeum vulgare L.). Plant Cell Tissue Organ Cult 12:291–297
Raghavan V (1990) From microspore to embryoid: faces of the angiosperm pollen grain. In: Nijkamp HJJ, van der Plas LHW, van Hartrigik J (eds) Progress in plant cellular Mol Biol. Kluwer, Dordrecht, pp 213–221
Rotino GL (1996) Haploidy in eggplant. In: Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants, vol 3. Kluwer, Dordrecht, pp 115–142
Ruiz C, Paz Breto M, Asins MJ (2000) A quick methodology to identify sexual seedlings in citrus breeding programs using SSR markers. Euphytica 112:89–94
Sangwan-Norreel BS (1983) Male gametophyte nuclear DNA content evolution during androgenic induction in Datura innoxia. Z Pflanzenphysiol 111:47–54
Sangwan-Norreel BS, Sangwan RS, Pare J (1986) Haploidie et embryogenèse provoquée in vitro. Bull Soc Bot Fr Actual Bot 133:7–39
Saunt J (1990) Citrus varieties of the world. Sinclair Int, UK
Sharma AK, Sharma A (1972) Chromosome techniques. Butterworths, London/University Park Press, Baltimore
Starrantino A (1986) Produzione di aploidi androgenetici di agrumi. In: Il recente contributo della ricerca allo sviluppo dell'agrumicoltura italiana. Delfino, Cagliari, pp.31–37
Sunderland N, Dunwell JM (1977) Anther and pollen culture. In: Street HE (ed) Plant tissue cell culture. Blackwell, Oxford, pp 223–265
Vasil IK (1980) Androgenic haploids. Intl Rev Cytol Suppl 11A:195–223
Wenzel G, Frei U, Jahoor A, Graner A, Foroughghi-Wehr B (1995) Haploids—an integral part of applied and basic research. In: Terzi M, Cella R, Falavigna A (eds) Current issues in plant molecular and cellular biology. Kluwer, Dordrecht, pp 127–135
Acknowledgements
The authors wish to thank Prof. K. Glimelius for his critical reading of the manuscript and Prof. H. Lorz for his helpful suggestions. This investigation was supported by the National Project 'Plant Biotechnology' of MiPAF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Peña
Both authors have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Germanà, M.A., Chiancone, B. Improvement of Citrus clementina Hort. ex Tan. microspore-derived embryoid induction and regeneration. Plant Cell Rep 22, 181–187 (2003). https://doi.org/10.1007/s00299-003-0669-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0669-7