Abstract
The effects of plant growth regulators, explant types, and culture regimens were investigated on in vitro shoot proliferation from terminal bud explants of Curcuma longa. Each bud was longitudinally divided into four equal pieces, each 1 cm in length, and used as explants. These were then cultured on MS medium supplemented with 18.17 μM thidiazuron for 4 weeks prior to transfer to MS medium without growth regulator for 8 weeks. Under these conditions, a shoot induction rate of 18.22±0.62 shoots/explant was obtained after 12 weeks of cultures. Spontaneous rooting was achieved. The regenerated plants were transferred to soil under greenhouse conditions and subsequently grown successfully in the field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Curcuma longa L. (Zingiberaceae), turmeric, is a tropical herb widely used both in food and traditional medicine. In Thai traditional medicine, turmeric is used as a carminative, for dyspepsia, and also externally for itching and infected wounds (Saralamp et al. 1996). Pharmacological and clinical studies have indicated the efficacy of turmeric for the treatment of dyspepsia (Thamlikitkul et al. 1989), peptic ulcers (Prucksunand et al. 2001), and gastric ulcers (Muderji et al. 1981; Sakai et al. 1989; Rafatullah et al. 1990; Kositchaiwat et al. 1993; Masuda et al. 1993). For medicinal purposes, the quality of turmeric is based on the amounts of curcuminoids and volatile oil present, both pharmacologically active compounds (World Health Organization 1999).
Although the cultivation of medicinal plants has many advantages, little effort has been expended with respect to relevant agronomic research in that direction (Palevitch 1988; Tyler 1988). The plants continue to be cultivated in the same way as they always have been. Most cultivated medicinal plants, excluding Papaver somniferum, P. bracteatum, Cinchona spp., Digitalis lanata, Chamomilla recutita, and Mentha piperita, are still genetically "wild" types (Tyler 1988; Chomchalow 1993). We have found a large variation in curcuminoid and volatile oil contents among turmeric grown in different parts of Thailand (unpublished data). Consequently, we believe that there is a strong need to genetically improve and cultivate turmeric with high and stable contents of curcuminoids and volatile oil.
In vitro propagation offers many advantages over conventional propagation methods. True-to-type multiplication provides uniform plants with genetic identity. Morphological and chemical uniformity among plants regenerated by this technique has been reported in various species of medicinal plants, such as shoot-tip and axillary bud cultures of Aconitum carmichaeli (Hatano et al. 1988), shoot-tip cultures of Atractylodes spp. (Hatano et al. 1990), node cultures of Gentiana scabra (Yamada et al. 1991), and stem-tip cultures of Stevia rebaudiana (Tamura et al. 1984).
Our investigations are aimed at applying plant tissue culture techniques to generate high-quality somaclones of turmeric with an increased, constant levels of curcuminoids and volatile oils for phytomedicine production. Although a number of protocols for in vitro propagation of C. longa have been published using bud or shoot-tip explants (Yasuda et al. 1988; Keshavachandran and Khader 1989; Winnaar 1989; Balachandran et al. 1990; Rajan 1997; Shirgurkar et al. 2001; Sunitibala et al. 2001; Salvi et al. 2002), leaf-base explants (Salvi et al. 2001), and immature inflorescence explants (Salvi et al. 2000), these procedures are inadequate to meet the need in time. We report here a system for high-frequency shoot multiplication of C. longa terminal bud explants using TDZ.
Materials and methods
Plant materials
Shoots were collected from 2-month-old Curcuma longa plants and washed thoroughly in running tap water. Sections of shoots 2 cm long were treated with 70% ethanol for 1 min, rinsed with distilled water, immersed for 20 min in a commercial bleach solution of 1.5% sodium hypochlorite containing 50 μl Tween 80, and finally rinsed three times in sterile distilled water under sterile conditions. Leaf sheaths of the shoots were trimmed off. Only terminal buds were collected and surface sterilized with a commercial bleach solution of 0.75% sodium hypochlorite containing 50 μl Tween 80 for 10 min, then rinsed three times in sterile distilled water. The terminal buds were cultured on MS medium (Murashige and Skoog 1962) supplemented with 13.32 μM BA, 3% sucrose, and 0.55% Agargel for 4 weeks, then transferred to PGR-free MS medium. The plants were subcultured every 4 weeks until the beginning of the experiments.
Explants, media, and culture conditions
The effects of PGRs, explant type, and explant age on shoot regeneration of C. longa were studied in three parallel experiments.
-
1.
To study the effect of TDZ alone and in combination with NAA on shoot multiplication, we used both undivided and divided terminal bud explants. For divided terminal bud explants, each bud from in vitro donor plants was longitudinally divided into four equal pieces, each 1 cm in length (Fig 5A). The explants were inoculated vertically on MS medium supplemented with TDZ (0.23–18.17 μM), either alone or in combination with 0.54 μM NAA (Fig 5B). Eight weeks after culture initiation, the explants were transferred to MS medium without PGR (experiment 1).
-
2
To study the effect of a high concentration of cytokinins on shoot multiplication, we used divided terminal bud explants. The explants were cultured on MS medium supplemented with 35.51 μM BA, 37.17 μM Kin, 39.37 μM 2iP, and 18.17 μM TDZ for 8 weeks (experiment 2A) or 4 weeks (experiment 2B), followed by transfer to MS medium without PGR for 2 weeks.
-
3.
To establish and prove the efficiency of a novel protocol for rapid micropropagation of C. longa, we used the divided terminal buds from 1- to 3-month-old donor plants as explants. The explants were cultured on MS medium supplemented with 18.17 μM TDZ for 4 weeks, followed by transfer to MS medium without PGR for 8 weeks (experiment 3).
All media contained 3% sucrose and 0.55% Agargel, and the pH was adjusted to 5.8 before autoclaving. For each treatment, a total of 12 replications each with four explants were inoculated; therefore, 48 explants per treatment were tested. Experiment 3 was repeated five times. Cultures were maintained at 25°C under a 16/8-h (light/dark) photoperiod with a light intensity of 3,000 lux.
Acclimatization and field experiment
After rooting on PGR-free MS medium, regenerated plants were transferred to small pots containing sand and rice shell ash (1:1) under greenhouse conditions for 1 month. The regenerants were then transplanted to the field at the Salaya campus of the Mahidol University, Thailand, and grown for 8 months.
Results and discussion
The incubation of C. longa terminal bud explants on MS media supplemented with TDZ (0.23–18.17 μM) for 8 weeks resulted in 3.42–5.08 shoots/explant for undivided explants (Fig. 1A) and 3.47–4.36 shoots/explant for divided explants (Fig. 2A). Balachandran et al. (1990) reported a proliferation rate of 3.43 shoots/bud after growing terminal buds on MS supplemented with 13.32 μM BA for 4 weeks. Salvi et al. (2002) also reported shoot multiplication rates of 4.2, 3.5, and 6.6 shoots/explant following the culture of shoot-tip explants for 8 weeks in liquid medium supplemented with 1 μM NAA and BA, Kin, or 2iP (10 μM each), respectively. Although we obtained lower shoot induction rates than those reported in previous publications, we were able to demonstrate that the terminal buds of C. longa can be divided into four parts and used as explants for in vitro propagation.
Effect of TDZ alone and in combination with NAA on the shoot induction of undivided terminal bud explants. A Eight weeks after inoculation on MS media supplemented with TDZ and NAA, B 4 weeks after transfer to PGR-free MS medium. Vertical lines Standard error, single asterisk non-significant differences, double asterisk significant differences (P=0.023) using polynomial regressions
Effect of TDZ alone and in combination with NAA on the shoot induction of divided terminal bud explants. A Eight weeks after inoculation on MS media supplemented with TDZ and NAA, B 4 weeks after transfer to PGR-free MS medium. Vertical lines Standard error, single asterisk non-significant differences, double asterisk significant differences (P=0.032 and P=0.046 for TDZ treatment and TDZ and NAA treatment, respectively) using polynomial regressions
Four weeks after the transfer of the explants to MS medium without PGR for 4 weeks, both the shoot induction rate and shoot length had increased in all treatments (Figs. 1B, 2B). In both explant types, the maximum shoot induction rates were obtained at a TDZ concentration of 18.17 μM (13.25±0.70 and 11.33±0.86 shoots/explant for undivided and divided explants, respectively). TDZ, a non-purine cytokinin-like compound, has been shown to exhibit stronger effects than conventional cytokinins over a wide range species. It is effective for axillary shoot proliferation and adventitious shoot organogenesis (Huetteman and Preece 1993). Its mode of action may be attributed to its ability to induce cytokinin accumulation (Victor et al. 1999) and also enhance the accumulation and translocation of auxin within TDZ-exposed tissue (Murch and Saxena 2001).
Our results indicate that exposure of the explants to a high concentration of cytokinins prior to transfer to PGR-free MS medium led to the increased shoot proliferation of C. longa. To determine the optimum period that C. longa cultures should be exposed to cytokinins, we carried out experiments 2A and 2B. The average frequency of explants responding in the control, BA, Kin, 2iP, and TDZ treatments were 68%, 51%, 58%, 64%, and 74%, respectively, calculated across both experiments. In experiment 2A, after the divided explants were cultured on MS medium supplemented with 35.51 μM BA, 37.17 μM Kin, 39.37 μM 2iP, or 18.17 μM TDZ for 8 weeks, the highest shoot multiplications were obtained in the TDZ and BA treatments [3.54±0.42 and 3.01±0.44 shoots/explant, respectively (Fig. 3A)]. When the explants were transferred to MS medium without PGR for 2 weeks, the number of induced shoot per explant increased markedly in both the TDZ and BA treatments [5.58±0.29 and 4.81±0.72 shoots/explant, respectively (Fig. 3B)].
Effect of cytokinins on the shoot induction of divided terminal bud explants. A Eight weeks after inoculation on PGR-MS medium, B 2 weeks after transfer to PGR-free MS medium. Vertical lines Standard error. Bars with the same letter are not significantly different at the 5% level by the Student-Newman-Keuls test
In experiment 2B, the length of the incubation period of the divided explants on the PGR-supplemented MS media was reduced to 4 weeks. The shoot multiplication rates were between 1.11 shoots and 2.05 shoots per explant (Fig 4A). When the explants were transferred to PGR-free MS medium for 2 weeks, only the TDZ treatment demonstrated the dramatically increased shoot induction rate to 5.67±0.76 shoots/explant (Fig. 4B).
Effect of cytokinins on the shoot induction of divided terminal bud explants. A Four weeks after inoculation on PGR-MS medium, B 2 weeks after transfer to PGR-free MS medium. Vertical lines Standard error. Bars with the same letter are not significantly different at the 5% level by the Student-Newman-Keuls test
After transfer of the explants to PGR-free MS medium, the maximum shoot induction rates were essentially the same for those cultured 8 weeks or 4 weeks in TDZ-supplemented MS medium (5.58±0.29 and 5.67±0.76 shoots/explant, respectively). Although the cluster of shoots induced by TDZ were about 1 cm in length, they elongated to almost 3 cm following their transfer to PGR-free MS medium. In comparison with TDZ, only BA could increase shoot induction rate when the explants were incubated 8 weeks. No increased shoot proliferation effect was observed after the Kin and 2iP treatment of 4 weeks or 8 weeks.
In general, a low range of concentrations of TDZ—from 1 nM to 10 μM—has been recommended for shoot proliferation (Huetteman and Preece 1993). High levels of TDZ cause abnormalities or are toxic (Kim et al. 1997; Chand et al. 1999). Our results indicate that an optimum exposure time of explants in TDZ-supplemented medium followed by the withdrawal of PGR effectively triggered shoot multiplication in C. longa. The PGR may be needed for initiating the multiplication of bud meristems. Subsequently, incubation on PGR-free MS medium led the explants to elongation of regenerated shoots as well as induction of more shoot initials.
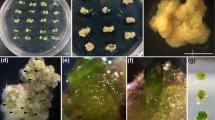
In experiment 3, we established an improved protocol for the micropropagation of C. longa. The divided terminal bud explants were cultured on MS medium supplemented with 18.17 μM TDZ for 4 weeks prior to their transfer to MS medium without PGR for 8 weeks. After 4 weeks of incubation on TDZ, the shoot induction rate was 2.22±0.14 (Fig. 5C), and this increased to 7.12±0.52 and 18.22±0.62 shoots/explant at the 8th week and 12th week, respectively (Fig. 5D). The average shoot length was 3.85 cm after 12 weeks of culture. Rooting was spontaneously achieved (Fig 5E), with an average frequency of 88.4±2.6% (Table 1). The induced shoots that did not produce any roots were transferred to MS medium without PGR for another 4 weeks; every one of those shoots subsequently produced roots. The regenerated plants were transferred to soil under greenhouse conditions and successfully grown under the field conditions (Fig 5F–H). They did not show any detectable variation in morphology or growth characteristics compared to their donor plant.
A protocol developed for rapid micropropagation of Curcuma longa. A Divided terminal bud explant, B inoculated explant, C explants with newly developed shoots, D a cluster of shoots after transfer to PGR-free medium, E, F rooted plantlets, G C. longa plantlets after acclimatization, H micropropagated C. longa in the field. Bars: A–F 1 cm, G 5 cm, H 50 cm
Although, we used explants derived from 1 to 3-month-old donor plants, no significant difference among shoot induction rates from explants of different ages was found at week 12. Our results demonstrate that the age of terminal bud explants does not affect the shoot induction rate of C. longa, which is contrary to those effects reported in woody plants such as Corylus avellana (Betulaceae) (Messeguer and Mele 1987), Wrightia tomentosa (Apocynaceae) (Purohit et al. 1994), Maytenus ilicifolia (Celastraceae) (Pereira et al. 1995), and Morus (Moraceae) (Pattnaik and Chand 1997).
Abbreviations
- BA :
-
6-Benzylaminopurine
- 2iP :
-
N6-(2-Isopentyl)adenine
- Kin :
-
Kinetin
- NAA :
-
α-Naphthaleneacetic acid
- PGR :
-
Plant growth regulator
- TDZ :
-
Thidiazuron
References
Balachandran SM, Bhat SR, Chandel KPS (1990) In vitro clonal multiplication of turmeric (Curcuma spp.) and ginger (Zingiber officinale Rosc.). Plant Cell Rep 8:521–524
Chand H, Pearson MN, Lovell PH (1999) Rapid vegetative multiplication in Colocasia esculenta (L) Schott (taro). Plant Cell Tissue Organ Cult 55:223–226
Chomchalow N (1993) Medicinal and aromatic plants germplasm conservation and utilization in Asia. In: Chomchalow N, Henle HV (eds) Proc Regional Expert Consultation Breed Improve Med Aromatic Plants Asia. FAO regional office for Asia and the Pacific, Bangkok, pp 1–5
Hatano K, Kamura K, Shoyama Y, Nishioka I (1988) Clonal multiplication of Aconitum carmichaeli by tip tissue culture and alkaloid contents of clonally propagated plant. Planta Med 54:152–155
Hatano K, Shoyama Y, Nishioka I (1990) Clonal propagation of Atractylodes japonica and A. ovata by tip tissue culture and atractylon content of clonally propagated plants. Planta Med 56:131–132
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Keshavachandran R, Khader MA (1989) Tissue culture propagation of turmeric. S Indian Hortic 37:101–102
Kim MK, Sommer HE, Bongarten BC, Merkel SA (1997) High-frequency induction of adventitious shoots from hypocotyl segments of Liquidambar styraciflua L. by thidiazuron. Plant Cell Rep 16:536–540
Kositchaiwat C, Kositchaiwat S, Havanondha J (1993) Curcuma longa Linn. in the treatment of gastric ulcer comparison to liquid antacid: a controlled clinical trial. J Med Assoc Thailand 76:601–605
Masuda T, Jitoe A, Isobe J, Nakatani N, Yonemori S (1993) Anti-oxidative and anti-inflammatory curcumin-related phenolics from rhizome of Curcuma domestica. Phytochemistry 32:1557–1560
Messeguer J, Mele E (1987) In vitro propagation of adult material and seedlings of Corylus avellana. Acta Hortic 212:499–503
Muderji B, Zaidi SH, Singh GB (1981) Spices and gastric function. Part I. Effect of Curcuma longa on the gastric secretion in rabbits. J Sci Indian Res 20:25–28
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murch SJ, Saxena PK (2001) Molecular fate of thidiazuron and it effects on auxin transport in hypocotyls tissues of Pelargonium × hortorum Bailey. Plant Growth Regul 35:269–275
Palevitch D (1988) Agronomy applied to medicinal plant conservation. In: Akerele O, Heywood V, Synge H (eds) The conservation of medicinal plants. Cambridge University Press, Cambridge, pp 167–178
Pattnaik S, Chand P (1997) Rapid clonal propagation of three mulberries, Morus cathayana Hemsl., M. lhou Koiz. and M. serrata Roxb., through in vitro culture of apical shoot buds and nodal explants from mature trees. Plant Cell Rep 16:503–508
Pereira A, Moro J, Cerdeira R, Franca S (1995) Effect of phytoregulators and physiological characteristics of the explants on micropropagation of Maytenus ilicifolia. Plant Cell Tissue Organ Cult 42:295–297
Prucksunand C, Indrasukhsri B, Leethochawalit M, Hungspreugs K (2001) Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J Trop Med Public Health 32:208–215
Purohit S, Kukda G, Sharma P, Tak K (1994) In vitro propagation of an adult tree Wrightia tomentosa through enhanced axillary branching. Plant Sci 103:67–72
Rafatullah S, Tariq M, Al-Yahya MA, Mossa JS, Ageel AM (1990) Evaluation of turmeric (Curcuma longa) for gastric and duodenal antiulcer activity in rats. J Ethnopharmacol 29:25–34
Rajan VR (1997) Micropropagation of turmeric (Curcuma longa L.) by in vitro microrhizomes. In: Edison S, Ramana KV, Sasikumar B, Baba KN, Eapen SJ (eds) Biotechnology of spices, medicinal and aromatic plants. Indian Society for Spices, Calicut, pp 25–28
Sakai K, Miyazaki Y, Yamane T, Saitoh Y, Ikawa C, Nishihata T (1989) Effect of extracts of Zingiberaceae herbs on gastric secretion in rabbits. Chem Pharm Bull 37:215–217
Salvi N, George L, Eapen S (2000) Direct regeneration of shoots from immature inflorescence cultures of turmeric. Plant Cell Tissue Organ Cult 62:235–238
Salvi N, George L, Eapen S (2001) Plant regeneration from leaf base callus of turmeric and random amplified polymorphic DNA analysis of regenerated plants. Plant Cell Tissue Organ Cult 66:113–119
Salvi N, George L, Eapen S (2002) Micropropagation and field evaluation of micropropagated plants of turmeric. Plant Cell Tissue Organ Cult 68:143–151
Saralamp P, Chuakul W, Temsiririrkkul R, Clayton T (1996) Medicinal plants in Thailand. Amarin, Bangkok
Shirgurkar M, John C, Nadgauda R (2001) Factor affecting in vitro microrhizome production in turmeric. Plant Cell Tissue Organ Cult 64:5–11
Sunitibala H, Damayanti M, Sharma G (2001) In vitro propagation and rhizome formation in Curcuma longa Linn. Cytobios 105:71–82
Tamura Y, Nakamura S, Fukui H, Tabata M (1984) Comparison of Stevia plants grown from seeds, cuttings and stem-tip cultures for growth and sweet diterpene glucosides. Plant Cell Rep 3:180–182
Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, Theerapong S, Chantrakul C, Thanaveerasuwan T, Nimitnon S, Boonroj P, Punkrut W, Gingsungneon V, Wongkonkatape S, Ekpalakorn W, Boontaeng N, Jesadaporn U, Taechaiya S, Petcharoen S, Riewpaiboon A, Adthasit R, Timsard S, Tenambergen ED (1989) Randomized double blind study of Curcuma domestica Val. for dyspepsia. J Med Assoc Thailand 72:613–620
Tyler VE (1988) Medicinal plant research: 1953–1987. Planta Med 54:95–100
Victor JMR, Murthy BNS, Murch SJ, Krishnaraj S, Saxena PK (1999) Role of endogenous purine metabolism in thidiazuron-induced somatic embryogenesis of peanut (Arachis hypogaea L.). Plant Growth Regul 28:41–47
Winnaar E de (1989) Turmeric successfully established in tissue culture. Inf Bull Citrus Subtrop Fruit Res Inst, South Africa 199:1–2
World Health Organization (1999) WHO monographs on selected medicinal plants, vol 1. WHO, Geneva
Yamada Y, Shoyama Y, Nishioka I, Kohda H, Namera A, Okamoto T (1991) Clonal micropropagation of Gentiana scabra Bunge var. buergeri Maxim. and examination of the homogeneity concerning the gentiopicroside content. Chem Pharm Bull 39:204–206
Yasuda K, Tsuda T, Shimizu H, Sugaya A (1988) Multiplication of Curcuma species by tissue culture. Planta Med:75–78
Acknowledgements
This investigation was supported by the National Research Council of Thailand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Lörz
Rights and permissions
About this article
Cite this article
Prathanturarug, S., Soonthornchareonnon, N., Chuakul, W. et al. High-frequency shoot multiplication in Curcuma longa L. using thidiazuron. Plant Cell Rep 21, 1054–1059 (2003). https://doi.org/10.1007/s00299-003-0629-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0629-2