Abstract
To identify the association between traditional cardiovascular risk factors, diseases related factors, body composition and adipokines with high cardiovascular risk (HCVR) in psoriatic arthritis and non-psoriatic spondyloarthritis. This was a cross-sectional study involving age and BMI matched adults with psoriatic arthritis (PsA) (n = 56) and non-psoriatic spondyloarthritis (nPsA–SpA) (n = 58). Body composition using whole-body dual energy X-ray absorptiometry, adipokines and disease characteristics along with cardiovascular risk scoring QRISK3 and carotid intimal medial thickness (CIMT) was collected. Individuals with a QRISK3 ≥ 10% or CIMT of ≥ 75 percentile of the general population were categorised as HCVR. Predictors of HCVR were determined by logistic regression. HCVR was detected in 39 (34.2%) of the patients. After adjusting for all the factors, sarcopenia (aOR-15.83; 95% CI 1.16–215.48; p = 0.038) and presence of any traditional CV comorbidity (aOR: 18.97; 95% CI 1.63–221.29; p = 0.019) were associated with HCVR. nPsA–SpA had a 97% lesser chance of having HCVR as compared to PsA. The ROC curve analysis for the multiple logistic regression model which estimated the AUC as 0.787 (95% CI 0.701–0.874) and a P value < 0.001. Adipokine levels correlated well with body composition, but not with HCVR. PsA has a higher CV risk and the mechanisms for the same are poorly understood. Sarcopenia is an important determinant of HCVR and may be due to ectopic adipose tissue deposition in skeletal muscles. Focused physical therapy to prevent sarcopenia, optimum treatment of traditional CV risk factors and adequate disease control may help in preventing atherosclerosis in SpA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advent of newer therapeutic options along with treat to target approach have improved the disease outcomes and quality of life in spondyloarthritis (SpA) [1]. These improved outcomes have however unearthed new challenges like accumulation of metabolic and cardiovascular (CV) comorbidities [2, 3]. There is an increased risk of myocardial infarction (RR: 1.41, 95% CI 1.17–1.69) and stroke (RR = 1.33, 95% CI 1.22–1.45) in psoriatic arthritis (PsA) and in ankylosing spondylitis (AS) (RR: 1.24, 95% CI 0.93–1.65) and (RR = 1.49, 95% CI 1.25–1.77) [4]. International guidelines have identified CV risk to be an important outcome in autoimmune rheumatic diseases (ARD) including SpA and have chalked out specific steps for their management. In their current iterations, these guidelines have suggested screening and managing CV and metabolic comorbidities in accordance to the respective national guidelines [5]. Currently there are two major challenges that are prevalent in identifying and managing CV risk in SpA. First being the absence of good surrogate markers that can identify at high-risk individuals for CV disease, as assessment of the traditional CV risk factors in isolation are inadequate. Second, majority of the CV risk predication scores have not considered the chronic inflammatory status, drugs used and their related effects in spondyloarthritis while calculating CV risk. It is well known that risk factors like obesity, atherogenic lipid profile, metabolic syndrome along with comorbidities like hypertension (HTN) and diabetes mellitus (DM) determines CV risk in healthy control. These factors have proven to be grossly inadequate in determining the CV risk in ARDs [6]. An alternative method to determine CV risk is by the ultrasound-based carotid intima medial thickness (CIMT), which shows strong correlation with long term CV outcome, but in isolation may not be adequate. An alternative to these would be a combination of the risk predication scores, which has shown to better predict CV risk in ARDs [7]. Similarly a combination of one CV risk predictor score along with CIMT measurement has also been proposed to improve the chances of identifying CV risk in rheumatoid arthritis [8].
Spondyloarthritis is a heterogenous group that mainly comprises ankylosing spondylitis (AS), reactive arthritis (ReA), undifferentiated SpA (USpA) and enteropathy-related arthritis (EA) [9] beyond psoriatic arthritis (PsA). They share similar pathophysiological and clinical features with each other besides a higher association with HLA-B*27. The traditional CV risk factors are reported to be higher in PsA [10,11,12] and is mainly attributed to higher BMI among those individuals. Hence, it is also necessary to study the two groups by offsetting the overwhelming effect of BMI between PsA and non-psoriatic spondyloarthritis (nPsA–SpA). Several factors, such as diseases activity [13], body composition and adipokines are interdependent and may determine the cardiovascular risk in these group of patients, but have not been systematically evaluated. The interdependence if these factors need to be studied to find common mechanisms influencing the occurrence and progression of CV risk in spondyloarthritis. Therefore, we designed this study with an objective to identify the association between traditional CV risk factors, body composition parameters and serum adipokines with high cardiovascular risk (HCVR) in psoriatic arthritis (PsA) and non-psoriatic spondyloarthritis (nPsA–SpA).
Materials and methods
This cross-sectional study was conducted at a tertiary care, teaching hospital in India. Ethical approval for the study was obtained from the institute ethics committee (JIP/IEC/2018/349) and the study was conducted in accordance with the Indian Council for Medical Research Guidelines for Biomedical Research on Human Participants. Written consent from patients were obtained before recruiting into the study.
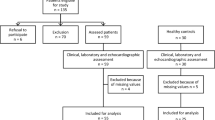
Consecutive age and BMI matched adults who were not on lipid lowering drugs or oral glucocorticoids in the past 6 months with psoriatic arthritis (PsA) satisfying the classification criteria for psoriatic arthritis (CASPAR) criteria (n = 56) and nPsA–SpA satisfying the Assessment of Spondyloarthritis International Society (ASAS) classification criteria [14] (n = 58) were included in the study. All the study subjects were less than 60 years of age and without a history of major or minor cardiovascular event, chronic liver disease, chronic kidney disease and endocrinopathies except type 2 diabetes mellitus.
Demographic variables including age, gender, family history of cardiovascular disease, smoking status and presence of co-morbidities were collected. Anthropometric measurements including weight, height, BMI and waist circumference (WC) and hip circumference (HC) were assessed. Diseases related information including duration of disease, type of SpA (axial versus peripheral), severity of disease Ankylosing Spondylitis Diseases Activity Score-C-reactive protein (ASDAS CRP) [15] and details of comorbidities were collected. Disease activity was assessed by using ASDAS CRP as it has been validated as a good outcome measure in AS, early forms of spondyloarthritis, non-radiographic axial–SpA and peripheral–SpA [16]. Metabolic syndrome was diagnosed as per the criteria of the National Cholesterol Education Program’s Adult Panel III with modifications for Asian population [17]. Blood samples were collected for analysis after overnight fasting for fasting blood sugars, lipid profile, adipokines and cytokine measurement and the serum was store in -400C till the date of analysis. Interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, leptin, resistin, omentin and adiponectin were measured using enzyme-linked immunosorbent assay (Wuhan Fine Biotech, China) as per manufacturers protocol.
Assessment of body composition
Body composition of all the participants was assessed by dual energy absorption spectrometry (DXA) Hologic Discovery Wi (S/N 87574). Sarcopenia was defined as appendicular lean mass (ALM)/height 2 as per Asian Working Group for Sarcopenia (AWGS) with cutoff values of 7.0 kg/m2 in men and 5.4 kg/m2 in women [18]. Quantity and distribution of fat and muscle content were standardised for individual height by dividing all values with height2 in meters.
Cardiovascular risk evaluation
For evaluation of cardiovascular risk, QRISK-3 score was used [19] and measurement of carotid intimal medial thickness (CIMT) was done. A single trained rheumatologist under the supervision of the radiologist performed all CIMT measurements using 7–13 MHz linear probe of MyLab 70 XVision (Esaote, Genoa, Italy). A detailed B mode scan of common carotid, internal carotid, external carotid and carotid bulb of both sides were made. The CIMT was measured from the far wall of the common carotid artery, at least 10 mm proximal to the carotid bulb. A total of 3 carotid intima-media thickness measurements were taken per side and the average value of 6 readings (3 from each side) was taken for further assessment. The average CIMT which was ≥ 75th percentile for age and gender matched Indian population, or presence of carotid plaque was considered as suggestive of subclinical atherosclerosis [20]. Individuals with a QRISK 3 score of ≥ 10 or those with a CIMT ≥ 75th percentile to their age matched scores were considered as those with high cardiovascular risk (HCVR).
Statistical analysis
Categorical variables were represented as frequency and percentages. All continuous variables were tested for normality using the Kolmogorov–Smirnov test. Normally distributed variables were summarised as the mean ± standard deviation (SD) and non-normally distributed variables as median with inter quartile range (IQR). Association of categorical variables with diseases PsA and SpA was tested using Chi-square test or Fisher's Exact Test. The potential categorical factors contributing for high cardiovascular risk were tested using Chi-square/Fishers exact test. The continuous factors were tested between those with and without high cardiovascular risk status was done by independent Student’s t test/Mann–Whitney U test depending upon the distribution of the variable. Bivariate correlation analysis was performed to assess for linear relationship between serum, adipokines, body composition, CIMT and diseases activity measures. 95% confidence interval of the correlation coefficient along with the P value was reported.
Individuals with a QRISK 3 score of ≥ 10 or those with a CIMT ≥ 75th percentile to their age matched scores were considered as those with high cardiovascular risk. Unadjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) were estimated to determine the association of high cardiovascular risk and various demographic, diseases related and body composition factors. Variables that were univariately associated with high cardiovascular risk with a P < 0.15 were considered to construct predictive models. Stepwise selection methods were used to determine the final model and all variables with a P < 0.05 was retained in the final model. Regression coefficients (β’s) along with their 95% confidence interval and P values are reported. A receiver-operating curve (ROC) was plotted to find the discriminant ability of the predictive model with important variables.
All analyses were performed using SPSS, version 19 (SPSS for Windows, Version 19.0. Chicago, SPSS Inc.). The analyses were performed at 5% level of significance and a P value < 0.05 was considered statistically significant.
Results
The important clinical, demographic and anthropometric features along with the difference in adipokine levels between cases of PsA and nPsA–SpA is represented in Table 1. The nPsA–SpA group had higher number of males 44 (75.9%) versus 28 (50%), positive HLA-B*27 [28 (48.3%) vs 8 (14.3%)] and axial spondyloarthritis 56 (96.6) versus 26 (46.45) as compared to PsA (p < 0.05). PsA males had a higher visceral adipose tissue/height2 of 238.11 ± 79.40 gm/m2 against 188.48 ± 75.88 gm/m2 in nPsA–SpA males (p = 0.01). PsA also had a higher average CIMT and higher number of people having population matched CIMT of ≥ 75th percentile 24 (43.6%) compared to nPsA–SpA [8 (13.8%) (p < 0.001)]. Disease activity measured by ASDAS CRP was higher nPsA–SpA 2.01(1.4–2.5) compared to PsA 1.6 (1.3–2.2) p = 0.016). Levels of leptin, adiponectin, IL-6 and MCP-1 were higher in PsA in comparison to those with non-PsA–SpA (p < 0.001). Among the 58 nPsA–SpA, 32(55%) satisfied modified New York criteria for ankylosing spondylitis, 24 (41.4%) were non-radiographic axial SpA, and 2 were peripheral undifferentiated SpA.
We then classified patient as those with and without high risk cardiovascular (HCVR) status if they satisfied either of QRISK3 score ≥ 10% or CIMT ≥ 75th percentile as compared to age and gender matched Indian population. Table 2 shows comparison of various parameters between these groups. We had 39 (34.2%) in the HCVR group and 75 (65.8%) in the non-cardiovascular risk group. QRISK3 could identify HCVR in 10 (25.6%) patients and all of them were in the age group of 50–59 years. CIMT on the other hand detected HCVR in 18 (46.2%), 9 (23.1%) and 5 (12.8%) in < 39, 40–49 and 50–59 years age groups, respectively. Individuals with HCVR status had a higher median age 42(IQR-35,52) years as compared to 37(IQR-33,43) years with non-HCVR status (p = 0.006). HCVR was seen more in PsA (n = 27, 69.2%) as compared to nPsA–SpA (n = 12, 30.8%). More individuals (48.7%) with HCVR had a higher incidence of one of the traditional CV comorbidities (p = 0.041). Trunk fat/limb fat and visceral adipose tissue/Ht2 was higher in HCVR group 1.13 ± 0.25 and 245.07 ± 89.44 gm/m2 then in others 1.03 ± 0.23 (p = 0.028) and 198.29 ± 74.93 gm/m2 (p = 0.004), respectively. Adiponectin and MCP-1 were significantly higher in HCVR and Resistin was lower in HCVR (p < 0.05) (Table 2).
On multifactorial logistic regression (Table 3) to identify the factors independently associated with HCVR. We found sarcopenia was associated with HCVR (aOR-15.83; 95% CI 1.16–215.48; p = 0.038). We also found that combined comorbidities of diabetes, hypertension and metabolic syndrome had higher cardiovascular risk (aOR: 18.97; 95% CI 1.63–221.29; p = 0.019). Further, it was observed that SpA patients had 97% lesser chance of having HCVR as compared to patients with PsA. The ROC curve analysis for the multiple logistic regression model which estimated the AUC as 0.787 (95% CI 0.701–0.874) and a P value < 0.001.
Upon Pearson’s correlation (Supplementary Table1) it was found that age had moderate correlation with CIMT (r = 0.46 (0.29,0.62); p < 0.001) and weak correlation with visceral adipose tissue standardized to height (VAT/Ht2) (r = 0.31 (0.13, 0.49); p = 0.001). Appendicular lean mass standardized to height (ALM/ht2) had significant negative correlation with total body fat (r = − 0.32 (− 0.15, − 0.50); p < 0.001) and positive correlation with trunk fat/limb fat (r = 0.45 (0.29, 0.62); p < 0.001). The VAT/Ht2 had strong and moderate correlation with BMI (r = 0.70 (0.57, 0.83); p < 0.001) and total body fat (r = 0.55 (0.39, 0.70); p < 0.001), respectively. We performed Spearman’s rho to look for correlation between adipokines and body composition (Supplementary Table 2). Leptin correlated highly with all the parameters measuring body fat and moderately with BMI and hip circumference (p < 0.01) and had a mild negative correlation with ALM/ht2 (r = − 0.220; p = 0.047).
Discussion
In this cross-sectional study, we assessed age and BMI matched PsA and nPsA–SpA for determinants of high cardiovascular risk. We found that a diagnosis of psoriatic arthritis, presence of traditional cardiovascular risk factors and sarcopenia independently determined high cardiovascular risk.
The two subgroups (PsA and nPsA–SpA) were similar to the representative samples encountered in routine clinical practice. The nPsA–SpA mainly comprises axial spondyloarthritis (axSpA) with majority being males, with a higher HLA-B*27 positivity, axial disease and a higher disease activity reflecting similarity with other larger cohorts [21]. The comorbidity status was similar across both the groups possibly because of the matching done for BMI during enrolment. Despite having comparable BMI when gender-specific assessment of body composition was analysed, we found a higher visceral adipose tissue among PsA males as compared to nPsA–SpA. Visceral adipose tissue correlates with deposition of fat in liver [22]. A similar finding with higher visceral adipose tissue and liver fat was also described using MRI [10] in PsA and computed tomography studies in psoriasis [23].
Identification of cardiovascular risk status and their pathophysiology in inflammatory diseases is an ongoing topic of research. For optimum detection of subclinical atherosclerosis, it is often suggested to use vascular ultrasound based CIMT measurement or detect atherosclerotic plaques in combination with risk scores. This approach has consistently improved detection of high cardiovascular risk in individuals with spondyloarthritis [24, 25]. High cardiovascular risk was detected in over 34% of our patients which is slightly lower in comparison to other cohorts of spondyloarthritis where the point prevalence was > 40% [24, 26]. The difference may be due to different CIMT cut offs used for detecting atherosclerosis and a lower number of individuals with traditional CV risk factors in our study.
Upon analysing the determinants of HCVR, one of the key findings in the univariate analysis that lost significance on multivariate analysis was the visceral adipose tissue/ht2. It is well known that increase in total and trunk fat mass increases the chance of developing prediabetes in at risk individuals when followed up for over 5 years [27]. Such ectopic fat deposition also is known to be associated with cardiometabolic risks in individuals with inflammatory arthritis and in general population [28]. Abnormal or ectopic fat deposition is associated with ectopic intramuscular fat deposition resulting in sarcopenia [10]. Sarcopenia is associated with metabolic syndrome [29] and subclinical atherosclerosis [30, 31]. Interestingly, close to 30% of our cohort had sarcopenia but the incidence was similar across both PsA and nPsA–SpA subgroups. We have found sarcopenia to be a significant factor determining HCVR status independent of age, duration of illness, subtype of spondyloarthritis and visceral fat. Even though conclusive proof that ectopic fat deposition was associated with sarcopenia needs MRI, our study has shown some leads. We have found indirect evidence in the form of a negative correlation between total fat and ALM/ht2 (a measure of sarcopenia) and positive correlation with Trunk fat/Limb fat suggesting abnormal intramuscular fat deposition. Such intramuscular fats predisposing to sarcopenia are known to result in high cardiovascular risk [32].
Besides sarcopenia, PsA itself seems to contribute to HCVR, suggesting that there are additional unidentified factors in PsA like added inflammatory burden due to skin involvement resulting in CV morbidity. Such additional factors may not be optimally captured by the disease activity measures and inflammatory markers that was used in our study. This additional risk suggests that the guidelines and CV risk scores may have to be revised to include an additional multiplication factor while assessing CV risk in PsA [5], [19] akin to that currently being done for rheumatoid arthritis and lupus.
Adipose tissue is known to secrete several adipokines which may play a role in developing insulin resistance and cardiovascular risk. Although we did not find any association of adipokines with high cardiovascular risk, we found abnormally high levels of adipokines in PsA and nPsA–SpA. Levels of proinflammatory adipokines like leptin, MCP 1 and IL-6 were elevated in PsA. There was high correlation of leptin levels with total body fat. Besides correlating with metabolic syndrome leptin is proposed to be involved in osteoclast activation and disease progression in PsA [33] suggesting that leptin levels may be regulated more by the inflammatory status and the fat distribution rather than other factors. Similar to our findings, higher levels of adiponectin are often found in inflammatory arthritis including in PsA [34] and rheumatoid arthritis [35] again suggesting close interaction between adipokines and the inflammatory pathways. Resistin which is a pro-inflammatory adipokine and the anti-inflammatory omentin were higher in nPsA–SpA in our study. Higher levels of resistin are found in AS with high disease activity [36] suggesting a higher dependence of the levels on diseases activity. High omentin levels are associated with cardiovascular risk factors in both AS [37] and PsA [38], but such associations were not found in our study. Even though several studies have associated these adipokines with higher disease activity [39] and cardiovascular risk [37] we did not find any such correlations suggesting that the levels depend on a variety of factors including body composition, disease activity, drugs used, other comorbidities and probable adipokine resistance. Moreover, the levels of these adipokines are dynamic and a prospective study design may be required to make any conclusive attributions to cardiovascular risk especially in an inflammatory disease.
Our study demonstrates that visceral adiposity, sarcopenia and cardiovascular risk are interrelated. This enables clinicians to address some of these aspects at the clinics. For instance, identifying and addressing abnormal anthropometric measures along with optimum disease control may help prevent development of atherosclerosis. The strong correlation of WC with visceral adipose tissue/Ht2 found in our study suggests that WC can be a good clinical marker to initiate preventive measures against atherosclerosis but for optimising treatment one may need to test for sarcopenia using advanced measures. Exercise regimens or activities aimed at preventing sarcopenia have shown benefit in elderly [40] and such effects need to be systematically evaluated in ARDs. Besides, the role of improvement in sarcopenia in preventing atherosclerosis also needs to be evaluated.
The major strength of our study is the large sample who were matched for BMI and age. This is to the best of our knowledge the first attempt to determine the combined role of body composition, clinical parameters and adipokines in determining cardiovascular risk in a large cohort of spondyloarthritis. The finding of sarcopenia as a CV risk determinant in spondyloarthritis is also a novel finding. There are a few limitations in our study. First, we were unable to recruit equal number of females in both groups, but the regression model seems to have adequately controlled for this deficit. Second, we have not assessed the plaques in the carotids which could have been a better marker of high-risk cardiovascular status, nonetheless using CIMT cutoffs to classify cardiovascular risk is a time tested and reliable strategy. This may be the only option in a relatively young group of patients where carotid plaques may be minimal.
Conclusion
High CV risk is seen in ~ 35% of patients with spondyloarthritis and is more in PsA due to poorly understood mechanisms. Sarcopenia is often neglected but an important determinant of HCVR status and may be a result of ectopic adipose tissue deposition in skeletal muscles. Focused physical therapy to prevent sarcopenia along with optimum treatment of traditional CV risk factors in addition to adequate disease control may help in preventing atherosclerosis.
Data availability
Data of this study is available with the corresponding author and will be made available upon reasonable request after due permission from the institute ethics committee.
References
Molto A, López-Medina C, Van den Bosch FE, Boonen A, Webers C, Dernis E et al (2021) Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2020-219585
Bengtsson K, Forsblad-d’Elia H, Lie E, Klingberg E, Dehlin M, Exarchou S et al (2017) Are ankylosing spondylitis, psoriatic arthritis and undifferentiated spondyloarthritis associated with an increased risk of cardiovascular events? A prospective nationwide population-based cohort study. Arthritis Res Ther 19(1):102
Huang Y-P, Wang Y-H, Pan S-L (2013) Increased risk of ischemic heart disease in young patients with newly diagnosed ankylosing spondylitis–a population-based longitudinal follow-up study. PloS One 8(5):e64155
Liu W, Ma W, Liu H, Li C, Zhang Y, Liu J et al (2021) Stroke risk in arthritis: a systematic review and meta-analysis of cohort studies. PloS One 16(3):e0248564
Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJL et al (2017) EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 76(1):17–28
Rueda-Gotor J, Quevedo-Abeledo JC, Corrales A, Genre F, Hernández-Hernández V, Delgado-Frías E et al (2020) Reclassification into very-high cardiovascular risk after carotid ultrasound in patients with axial spondyloarthritis. Clin Exp Rheumatol 38(4):724–731
Corrales A, Vegas-Revenga N, Atienza-Mateo B, Corrales-Selaya C, Prieto-Peña D, Rueda-Gotor J et al (2021) Combined use of QRISK3 and SCORE as predictors of carotid plaques in patients with rheumatoid arthritis. Rheumatol Oxf Engl 60(6):2801–2807
González-Gay MA, González-Juanatey C, Llorca J (2012) Carotid ultrasound in the cardiovascular risk stratification of patients with rheumatoid arthritis: when and for whom? Ann Rheum Dis 71(6):796–798
Kavadichanda CG, Geng J, Bulusu SN, Negi VS, Raghavan M (2021) Spondyloarthritis and the human leukocyte antigen (HLA)-B* 27 connection. Front Immunol 12:497
Ferguson LD, Linge J, DahlqvistLeinhard O, Woodward R, Hall Barrientos P, Roditi G et al (2021) Psoriatic arthritis is associated with adverse body composition predictive of greater coronary heart disease and type 2 diabetes propensity - a cross-sectional study. Rheumatol Oxf Engl 60(4):1858–1862
Castañeda S, Martín-Martínez MA, González-Juanatey C, Llorca J, García-Yébenes MJ, Pérez-Vicente S et al (2015) Cardiovascular morbidity and associated risk factors in Spanish patients with chronic inflammatory rheumatic diseases attending rheumatology clinics: baseline data of the CARMA Project. Semin Arthritis Rheum 44(6):618–626
Mok CC, Ko GTC, Ho LY, Yu KL, Chan PT, To CH (2011) Prevalence of atherosclerotic risk factors and the metabolic syndrome in patients with chronic inflammatory arthritis. Arthritis Care Res 63(2):195–202
Valero-Jaimes JA, López-González R, Martín-Martínez MA, García-Gómez C, Sánchez-Alonso F, Sánchez-Costa JT et al (2021) Body mass index and disease activity in chronic inflammatory rheumatic diseases: results of the cardiovascular in rheumatology (Carma) project. J Clin Med 10(3):382
Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT et al (2011) The assessment of spondyloarthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70(1):25–31
Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J et al (2009) Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 68(1):18–24
Fernández-Espartero C, de Miguel E, Loza E, Tomero E, Gobbo M, Descalzo MA et al (2014) Validity of the ankylosing spondylitis disease activity score (ASDAS) in patients with early spondyloarthritis from the Esperanza programme. Ann Rheum Dis 73(7):1350–1355
Alberti KGMM, Zimmet P, Shaw J (2006) Metabolic syndrome–a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med J Br Diabet Assoc. 23(5):469–80
Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS et al (2014) Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 15(2):95–101
QRISK3 (2021) [Internet]. [Cited 2021 Feb 26]. Available from: https://qrisk.org/three/. Accessed 14 Oct 2020
Kasliwal RR, Bansal M, Desai N, Kotak B, Raza A, Vasnawala H et al (2016) A Study to derive distribution of carotid intima media thickness and to determine its correlation with cardiovascular Risk factors in asymptomatic nationwidE Indian population (SCORE-India). Indian Heart J 68(6):821–827
Zhang S, Wang Y, Peng L, Su J, Zeng X, Li M et al (2020) Comparison of clinical features in HLA-B27 positive and negative patients with axial spondyloarthritis: results from a cohort of 4131 patients. Front Med 7:609562
Jakobsen MU, Berentzen T, Sørensen TIA, Overvad K (2007) Abdominal obesity and fatty liver. Epidemiol Rev 29:77–87
Balci A, Balci DD, Yonden Z, Korkmaz I, Yenin JZ, Celik E et al (2010) Increased amount of visceral fat in patients with psoriasis contributes to metabolic syndrome. Dermatol Basel Switz 220(1):32–37
González Mazón I, Rueda-Gotor J, Ferraz-Amaro I, Genre F, Corrales A, Calvo Rio V et al (2021) Subclinical atherosclerotic disease in ankylosing spondylitis and non-radiographic axial spondyloarthritis. A multicenter study on 806 patients. Semin Arthritis Rheum 51(2):395–403
Lam SHM, Cheng IT, Li EK, Wong P, Lee J, Yip RM-L et al (2020) DAPSA carotid plaque and cardiovascular events in psoriatic arthritis: a longitudinal study. Ann Rheum Dis 79(10):1320–1326
Rueda-Gotor J, Corrales A, Blanco R, Fuentevilla P, Portilla V, Expósito R et al (2015) Atherosclerotic disease in axial spondyloarthritis: increased frequency of carotid plaques. Clin Exp Rheumatol 33(3):315–320
Al Hommos NA, Ebenibo S, Edeoga C, Dagogo-Jack S (2021) Trajectories of body weight and fat mass in relation to incident prediabetes in a biracial cohort of free-living adults. J Endocr Soc. 5(2):bvaa164
Després J-P (2012) Body fat distribution and risk of cardiovascular disease: an update. Circulation 126(10):1301–1313
Kim SH, Jeong JB, Kang J, Ahn D-W, Kim JW, Kim BG et al (2021) Association between sarcopenia level and metabolic syndrome. PloS One 16(3):e0248856
Kang M-K, Park J-G (2021) Low skeletal muscle mass is a risk factor for subclinical atherosclerosis in patients with nonalcoholic fatty liver disease. Diagn Basel Switz 11(5):854
Lai S, Muscaritoli M, Andreozzi P, Sgreccia A, De Leo S, Mazzaferro S et al (2019) Sarcopenia and cardiovascular risk indices in patients with chronic kidney disease on conservative and replacement therapy. Nutr Burbank Los Angel Cty Calif 62:108–114
Katsiki N, Athyros VG, Mikhailidis DP (2016) Abnormal peri-organ or intra-organ fat (APIFat) deposition: an underestimated predictor of vascular risk? Curr Vasc Pharmacol 14(5):432–441
Pina T, Genre F, Lopez-Mejias R, Armesto S, Ubilla B, Mijares V et al (2015) Relationship of leptin with adiposity and inflammation and resistin with disease severity in psoriatic patients undergoing anti-TNF-alpha therapy. J Eur Acad Dermatol Venereol JEADV 29(10):1995–2001
Xue Y, Jiang L, Cheng Q, Chen H, Yu Y, Lin Y et al (2012) Adipokines in psoriatic arthritis patients: the correlations with osteoclast precursors and bone erosions. PloS One 7(10):e46740
Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gómez-Reino JJ et al (2006) Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis 65(9):1198–1201
Genre F, López-Mejías R, Miranda-Filloy JA, Ubilla B, Carnero-López B, Blanco R et al (2014) Adipokines, biomarkers of endothelial activation, and metabolic syndrome in patients with ankylosing spondylitis. BioMed Res Int 2014:860651
Genre F, Rueda-Gotor J, Remuzgo-Martínez S, Pulito-Cueto V, Corrales A, Mijares V et al (2020) Omentin: a biomarker of cardiovascular risk in individuals with axial spondyloarthritis. Sci Rep 10(1):9636
Dağdelen D, Karadag AS, Kasapoğlu E, Wang JV, Erman H (2020) Correlation of metabolic syndrome with serum omentin-1 and visfatin levels and disease severity in psoriasis and psoriatic arthritis. Dermatol Ther 33(6):e14378
Kononoff A, Vuolteenaho K, Hämäläinen M, Kautiainen H, Elfving P, Savolainen E et al (2020) Metabolic syndrome, disease activity, and adipokines in patients with newly diagnosed inflammatory joint diseases. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. https://doi.org/10.1097/RHU.0000000000001412
Zhang Y, Zou L, Chen S-T, Bae JH, Kim DY, Liu X et al (2021) Effects and moderators of exercise on sarcopenic components in sarcopenic elderly: a systematic review and meta-analysis. Front Med 8:649748
Acknowledgements
The research was done with the aid of the JIPMER intramural grant (JIP/Res/Intramural/phs2/2018-19 Dated: 18.01.2019)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kavadichanda, C., Shanoj, K.C., Ganapathy, S. et al. Factors associated with high cardiovascular risk in psoriatic arthritis and non-psoriatic spondyloarthritis. Rheumatol Int 42, 251–260 (2022). https://doi.org/10.1007/s00296-021-05064-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-021-05064-2