Abstract
Hairy root cultures of diploid Artemisia annua L. (clone YUT16) grow rapidly and produce the antimalarial sesquiterpene artemisinin. Little is known about how polyploidy affects the growth of transformed hairy roots and the production of secondary metabolites. Using colchicine, we produced four stable tetraploid clones of A. annua L. from the YUT16 hairy root clone. Analysis showed major differences in growth and artemisinin production compared to the diploid clone. Tetraploid clones produced up to six times more artemisinin than the diploid parent. This study provides an initial step in increasing our understanding of the role of polyploidy in secondary metabolite production, especially in hairy roots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most plant species, artificial polyploidy increases the size of the cells, leading to larger reproductive and vegetative organs (Adaniya and Shira 2001; Chen and Goeden-Kallemeyn 1979; Watrous and Wimber 1988). Vegetative plant organs, such as roots, are the source of many commercially important secondary metabolites (Pal Bais et al. 2001). The ginkgolides of Ginkgo biloba and forskolin from the Indian herb Coleus forskohlii, both traditionally used to treat heart and respiratory diseases, are just two examples of the many root-specific secondary metabolites that have medicinal applications. Tropane alkaloids, terpenoids, and isoflavonoids are among the most important medicinal compounds extracted from plant roots.
The induction of artificial polyploidy may prove useful in increasing the production of important medicinal compounds (Dhawan and Lavania 1996) as this has been shown to increase the production of secondary metabolites in many plants compared to their diploid parent. For instance, tetraploids Chamomilla recutita, Petunia Mitchell and Salvia miltiorrhiza Bge produce more flavonoids and terpenoids per gram of tissue than their diploid counterparts (Gao et al. 1996; Griesbach and Kamo 1996; Švehlíková and Repčák 2000). Although the physiological effects of polyploidy are not generally predictable, and the responses are often species-specific, doubling the chromosome number of a plant species that produces useful compounds can enhance overall secondary metabolism.
Artemisia annua L. produces, along with many other terpenoids, the sesquiterpene antimalarial drug artemisinin (Meshnick et al. 1991). Transformation of A. annua plants with Agrobacterium rhizogenes results in the formation of transformed (hairy) roots that can produce high levels of artemisinin (Jaziri et al. 1995; Weathers et al. 1994). A. annua, therefore, offers a useful model system for studying the role of artificial polyploidy on terpenoid production.
Previously, Wallaart et al. (1999) induced polyploidy in A. annua plants, from 18 to 36 chromosomes, by using the mitotic inhibitor colchicine. They reported a polyploidy induction efficiency of 20% and an average specific artemisinin level (μg artemisinin g-1 DW) in the tetraploids that was 38% higher than that of the diploid plants. However, biomass accumulation of the tetraploid plants was lower than that of the diploid plants, so the net yield of artemisinin per square meter of field-grown plants decreased by 25% (Wallaart et al. 1999). Even though the overall artemisinin production was lower in tetraploid whole plants, the study suggested the possibility of using hairy root cultures for the optimization of growth and artemisinin production. In the investigation reported here, we compared the growth and artemisinin production of tetraploid A. annua hairy root cultures with those of their diploid parent.
Materials and methods
Plant material
The transformed root culture used was the Artemisia annua YUT16 clone described by Weathers et al. (1994). Cultures were maintained by subculturing 0.5 g FW of roots every 2 weeks into a 125-ml Erlenmeyer flask containing 50 ml of Gamborg's B5 medium (pH 5.7) (Gamborg et al. 1968) supplemented with 3% sucrose. The culture flasks were kept at 25°C, in shake culture (100 rpm) under continuous cool-white fluorescent light (5 μE m-2 s-1).
Induction of polyploidy in A. annua hairy roots
Filtered-sterilized (0.2 μm) colchicine (Sigma-Aldrich, St. Louis, Mo.), at concentrations of 0, 0.5%, 0.25%, 0.1%, or 0.05% (w/v) in water, was added to filter-sterilized culture medium (Gamborg's B5+3% sucrose, pH 5.7). To induce polyploidy, we placed single 2-cm-long root tips from 14-day-old cultures in uncoated polystyrene six-well plates (Falcon no. 351146) containing 5 ml of colchicine-containing media per well and incubated them at room temperature for 1-7 days. Following the colchicine treatment, the root tips were rinsed twice with approximately 10 ml fresh B5 medium, transferred to fresh B5 semi-solid medium (0.2% w/v GelRite, Sigma-Aldrich), and incubated at 25°C in the dark for 30 days. For determination of survival, the roots were removed from colchicine medium every other day for 7 days. Subsequent root lines were initiated from any lateral root tip growing from the original colchicine-treated roots. These new root lines were examined on a monthly basis for ploidy-level stability. The diploid A. annua YUT-16 clone was grown in the same way as the colchicine-treated root tips, but without colchicine.
Preparation of chromosomes for ploidy-level determination
Before polyploidy was induced, the YUT16 clone of A. annua was cytologically examined to confirm its diploid number of chromosomes (2n=18). Samples of single hairy roots from all cultures were pretreated in a saturated α-bromonaphthalene solution for 24 h at 4°C to accumulate cells in metaphase. At least ten roots were then fixed overnight in cold Carnoy's solution (3:1, 100% ethanol:glacial acetic acid). After rinsing twice with deionized water, root tips were hydrolyzed with 1 N HCl at 60°C for 8 min and then rinsed in deionized water. Excess water was removed by blotting, and the roots were stained for 2 h in Feulgen solution (Singh 1993) at room temperature. Stained root-tip meristems (about 3 mm long) were removed, placed on a clean slide, and squashed in 1% (w/v) aceto-carmine (Singh 1993). The preparations were observed with an optical microscope at a magnification of 1,000×. The best metaphase views were observed with a Nikon Coolpix digital camera (Nikon, Japan). At least twenty meristems were analyzed for each clone. Each putative tetraploid clone was validated as polyploid at least 20 times over 40 subcultures.
Biomass analysis
FW was measured after the roots were washed with deionized water and blotted dry. DW was measured after the roots were oven-dried at 60°C for at least 24 h or until the weight no longer fluctuated.
Extraction and analysis of artemisinin
Artemisinin (AN) was extracted and assayed at 260 nm using HPLC according to the method described by Smith et al. (1997) with the following modifications. One gram of roots (blotted FW) was extracted twice with 3 ml of scintanalyzed toluene in an ultrasonic bath for 30 min in ice-cold water. The extracts were centrifuged at 4,390 g for 10 min and the supernatants subsequently decanted, pooled, dried under nitrogen and stored at –20°C for later analysis by HPLC.
For HPLC analysis, the AN samples were first converted to their Q260 derivatives (Smith et al. 1997) and then applied to a 15-cm Microsorb-MV C-18 column with a 4.6 mm internal diameter that contained 5 μm silica beads (10-nm pore size) (Varian, Walnut Creek, Calif.). The mobile phase consisted of 0.01 M sodium phosphate buffer:methanol [55:45 (v/v)] pH 7.0, at a flow rate of 1.0 ml/min. A linear calibration curve of AN (Sigma-Aldrich) was measured in the 0.1- to 0.5-μg/ml range. The retention time of AN under these conditions was about 12.0 min. Each sample was co-injected a second time with a known amount of artemisinin to validate peak identification.
Single-root growth studies
Single hairy roots, 2 cm in length, from the established diploid (YUT16) and tetraploid clones (Fig. 1) were inoculated into six-well polystyrene plates (one root per well) and grown as described above for cultures in the shake flasks (Kim et el. 2002; Srinivasan et al. 1997). Root lengths, number of laterals, and general appearance of roots were recorded. To compare results from different tetraploid clones directly, we calculated the fractional increase in length of each root by scaling the total length of the primary root by the number of root tips (Yu and Doran 1994; Wyslouzil et al. 2000). This is defined as the root growth unit (RGU): RGU = (Σlength of all laterals + length of primary root) (number of root tips)-1
For the root diameter measurements, single roots from 14-day-old shake flask cultures were mounted on microscope slides and their diameter measured 1 cm from the root tip using an optical microscope with a 40× objective plus a micrometer in a 10× ocular; ten measurements per clone were obtained.
Statistical analysis
The data from all experiments were analyzed using Student's t-test and analysis of variance (ANOVA). All experiments had at least three to six replicates, and each experiment was conducted at least twice.
Results and discussion
Confirmation and stability of polyploidy
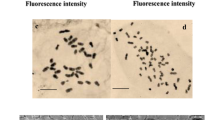
Cytological examination of root tips of the YUT16 parent clone verified it as being diploid, with 18 chromosomes (Fig. 1A) as previously determined for A. annua by others (Wallaart et al. 1999). During colchicine treatment, about 25% of the root tips died regardless of the concentration of colchicine used. The surviving colchicine-treated roots developed new lateral root tips that were used for the creation of subsequent root lines. In total, more than 40 root lines were generated by this method. These roots were prepared for microscopic examination of chromosomes, and at least 20 well-defined metaphase displays per root tip were counted (Fig. 1). Treatments consisting of colchicine concentrations of 0.25% or 0.5% (w/v) for 7 days were the most effective in producing tetraploid hairy root clones. The efficiency of polyploidy induction using colchicine in this method was low. Of the 40 root lines screened, only four (YUT16-9Lb, YUT16-8LT, YUT16-6P and YUT16-7P; Fig. 1) were determined to be stable tetraploids with n=36. Only one of the tetraploid clones, YUT16-6P, is shown since all four clones studied were morphologically indistinguishable from one another. The tetraploids and their diploid parent were cytologically examined monthly for 18 months to validate ploidy stability (Fig. 1).
Root morphology (as seen from the bottom of a 125-ml flask) and chromosome number of diploid and a representative tetraploid clone of Artemisia annua. A Cells from YUT16 showing the normal diploid number of chromosomes (2n=18). C Cells from tetraploid YUT16-6P showing 4n=36 chromosomes. B, D Root morphology: B diploid YUT16, D tetraploid YUT16-6P. Bar: 10 μm
Although no obvious morphological differences were observed between the diploid and the tetraploid clones (Fig. 1, B–D), one clone (not shown), YUT16-7LT, produced very thick curled roots. Despite its slow growth, YUT16-7LT required weekly medium exchange to maintain viability compared to the biweekly culturing required by the other clones. The poor growth of this unusual clone made it difficult to study and, consequently, chromosome analyses were not carried out (data not shown).
Growth of diploid and tetraploid clones
To compare the growth of the tetraploid clones with their diploid parent, we grew roots both in flasks and singly in six-well plates. Initially we used inoculum from late-log cultures (14 day) to compare biomass yields in flasks. Table 1 shows that all tetraploid clones produced significantly less biomass (FW, DW, and %DW) than the diploid YUT16 when the cultures were initiated with a 14-day inoculum. A common result of polyploidy in plants is a reduced number of cell divisions during growth and development (Adaniya and Shira 2001; Otto and Whitton 2000), so overall reduced growth was not surprising. However, previous studies with hairy root cultures showed that the age of the inoculum culture might affect root growth as well as secondary metabolite production (Takahashi et al. 2001; Weathers et al. 1996, 1997). Since high growth rates are just as important in achieving high overall secondary metabolite productivity as is specific product yield, we therefore decided to use an older inoculum (20 day) to see if there was an age effect on biomass accumulation. When a 20-day inoculum was used, the diploid YUT16 still grew better than the tetraploids (Table 1). Thus, inoculum age did not appear to alter the growth yields of the tetraploids. Even though the tetraploid clones all accumulated less biomass than the diploid, one clone, YUT16-6P, consistently grew better than the other three tetraploids. Indeed, the growth yield of this clone was only 12-20% less than that of the diploid YUT16 (Table 1).
Although the total biomass and metabolite production of a particular root clone can be routinely determined in shake flask experiments, measuring the growth of individual roots is difficult. A better method for understanding root growth behavior in a liquid environment is to follow elongation and branching of individual roots (Kim et al. 2002; Wyslouzil et al. 2000). This allows a more detailed understanding of root growth kinetics (Yu and Doran 1994), and variation in morphology may be related to such root characteristics as growth rate and secondary metabolite production level, among other factors (Bhadra et al. 1993). The use of six-well plates has proved useful for such a study (Kim et al. 2002; Srinivasan et al. 1997). Each six-well plate provides six replicate experiments with only a minimal use of medium and space and can provide a considerable amount of information (Table 2).
Our comparative single-root growth study showed that all of the tetraploid clones had root diameters considerably larger than that of the diploid YUT-16 (Table 2). This was not a surprising observation since larger plant organs are often the result of polyploidy (Otto and Whitton 2000). Interestingly, all of the tetraploid clones showed lower growth values for most of the other characteristics measured when compared to the diploid YUT16 (Table 2). However, when the tetraploids were compared to each other, clone YUT16-9Lb produced the highest number of lateral roots and lateral roots per centimeter (i.e., lateral density). Likewise, clone YUT16-6P had the greatest total lateral length and clone YUT16-8LT yielded the greatest total root length (Table 2).
When RGU values were compared, a major difference was observed between the diploid parent and the tetraploids. Three tetraploid clones, YUT16-9Lb, YUT16-8LT, and YUT16-7P showed final RGU values higher than that of the diploid YUT16. YUT16-6P was the only clone with a final RGU value equivalent to that of the diploid clone. The RGU unit is a morphological parameter indicating the branching activity of the root system. YUT16-6P grew with a mean length per root tip of about 1 cm, which is a typical value for roots grown in shake flasks (Wyslouzil et al. 2000), suggesting that it grows nearly as fast as the diploid YUT16 (Table2). This is consistent with the biomass data (Table 1), which showed that YUT16-6P had the highest biomass accumulation of the tetraploid clones tested.
Artemisinin production of diploid and tetraploid clones
When the concentration of artemisinin in the clones was analyzed by HPLC (Table 1), all of the tetraploid cultures initiated with a 14-day-old inoculum were found to have produced more artemisinin per gram DW than the diploid YUT16, even though the former produced less biomass. Clone YUT16-8LT, in particular, produced about five times the specific artemisinin level (μg artemisinin g-1 DW) than the diploid YUT16, and it was about twice as productive as the other tetraploid clones similarly inoculated. The overall productivity per culture for YUT16-8LT was three times that of the diploid clone (Table 1). Similar increases in artemisinin levels were also reported by Wallaart et al. (1999) in tetraploid whole A. annua plants. Unfortunately, in their case, the overall yield of artemisinin per tetraploid plant was effectively the same as that of the diploid because the tetraploid plants were proportionately smaller.
Surprisingly, the artemisinin production profile was quite different with the tetraploid cultures started from a 20-day-old inoculum. In this case, a different tetraploid clone, YUT16-7P, was the highest artemisinin producer when compared to results from the experiment using a 14-day-old inoculum. YUT16-7P produced six times the specific artemisinin level produced by the diploid (Table 1). Overall, the YUT16-7P tetraploid was about four times as productive as the diploid (Table 1). These results suggest a possible effect of inoculum age on secondary metabolite production in two of the tetraploid clones, YUT16-7P and YUT16-8LT.
Unfortunately, the increased yields of the tetraploid clones have not reached commercially useful quantities (mg g-1 DW) of artemisinin (Table 1). Since YUT16 was first isolated in the early 1990s, we have noticed a steady decline in overall specific artemisinin productivity from more than 250 μg g-1 DW (Kim et al. 2001; Weathers et al. 1994) to about 3 μg g-1 DW reported here. It is not clear what selective pressure would be needed to recover the original high production of the diploid YUT16 clone (Weathers et al. 1994).
Conclusions
To our knowledge, this is the first report of the formation of tetraploid hairy roots. Similar to polyploid whole plants, polyploid hairy roots produced significantly more artemisinin than their diploid counterparts. Although the artemisinin yields obtained from these tetraploid clones were not as high as previously reported from their parent clone when it was isolated 10 years ago (Weathers et al. 1994), some of them produced more artemisinin than the diploid. These results show that, although careful screening is still required of polyploids induced from an elite clone, there appears to be advantages in selecting for high-yielding polyploids artificially produced from hairy root cultures.
Abbreviations
- AN :
-
Artemisinin
- DW :
-
Dry weight
- FW :
-
Fresh weight
- HPLC :
-
High pressure liquid chromatography
References
Adaniya S, Shira D (2001) In vitro induction of tetraploid ginger (Zingiber officinalis Roscoe) and its pollen fertility and germinability. Sci Hortic 88:277–287
Bhadra R, Vani S, Shanks JV (1993) Production of indole alkaloids by selected hairy root lines of Catharanthus roseus. Biotechnol Bioeng 41:581–592
Chen CH, Goeden-Kallemeyn YC (1979) In vitro induction of tetraploid plants from colchicine-treated diploid daylily callus. Euphytica 28:705–709
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87:81–89
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gao SL, Zhu DN, Cai ZH, Xu DR (1996) Autotetraploid plants from colchicines-treated bud culture of Salvia miltiorrhiza Bge. Plant Cell Tissue Organ Cult 47:73–77
Griesbach RJ, Kamo KK (1996) The effect of induced polyploidy on the flavonoids of Petunia Mitchell. Phytochemistry 42:361–363
Jaziri M, Shimomura K, Yoshimatsu K, Fauconnier ML (1995) Establishment of normal and transformed root cultures of Artemisia annua L. for artemisinin production. J Plant Physiol 145:175–177
Kim Y, Wyslouzil BE, Weathers PJ (2001) A comparative study of mist and bubble column reactors in the in vitro production of artemisinin. Plant Cell Rep 20:451–455
Kim Y, Wyslouzil BE, Weathers PJ (2002) Secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell Dev Biol Plant 38:1–10
Meshnick SR, Thomas A, Ranz A, Xu CM, Pan HZ (1991) Artemisinin (Quinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol Biochem Parasitol 49:181–190
Otto SP, Whitton J (2000) Polyploid incidence and evolution. Annu Rev Genet 34:401–437
Pal Bais H, Loyola-Vargas VM, Flores HE, Vivanco JM (2001) Root-specific metabolism: the biology and biochemistry of underground organs. In Vitro Cell Dev Biol Plant 37:730–741
Singh RJ (1993) The handling of chromosomes. In: Singh RJ (ed) Plant cytogenetics. CRC Press, London, pp 7–12
Smith TC, Weathers PJ, Cheetham RD (1997) Effects of gibberellic acid on hairy root cultures of Artemisia annua: growth and artemisinin production. In Vitro Cell Dev Biol Plant 33:75–79
Srinivasan V, Roberts SC, Shuler ML (1997) Combined used of six-well polystyrene plates and thin layer chromatography for rapid development of optimal plant cell culture processes: application to taxane production by Taxus sp. Plant Cell Rep 16:600–604
Švehlíková V, Repčák M (2000) Variation of apigenin quantity in diploid and tetraploid Chamomilla recutita (L.) Rauschert. Plant Biol 2:403–407
Takahashi Y, Hitaka Y, Kino-oka M, Taya M, Tone S (2001) Evaluation of growth property of red beet hairy roots depending on condition of inocula and its application to culture control with fuzzy logic theory. Biochem Bioeng J 8:121–127
Wallaart TE, Pras N, Quax WJ (1999) Seasonal variations of artemisinin and its biosynthetic precursors in tetraploid Artemisia annua plants compared with the wild-type. Planta Med 65:723–728
Watrous SB, Wimber DE (1988) Artificial induction of polyploidy in Paphiopedilum. Lindleyana 34:177–183
Weathers PJ, Cheetham RD, Follansbee E, Teoh K (1994) Artemisinin production by transformed roots of Artemisia annua. Biotechnol Lett 16:1281–1286
Weathers P, Smith T, Hemmavanh D, Follansbee E, Ryan J, Cheetham R (1996) Production of the antimalarial artemisinin by transformed roots of Artemisia annua. Acta Hortic 426:157–163
Weathers PJ, Hemmavanh DD, Walcerz DB, Cheetham R.D, Smith TC (1997) Interactive effects of nitrate and phosphate salts, sucrose, and inoculum culture age on growth and sesquiterpene production in Artemisia annua hairy root cultures. In Vitro Cell Dev Biol Plant 33:306–312
Wyslouzil BE, Waterbury RG, Weathers PJ (2000) The growth of single roots of Artemisia annua in nutrient mist reactors. Biotechnol Bioeng 70:143–150
Yu S Doran PM (1994) Oxygen requirements and mass transfer in hairy root cultures. Biotechnol Bioeng 44:880–887
Acknowledgements
Our special thanks are extended to Prof. Kristin K. Wobbe, Chemistry and Biochemistry Department, WPI, for critical reading of the manuscript. We also thank Joseph F. Romagnano and Mark C. McCoy, Biology and Biotechnology Department, WPI, for assistance with the photography and HPLC, respectively. This research was supported by a National Needs Graduate Fellowship (USDA 98-38420-5818).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.E Horn
Rights and permissions
About this article
Cite this article
De Jesus-Gonzalez, L., Weathers, P.J. Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep 21, 809–813 (2003). https://doi.org/10.1007/s00299-003-0587-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0587-8