Abstract
Primary Sjӧgren’s syndrome (pSS) is an autoimmune-mediated, inflammatory, and systemic connective tissue disease (CTD), especially in middle-aged women, which often involves multiple systems and organs of the body. In fact, the heart is an important target organ in patients with pSS. In recent years, it has been confirmed that the morbidity of cardiac involvement has increased in patients with pSS, and cardiovascular disease (CVD) is one of the main causes of death. The increased risk of CVD in pSS patients is associated with a great variety of risk factors, such as age, gender, hypertension, diabetes mellitus, dyslipidemia, disease duration, extra-glandular manifestations, therapeutic drugs of pSS, and so on. Early recognition and effective treatment of CVD may play a crucial role in improving adverse cardiovascular prognosis. Whereas cardiac involvement is closely related to patient prognosis and survival, the cardiac involvement of patients with pSS remains poorly studied. Therefore, this article reviews the cardiovascular risk factors, clinical manifestations of cardiac involvement, cardiovascular biomarkers, and therapeutic strategies of pSS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary Sjӧgren’s syndrome (pSS) is a slowly progressive autoimmune inflammatory disease mainly characterized by lymphocyte infiltrating exocrine glands, primarily the salivary and lacrimal glands. The incidence of pSS is 6.92 per 100,000 person-years and the prevalence of pSS is 60.82 cases per 100,000 people, and it mostly affects middle-aged women [1]. The exact etiology and pathogenesis of pSS are still unknown, and infection, genetic, and environment may play a crucial role in the occurrence and development of pSS. More than one-third of pSS patients have extra-glandular involvement, and the heart is an important target organ [2]. All parts of the heart can be affected, including the pericardium, myocardium, conduction system, valves, and coronary arteries, which are mainly manifested as coronary heart disease (CHD), pulmonary arterial hypertension (PAH), pericarditis, cardiac arrhythmias, valvular regurgitation, autonomic dysfunction, and heart failure [2,3,4]. In particular, cardiac arrhythmias can be used as the initial manifestation of adult pSS, and female patients with anti-SSA/SSB antibodies have a significantly increased risk of giving birth to a neonatal complete congenital heart block [5]. In fact, cardiovascular disease (CVD) is common in pSS patients, which is one of the leading causes of mortality [6]. However, there is much less clarity regarding the underlying mechanism of CVD in pSS patients. The increasing evidence demonstrated that systemic inflammation and immune-mediated mechanisms may play a crucial role in the development of CVD [7]. Bartoloni et al. pointed that the occurrence of cardiovascular events was predicted by interleukin-6 (IL-6) and high-sensitive C reactive protein (hsCRP), both these markers of systemic inflammation, which further reinforce the strong interaction between inflammation and CVD [8]. In addition, patients with pSS had an increased risk of CVD compared to the general population, which was mainly related to the disease-related characteristics and traditional cardiovascular risk factors [3, 9]. It was worth mentioning that pSS was similar to rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) in terms of several clinical and immunological characteristics, but pSS was characterized by low-grade systemic inflammation and a slow and benign evolution, and usually not require immunosuppressive therapies or high doses of corticosteroid treatment [10].
Although cardiovascular comorbidities are frequently reported in patients with autoimmune diseases, CVD remains still underdiagnosed and undertreated. To date, a few studies have focused on cardiac involvement in pSS, but cardiovascular events may have a serious impact on patient prognosis and survival, so it is of great significance to strengthen the research on this disease [11]. Thus, this article reviews the cardiovascular risk factors, clinical manifestation of cardiac involvement, possible cardiovascular biomarkers, and therapeutic strategies of pSS patients, which may be helpful for clinicians to early identify the risk of cardiac involvement in pSS patients and take effective treatment measures to improve adverse cardiovascular prognosis.
Search strategy
We searched for literature through the MEDLINE/PubMed, EMBASE and Scopus database from inception to March 2021, using the following keywords combination: (Sjogren’s syndrome OR Sjӧgren’s syndrome OR sicca syndrome) AND (heart OR cardiac OR cardiovascular OR cardiology). And the relevant references in the literature were also included. We did not include the literature's inability to find full texts or obtain patients’ details from original articles or abstracts. The publication not in English and data from ongoing research were also excluded.
Cardiovascular morbidity and mortality
More recently, two meta-analyses have assessed the morbidity and mortality of cardiac involvement in pSS, and the results indicate that pSS patients have an increased risk of cardiovascular events [7, 12]. Yong et al. evaluated 10 studies and 165 292 participants found that cardiovascular or cerebrovascular events were indeed more prevalent in patients with pSS compared with the general population (OR = 1.28, 95%CI: 0.11–1.46) [12]. Another meta-analysis included 14 studies and 67 124 patients showed that there was an increased morbidity of coronary events (RR = 1.34, 95%CI: 1.06–1.38), cerebrovascular diseases (RR = 1.46, 95%CI: 1.43–1.49), heart failure (OR = 2.54, 95%CI: 1.30–4.97), and thromboembolic events (RR = 1.78, 95%CI: 1.41–2.25) in pSS patients versus the controls [7]. Besides, the risk was also elevated for cardiovascular mortality in pSS [7].
However, there were several differences between these two meta-analyses. First, the choice of included studies was inconsistent. Abstracts of American and European scientific meetings on rheumatism were not included in the study by Yong et al. Second, the main outcomes of included studies were different. Of note, the outcome of the studies included by Yong et al. was not exclusively cardiovascular events. For instance, three studies focused on cardiovascular risk factors, the association between viral hepatitis and pSS, and the risk of gastroesophageal reflux disease in pSS patients, respectively, were also included [13,14,15]. In these studies, CVD was only the initial characteristic. Third, Yong et al. did not report the mortality of CVD in pSS patients, which may be due to missing data.
All in all, these meta-analyses provide high levels of evidence that patients with pSS have higher morbidity and mortality of cardiac involvement compared with the controls. However, the reason for the increased risk of CVD is not clear, which is one of the limitations of the two studies.
Subclinical atherosclerosis
In patients with pSS, an increased prevalence of subclinical atherosclerosis and vascular damage may play an indispensable role in the development of CVD [8]. It has been accepted that atherosclerotic endothelial damage is the result of the interaction of multiple pathogenic factors in pSS patients, including immune-mediated mechanisms and traditional cardiovascular risk factors [16, 17]. Of note, inflammation also plays an important role in the pathogenesis of atherosclerosis in patients with RA and SLE, but the real contribution of inflammation to atherosclerotic damage in patients with pSS is still unclear [8]. The activation of T cells and B cells plays a pivotal role in the process of immune dysregulation. Abnormal proliferation of B cells can differentiate into plasma cells that produce a large number of immunoglobulins and autoantibodies, especially anti-SSA/SSB antibodies. Traditional cardiovascular risk factors, namely hypertension, diabetes mellitus, dyslipidemia, may promote the progression of atherosclerosis by triggering inflammatory processes and vascular damage [9]. Some studies have found that there is no significant difference in the levels of some inflammatory biomarkers, such as CRP and hsCRP, between pSS patients and normal controls [8, 18]. Moreover, CRP or hsCRP is not associated with an increased risk of subclinical atherosclerosis in pSS patients, which also supports the notion that systemic inflammation is relatively low in patients with pSS [8].
A large number of studies proposed that functional and organic subclinical atherosclerosis damage can be measured by different methods, including artery intima-media thickness (IMT), pulse wave velocity (PWV), asymmetric dimethyl arginine (ADMA), coronary flow reserve (CFR), ankle-brachial index (ABI), flow-mediated dilation (FMD) and nitrate-mediated vasodilation (NMV) [19, 20]. Increased artery wall thickening and endothelial damage often occurred in the early stage of subclinical cardiovascular organ damage, and the study found that two-thirds of patients with pSS had increased artery wall thickening (IMT > 0.9 mm) [19]. What’s more, higher levels of ADMA and PWV were more common in patients with pSS, and their levels were proportional to arterial stiffness [19]. Lower ABI (ABI < 0.9) and lower CFR (CFR < 2.5) were considered to be the sign of impaired coronary function and increased cardiovascular risk, respectively [21, 22]. In addition, impaired FMD and NMV were also frequent in pSS patients, which were associated with endothelial dysfunction [17].
On the whole, immune system disorders and cardiovascular risk factors play a crucial role in the pathogenesis of subclinical atherosclerosis, but the mechanism of their interaction remains to be further clarified.
Cardiovascular risk factors
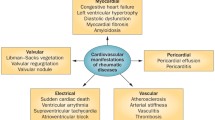
Traditional cardiovascular risk factors, such as age, sex, hypertension, dyslipidemia, and diabetes mellitus, are associated with the higher risk of CVD in pSS patients. However, these factors do not fully explain the increased risk of CVD, implying that other factors might contribute to the increased cardiovascular events burden in these patients. It has been found that the disease itself and disease-related features of pSS, for example, therapeutic drugs, extra-glandular manifestation and disease duration, play a role in the development of CVD [9, 13]. These risk factors of cardiac involvement in pSS patients are shown in Fig. 1.
Age and gender
Age and gender are well-recognized unalterable risk factors for CVD, and the prevalence of CVD increases with advancing age in pSS patients [23]. A large-scale cross-sectional study revealed that they were significantly older in patients with CVD than those without CVD (55.5 years vs. 46.0 years, P < 0.001), and it may be contributed to the increase of vascular endothelial injure and vascular stiffness with aging [24]. Despite the female predominance in patients with pSS, male appear to confer a higher risk of CVD, which may be due to the protective effect of estrogen on cardiovascular events in female [25].
Hypertension
Notably, hypertension was considered to be one of the most important cardiovascular risk factors for cardiovascular events, and the prevalence of hypertension in pSS patients is more than twice that in age-and sex-matched healthy controls (28–50% vs. 15.5–25.6%, P < 0.01) [4, 26]. The prevalence of hypertension in pSS was about 13% to 52%, and this high variability of prevalence may be related to the definition of hypertension, the different race of patients, the sex bias, and other concomitant factors such as reduced physical activity and use of some medications [9]. Hypertension can aggravate damage of the arterial wall by inducing the activation of inflammatory cytokines, complement, and innate immune system, resulting in hypertensive end-organ damage [9, 27]. A study demonstrated that hypertension was not only associated with subclinical atherosclerosis and asymptomatic cardiovascular damage with left ventricular dysfunction, but also with increased risk of cardiovascular and cerebrovascular events, such as myocardial infarction, heart failure, and stroke [27].
Dyslipidemia
Abnormal lipid metabolism of pSS patients was mainly characterized by increased total cholesterol (TC), increased triglyceride (TG), and reduced high-density lipoprotein cholesterol (HDL-C). Interestingly, the levels of low-density lipoprotein cholesterol (LDL-C) in pSS patients were significantly decreased compared with the controls [9]. The exact prevalence of impaired lipid profile in pSS was not certain, giving that the different definitions of dyslipidemia in some studies made data scarcely comparable. However, some studies demonstrated that pSS patients with CVD were prone to have altered lipid profile [9, 13]. Limited evidence has pointed out that anti-SSA/SSB antibodies are not only associated with dyslipidemia in pSS patients but also related to the pathogenesis of CVD in these patients [28]. Therefore, we infer that the autoantibody-inducted mechanism may play a certain role in the pathogenesis between dyslipidemia and CVD in patients with pSS, but further verification is still needed.
Diabetes mellitus
Diabetes mellitus is a CHD risk-equivalent, and the prevalence of diabetes mellitus in pSS patients varies greatly, ranging from 0 to 28% [9]. Most studies revealed that diabetes mellitus was more likely to occur in patients with pSS, but only one study showed that the prevalence of diabetes mellitus in pSS patients was lower than that in healthy subjects [11]. These conflicting data may be partly explained by different genetic and metabolic background, lifestyle habits, and local guidelines for diabetes mellitus screening [9, 11]. The possible mechanism between diabetes mellitus and CVD was involved in inflammation, vascular damage, and accelerated atherosclerosis [4]. In addition, a cohort study demonstrated that pSS patients with diabetes mellitus had an increased risk of CHD, with adjusted HR of 1.16 (95%CI: 1.01–1.34) [24].
Disease-related factors
Disease-related factors mainly include therapeutic drugs, extra-glandular manifestation, disease duration, and the disease itself of pSS. Although the therapeutic drugs of pSS, such as glucocorticoids (GC), hydroxychloroquine (HCQ), immunosuppressants (ISs), and B-cell targeted therapies, can bring some cardiovascular benefit, individual drugs also have specific adverse events and cardiovascular toxicity. For example, a rare and possibly unrecognized adverse event of HCQ is drug-induced cardiotoxicity, and restrictive cardiomyopathy and conduction abnormalities have been reported in the cases of long-term use of HCQ [29]. In addition, rituximab, an anti-CD20 monoclonal antibody, has been suggested as a treatment option for severe refractory patients. However, some serious cardiovascular events have occurred during the infusion of rituximab, including hypotension, arrhythmia, and angina [3]. A multicenter cohort study demonstrated that age at diagnosis of pSS (OR = 1.067, 95%CI: 1.039–1.095), longer disease duration (OR = 1.059, 95%CI: 1.015–1.104), lung involvement (OR = 1.567, 95%CI: 0.620–3.958), central nervous system involvement (OR = 5.666, 95%CI: 1.352–23.749), GC therapy (OR = 1.970, 95%CI: 1.083–3.582), and ISs therapy (OR = 1.966, 95%CI: 1.044–3.700) were significantly associated with an increased prevalence of CVD in pSS patients [11]. Besides, long-term use of GC may increase the frequency of cardiovascular risk factors, such as hypertension and diabetes mellitus. It was worth mentioning that pSS was also identified as an independent cardiovascular risk factor, which was in accordance with other CTD [3].
Cardiovascular biomarkers
With a deep knowledge of pathogenic mechanisms in pSS patients with CVD, some potential biomarkers evaluating cardiac involvement, disease severity, and risk of death have been proposed, although further verification is still needed. First, inflammatory markers, such CRP and IL-6, are involved in the pathogenesis of vascular damage and have been considered as independent predictors of cardiovascular events in pSS patients [8]. Second, the possible roles of calprotectin have been proposed as a marker of atherosclerosis and a prognostic factor for CVD [8]. Calprotectin is an important pro-inflammatory factor of innate immune that promotes vascular inflammation and endothelial damage [8]. Kunutsor et al. reported that calprotectin exerted an important role in the pathogenesis of CHD by inducing the inflammatory process [30]. Third, dickkopf-related protein 1 (DKK-1) is also identified as an independent predictor of CVD [8]. DKK-1, an antagonist Wingless-type signaling pathway, is usually overexpressed in endothelial cells and atherosclerotic plaques, enhancing the interaction between platelets and endothelial layer, driving local inflammation, and promoting plaque destabilization and rupture [31]. Moreover, plasma levels of DKK-1 correlate with hs-CRP and are connected with atherosclerosis [8]. Fourth, ADMA, an endogenous inhibitor of nitric oxide synthase, is not only a marker and mediator of endothelial dysfunction but also a predictor of subclinical atherosclerosis and cardiovascular risk stratification as well as all-cause mortality [19, 32]. It is worth mentioning that some specific biomarkers of cardiac damage, such as creatine kinase-MB (CK-MB), cardiac troponin I and T (cTnI and cTnT), brain natriuretic peptide (BNP), and pro-brain natriuretic peptide (pro-BNP), may also be considered as cardiovascular biomarkers in pSS patients, which are needed to rigorous validation processes.
Cardiovascular diseases in pSS
As well as other CTD, cardiac involvement in patients with pSS is often manifested as CHD, PAH, cardiac arrhythmia, pericardial and valvular diseases, cardiovascular autonomic dysfunction, and heart failure. The detailed clinical manifestations and related factors for cardiac involvement of pSS patients are shown in Table 1.
CHD
The incidence of CHD in pSS patients was 1.42 person-months, which was 1.36 times higher than in the controls [24]. The risk of CHD in patients with pSS increased with age, increasing by 6% per year, and patients aged 45–59 years had the highest risk of CHD (HR = 1.46, 95% CI: 1.20–1.79) [24]. Although pSS usually occurred in female patients, male patients were more like to have CHD than female patients [24]. In addition, patients with hypertension, diabetes mellitus, hyperlipidemia, and use of GC and nonsteroidal anti-inflammatory drugs (NSAIDs) also increased the risk of CHD, with adjusted HRs of 1.70 (95% CI: 1.51–1.92), 1.16 (95% CI: 1.01–1.34), 1.38 (95% CI: 1.21–1.57), 1.45 (95% CI: 1.07–1.97) and 1.31 (95% CI: 1.05–1.65), respectively [24]. Of note, the adjusted HR of CHD in pSS patients increased to 1.52 (95% CI: 1.21–1.92) after excluding the interference of the above-mentioned factors, which demonstrated that disease itself of pSS may be an independent risk factor for CHD [24]. However, some studies demonstrated that the risk of ischemic heart disease (IHD) and acute myocardial infarction (AMI) in pSS patients was lower than in the general population, and there was an insignificant link between pSS and AMI [33, 34]. It may be attributed to the different inclusion and exclusion criteria of the studies and different diagnostic criteria of diseases [33]. To date, the possible mechanisms involved CHD associated with pSS are still largely unknown, which need to be further explored.
PAH
pSS-PAH was a serious and relatively rare complication of patients that was characterized by increased pulmonary artery pressure and pulmonary vascular resistance, mainly manifesting with exertional dyspnea, dizziness, fainting, and right heart failure signs [3, 35]. Kobak et al. found that 23.4% (11/47) of pSS patients had PAH by using noninvasive echocardiograms [36]. The pathogenesis of pSS-PAH may be associated with pulmonary vasculitis, pulmonary vasospasm and reduced pulmonary vascular bed caused by pulmonary interstitial lesions, arterial occlusion and stenosis arising from pulmonary artery thrombosis and immune complex deposition [35, 37]. Some studies suggested that positive anti-SSB and anti-U1RNP antibodies, younger age at onset of pSS, the negative of corneal staining, Raynaud’s phenomenon, rheumatoid factor ≥ 200 U/ml, hepatic injury and pericardial effusion were employed as independent risk factors for pSS-PAH [35, 37, 38]. The overall one-, three- and five-year survival rates of pSS-PAH were 94.0%, 88.8% and 79.0%, respectively [35]. Moreover, the mortality of pSS-PAH was associated with a low cardiac index and increased damage index, thus the prognosis might be improved by optimizing cardiopulmonary function [35].
Cardiac arrhythmia
Cardiac arrhythmia was indeed more prevalent in adult patients with pSS compared with the general population (OR = 1.32, 95% CI: 1.03–1.71), and congenital heart block (CHB) was the main type of cardiac arrhythmia in pSS patients [39, 40]. The incidence of neonatal CHB in the offspring of mothers with anti-SSA antibody had been reported to be approximately 1% to 2%, and the risk of recurrence was 10 times higher in the subsequent pregnancies [41]. It had been proposed that immune-mediated tissue damage may be important in the pathogenesis of neonatal CHB [40]. Interestingly, the adult conduction system was thought to be able to tolerate the damage of anti-SSA/SSB antibodies, whereas the neonatal heart was affected by these antibodies. The autopsies results of infants affected with CHB revealed that there were no serious structural defects in the heart except atrioventricular node fibrosis [3]. It may be attributed to a cross-reaction with calcium channels on cardiac myocytes and the lower density of L-type calcium channels in neonatal cardiac myocytes [40]. L-type calcium channels were widely distributed in the cardiovascular system and were crucial for the propagation of action potential in the atrioventricular node. In addition, the presence of anti-idiotypic antibodies against anti-SSA/SSB antibodies in maternal serum could protect the fetus by blocking pathogenic maternal autoantibodies, which partially explains that there were no serious structural defects of neonatal CHB [3].
Pericardial and valvular diseases
It is well known that pSS is a low-grade systemic inflammation and a slow progressive disease, which has a less extend with systemic manifestations compared with other autoimmune diseases, especially RA and SLE [3]. Moreover, pericardial involvement is indeed not frequent involvement in pSS compared with RA, and pericardial and valvular diseases in pSS patients are often clinically silent, which are usually found by using echocardiography. Previous studies demonstrated that 10%-33% of pSS patients had signs of present or previous pericarditis, and pericardial effusion was present in 9 of 107 pSS patients (8.4%) [2, 42]. Patients with pericarditis were slightly older, had shorter disease duration, had significantly increased levels of orosomucoid and haptoglobin, and had an increased frequency of antinuclear-antibody positive than those patients without pericarditis [42]. Pericardial effusion was associated with cryoglobulinemia and primary biliary cirrhosis [2]. The possible mechanisms of pericarditis may be related to inflammatory cell infiltration in myocardial muscle bundles or systemic visceral vasculitis [3]. Although most patients with myocarditis were positive for anti-SSA antibody, the mechanism of autoantibody-inducted was not confirmed.
In fact, valvular regurgitation was the most common valvular lesions in pSS patients, and the frequency of mitral, aortic, and tricuspid valve regurgitation in patients with pSS was higher than that in healthy controls [2]. Age and low levels of C4 complement were the predictors of mitral valve regurgitation and aortic valve regurgitation, and tricuspid valve regurgitation was associated with PAH (OR = 13.33, 95%CI: 3.19–55.79) [2]. However, the mechanisms of valvular regurgitation remain elusive and merit exploration.
Cardiovascular autonomic dysfunction
An increasing number of studies indicated that the majority of pSS patients had mild or subclinical autonomic abnormalities, which often involved sympathetic and parasympathetic nerves and manifested as palpitation, disabling orthostatic hypotension and episodes of presyncope [3, 43, 44]. Heart rate and blood pressure variability, spontaneous baroreflex sensitivity, cardiovascular reflex tests, and composite questionnaires assessing autonomic symptoms have been used to evaluate the function of the autonomic nervous. In particular, heart rate variability may be more sensitive than cardiovascular reflex tests [45]. A case–control study demonstrated that the variability of heart rate and blood pressure in pSS patients were restricted, and the frequency of abnormal cardiovascular reflex tests in patients with pSS was higher than the healthy individuals, which was consistent with the study by Koh et al. [44, 45]. Moreover, Raynaud’s phenomenon was a more common clinical presentation in patients with autoimmune dysfunction than in subjects without autoimmune dysfunction (29.4% vs. 14.4%, P = 0.048), and chronic fatigue was related to autonomic dysfunction [44]. Inflammation, vasculitis, and autoantibody-mediated damage may play a significant role in the development of cardiovascular autonomic dysfunction [3].
Heart failure
Heart failure was a serious manifestation and the end stage of the development of various CVD, as well as one of the main causes of death. Heart failure was considered as the most common overt CVD, and the risk of heart failure was increased for pSS patients versus the general population [11]. A recent meta-analysis reported that patients with pSS were twice as likely to have heart failure as the general population [7]. The underlying pathogenesis of heart failure in pSS patients remains unclear. Some cases reported that the symptoms and laboratory abnormalities of heart failure disappeared after treatment with GC, thus we assumed that inflammation may be an important mechanism of heart failure in pSS patients. It was worth noting that diastolic heart failure was a common form of heart failure and characterized by left ventricular diastolic dysfunction, which was also relatively common in pSS patients [46]. Myocarditis, myocardial fibrosis, vasculitis, and microvascular dysfunction were important causes of left ventricular diastolic dysfunction.
Treatment
At present, the main purpose of the treatment of pSS is to relieve the symptoms of patients, prevent the progression of the disease, prolong the overall survival time, and improve the quality of life. Until now, there are no standard treatment guidelines for autoimmune diseases with cardiac involvement. A few studies have focused on the treatment of pSS patients with CVD, and the current treatment of disease is mainly empiric and often based on other systemic rheumatoid diseases with cardiac involvement. Table 2 summarizes the potential drugs for cardiac involvement of pSS. Some studies have shown that HCQ, low doses of GC (≤ 7.5 mg/day), and biological agents may bring some cardiovascular benefits [4, 35, 47]. It has been reported that approximately 40% of pSS patients are treated with HCQ, which has anti-thrombotic properties, improves lipid and glucose metabolism [11, 47]. Migkos et al. confirmed that the lipids profile in pSS patients treated with HCQ had a statistically significant improvement, and levels of TC and the atherogenic index (TC/HDL-C) were significantly decreased, while levels of HDL-C were increased [28]. The mechanism of the hypolipidemic action of HCQ may be related to interference with lysosomal activity, inhibition of antigen presentation and signal transduction of Toll-like receptor [28]. In addition, the improvement of glucose metabolism-related to HCQ treatment was associated with increased insulin secretion and peripheral insulin sensitivity [48]. These results demonstrated that HCQ may represent a relatively safe and inexpensive treatment to reduce the risk of CVD in pSS patients. However, myocarditis and arrhythmias may occur in the process of long-term use of HCQ, so attention should be paid to detecting its cardiotoxicity [29]. A multi-center cohort study showed that GC, ISs and PAH target therapy might be a beneficial treatment management strategy for pSS-PAH, and cyclophosphamide (CP) was the most commonly used immunosuppressant [35]. In recent years, biological agents may play a role in the treatment of pSS patients with severe involvement, but their exact therapeutic effect is still controversial. For instance, rituximab, a B-cell targeted therapy, has been successfully used in the treatment of pSS, as well as other rheumatic diseases [4]. Moreover, it has been confirmed that rituximab has the role of improving the lipid profiles, arterial stiffness, and carotid intima-media thickness in patients with RA [49]. Unfortunately, abatacept (a T-cell targeted therapy) and anti-cytokine therapy did not produce significant efficacy in patients with pSS [50, 51]. In addition, B-cell activating factor (BAFF) can promote the proliferation of B cells and the production of anti-SSA antibody, the latter plays an important role in the pathogenesis of pSS patients with CVD. Belimumab, an anti-BAFF agent, has proven its effectiveness in SLE patients, which may also be applicable to patients with pSS [52]. Some new biologic drugs are also under investigation which may open a new era in the treatment of cardiac involvement in patients with pSS.
Based on these findings, regularly evaluating the risk of CVD in pSS patients should be performed, and selecting the most appropriate therapeutic drugs to improve the cardiovascular prognosis. It is worth mentioning that the possible risks and benefits should be carefully balanced when choosing these drugs, and the potentially toxic side effects of drugs also should be closely monitored during the treatment.
Conclusion
It has a significantly increased risk of CVD in patients with pSS compared with the general population. Although the underlying cause is unknown, it is likely to be associated with subclinical atherosclerosis and vascular damage, traditional cardiovascular risk factors, and disease-related factors. Cardiovascular biomarkers allow the early identification of patients who are at high risk of cardiac involvement. Some intervention measures should be taken early to prevent or delay the occurrence of CVD for high-risk patients with pSS. Notably, the underlying mechanism of CVD and the treatment strategies of cardiac involvement in pSS patients are not clear, which needs further study.
Data availability
Not applicable.
Code availability
Not applicable.
References
Qin B, Wang J, Yang Z et al (2015) Epidemiology of primary Sjӧgren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis 74:1983–1989. https://doi.org/10.1136/annrheumdis-2014-205375
Vassiliou VA, Moyssakis I, Boki KA, Moutsopoulos HM (2008) Is the heart affected in primary Sjӧgren’s syndrome? An echocardiographic study. Clin Exp Rheumatol 26:109–112
Melissaropoulos K, Bogdanos D, Dimitroulas T, Sakkas LI, Kitas GD, Daoussis D (2020) Primary Sjӧgren’s syndrome and cardiovascular disease. Curr Vasc Pharmacol 18:447–454. https://doi.org/10.2174/1570161118666200129125320
Berardicurti O, Ruscitti P, Cipriani P et al (2018) Cardiovascular disease in primary Sjӧgren’s syndrome. Rev Recent Clin Trials 13:164–169. https://doi.org/10.2174/1574887113666180315130336
Liang M, Bao L, Xiong N et al (2015) Cardiac arrhythmias as the initial manifestation of adult primary Sjӧgren’s syndrome: a case report and literature review. Int J Rheum Dis 18:800–806. https://doi.org/10.1111/1756-185x.12616
Singh AG, Singh S, Matteson EL (2016) Rate, risk factors and causes of mortality in patients with Sjӧgren’s syndrome: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 55:450–460. https://doi.org/10.1093/rheumatology/kev354
Beltai A, Barnetche T, Daien C et al (2020) Cardiovascular morbidity and mortality in primary Sjӧgren’s syndrome: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 72:131–139. https://doi.org/10.1002/acr.23821
Bartoloni E, Alunno A, Cafaro G et al (2019) Subclinical atherosclerosis in primary Sjӧgren’s syndrome: does inflammation matter? Front Immunol 10:817. https://doi.org/10.1002/acr.23821
Bartoloni E, Alunno A, Valentini V et al (2018) The prevalence and relevance of traditional cardiovascular risk factors in primary Sjӧgren’s syndrome. Clin Exp Rheumatol 36(Suppl 112):113–120
Baldini C, Pepe P, Quartuccio L et al (2014) Primary Sjogren’s syndrome as a multi-organ disease: impact of the serological profile on the clinical presentation of the disease in a large cohort of Italian patients. Rheumatology (Oxford) 53:839–844. https://doi.org/10.1093/rheumatology/ket427
Bartoloni E, Baldini C, Schillaci G et al (2015) Cardiovascular disease risk burden in primary Sjӧgren’s syndrome: results of a population-based multicentre cohort study. J Intern Med 278:185–192. https://doi.org/10.1111/joim.12346
Yong WC, Sanguankeo A, Upala S (2018) Association between primary Sjӧgren’s syndrome, cardiovascular and cerebrovascular disease: a systematic review and meta-analysis. Clin Exp Rheumatol 36(Suppl 112):190–197
Pérez-De-Lis M, Akasbi M, Sisó A et al (2010) Cardiovascular risk factors in primary Sjӧgren’s syndrome: a case-control study in 624 patients. Lupus 19:941–948. https://doi.org/10.1177/0961203310367504
Yeh CC, Wang WC, Wu CS et al (2016) Association of Sjӧgren’s syndrome in patients with chronic hepatitis virus infection: a population-based analysis. PLoS ONE 11:e0161958. https://doi.org/10.1371/journal.pone.0161958
Chang CS, Liao CH, Muo CH, Kao CH (2016) Increased risk of concurrent gastroesophageal reflux disease among patients with Sjӧgren’s syndrome: a nationwide population-based study. Eur J Intern Med 31:73–78. https://doi.org/10.1016/j.ejim.2016.01.014
Bartoloni E, Shoenfeld Y, Gerli R (2011) Inflammatory and autoimmune mechanisms in the induction of atherosclerotic damage in systemic rheumatic diseases: two faces of the same coin. Arthritis Care Res (Hoboken) 63:178–183. https://doi.org/10.1002/acr.20322
Valim V, Gerdts E, Jonsson R et al (2016) Atherosclerosis in Sjӧgren’s syndrome: evidence, possible mechanisms and knowledge gaps. Clin Exp Rheumatol 34:133–142
Sezis Demirci M, Karabulut G, Gungor O, Celtik A, Ok E, Kabasakal Y (2016) Is there an increased arterial stiffness in patients with primary Sjӧgren’s syndrome? Intern Med 55:455–459. https://doi.org/10.2169/internalmedicine.55.3472
Atzeni F, Sarzi-Puttini P, Signorello MC et al (2014) New parameters for identifying subclinical atherosclerosis in patients with primary Sjӧgren’s syndrome: a pilot study. Clin Exp Rheumatol 32:361–368
Yong WC, Sanguankeo A, Upala S (2019) Association between primary Sjogren’s syndrome, arterial stiffness, and subclinical atherosclerosis: a systematic review and meta-analysis. Clin Rheumatol 38:447–455. https://doi.org/10.1007/s10067-018-4265-1
Doobay AV, Anand SS (2005) Sensitivity and specificity of the ankle-brachial index to predict future cardiovascular outcomes: a systematic review. Arterioscler Thromb Vasc Biol 25:1463–1469. https://doi.org/10.1161/01.atv.0000168911.78624.b7
Rachapalli SM, Kiely PD, Bourke BE (2009) Prevalence of abnormal ankle brachial index in patients with primary Sjogren’s syndrome. Clin Rheumatol 28:587–590. https://doi.org/10.1007/s10067-009-1099-x
Cai X, Luo J, Wei T, Qin W, Wang X, Li X (2019) Risk of cardiovascular involvement in patients with primary Sjӧgren’s syndrome: a large-scale cross-sectional cohort study. Acta Reumatol Port 44:71–77
Wu XF, Huang JY, Chiou JY, Chen HH, Wei JC, Dong LL (2018) Increased risk of coronary heart disease among patients with primary Sjӧgren’s syndrome: a nationwide population-based cohort study. Sci Rep 8:2209. https://doi.org/10.1038/s41598-018-19580-y
Zhang Y, Li M, Zhang L et al (2020) Association between comorbidities and extraglandular manifestations in primary Sjӧgren’s syndrome: a multicenter cross-sectional study. Clin Rheumatol 39:2677–2688. https://doi.org/10.1007/s10067-020-04992-x
Juarez M, Toms TE, de Pablo P et al (2014) Cardiovascular risk factors in women with primary Sjӧgren’s syndrome: United Kingdom primary Sjӧgren’s syndrome registry results. Arthritis Care Res (Hoboken) 66:757–764. https://doi.org/10.1002/acr.22227
Bartoloni E, Alunno A, Gerli R (2018) Hypertension as a cardiovascular risk factor in autoimmune rheumatic diseases. Nat Rev Cardiol 15:33–44. https://doi.org/10.1038/nrcardio.2017.118
Migkos MP, Markatseli TE, Iliou C, Voulgari PV, Drosos AA (2014) Effect of hydroxychloroquine on the lipid profile of patients with Sjӧgren’s syndrome. J Rheumatol 41:902–908. https://doi.org/10.3899/jrheum.131156
Nadeem U, Raafey M, Kim G et al (2021) Chloroquine- and hydroxychloroquine-induced cardiomyopathy: a case report and brief literature review. Am J Clin Pathol 155:793–801. https://doi.org/10.1093/ajcp/aqaa253
Kunutsor SK, Flores-Guerrero JL, Kieneker LM et al (2018) Plasma calprotectin and risk of cardiovascular disease: findings from the PREVEND prospective cohort study. Atherosclerosis 275:205–213. https://doi.org/10.1016/j.atherosclerosis.2018.06.817
Ueland T, Otterdal K, Lekva T et al (2009) Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol 29:1228–1234. https://doi.org/10.1161/atvbaha.109.189761
Németh B, Ajtay Z, Hejjel L et al (2017) The issue of plasma asymmetric dimethylarginine reference range - a systematic review and meta-analysis. PLoS ONE 12:e0177493. https://doi.org/10.1371/journal.pone.0177493
Luni FK, Malik SA, Khan AR et al (2017) Risk of ischemic heart disease in patients with Sjӧgren’s syndrome. Am J Med Sci 354:395–398. https://doi.org/10.1016/j.amjms.2017.05.001
Chiang CH, Liu CJ, Chen PJ et al (2013) Primary Sjӧgren's syndrome and the risk of acute myocardial infarction: a nationwide study. Acta Cardiol Sin 29:124–131. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc4804774/
Wang J, Li M, Wang Q et al (2020) Pulmonary arterial hypertension associated with primary Sjogren’s syndrome: a multicentre cohort study from China. Eur Respir J 56:1902157. https://doi.org/10.1183/13993003.02157-2019
Kobak S, Kalkan S, Kirilmaz B, Orman M, Ercan E (2014) Pulmonary arterial hypertension in patients with primary Sjӧgren’s syndrome. Autoimmune Dis 2014:710401. https://doi.org/10.1155/2014/710401
Yan S, Li M, Wang H et al (2018) Characteristics and risk factors of pulmonary arterial hypertension in patients with primary Sjӧgren’s syndrome. Int J Rheum Dis 21:1068–1075. https://doi.org/10.1111/1756-185x.13290
Zhang N, Zhao Y, Wang H et al (2019) Characteristics and risk factors for pulmonary arterial hypertension associated with primary Sjӧgren’s syndrome: 15 new cases from a single center. Int J Rheum Dis 22:1775–1781. https://doi.org/10.1111/1756-185x.13671
Kang JH, Lin HC (2010) Comorbidities in patients with primary Sjӧgren’s syndrome: a registry-based case-control study. J Rheumatol 37:1188–1194. https://doi.org/10.3899/jrheum.090942
Steele JC, Dawson LJ, Moots RJ, Field EA (2005) Congenital heart block associated with undiagnosed maternal primary Sjӧgren’s syndrome – a case report and discussion. Oral Dis 11:190–192. https://doi.org/10.1111/j.1601-0825.2005.01050.x
Brucato A (2008) Prevention of congenital heart block in children of SSA-positive mothers. Rheumatology (Oxford) 47(Suppl 3):iii35–iii37. https://doi.org/10.1093/rheumatology/ken153
Rantapää-Dahlqvist S, Backman C, Sandgren H, Ostberg Y (1993) Echocardiographic findings in patients with primary Sjӧgren’s syndrome. Clin Rheumatol 12:214–218. https://doi.org/10.1007/bf02231529
Cai FZ, Lester S, Lu T et al (2008) Mild autonomic dysfunction in primary Sjӧgren’s syndrome: a controlled study. Arthritis Res Ther 10:R31. https://doi.org/10.1186/ar2385
Koh JH, Kwok SK, Lee J, Park SH (2017) Autonomic dysfunction in primary Sjogren’s syndrome: a prospective cohort analysis of 154 Korean patients. Korean J Intern Med 32:165–173. https://doi.org/10.3904/kjim.2015.219
Kovács L, Paprika D, Tákacs R et al (2004) Cardiovascular autonomic dysfunction in primary Sjӧgren’s syndrome. Rheumatology (Oxford) 43:95–99. https://doi.org/10.1093/rheumatology/keg468
Akyel A, Tavil Y, Tufan A et al (2012) Atrial electromechanical delay and diastolic dysfunction in primary Sjӧgren’s syndrome. Clin Invest Med 35:303. https://doi.org/10.25011/cim.v35i5.18703
Gottenberg JE, Ravaud P, Puéchal X et al (2014) Effects of hydroxychloroquine on symptomatic improvement in primary Sjӧgren syndrome: the JOQUER randomized clinical trial. JAMA 312:249–258. https://doi.org/10.1001/jama.2014.7682
Rempenault C, Combe B, Barnetche T et al (2018) Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis 77:98–103. https://doi.org/10.1136/annrheumdis-2017-211836
Novikova DS, Popkova TV, Lukina GV et al (2016) The effects of rituximab on lipids, arterial stiffness and carotid intima-media thickness in rheumatoid arthritis. J Korean Med Sci 31:202–207. https://doi.org/10.3346/jkms.2016.31.2.202
Baer AN, Gottenberg JE, St Clair EW et al (2020) Efficacy and safety of abatacept in active primary Sjӧgren’s syndrome: results of a phase III, randomised, placebo-controlled trial. Ann Rheum Dis 80:339–348. https://doi.org/10.1136/annrheumdis-2020-218599
Roescher N, Tak PP, Illei GG (2010) Cytokines in Sjogren’s syndrome: potential therapeutic targets. Ann Rheum Dis 69:945–948. https://doi.org/10.1136/ard.2009.115378
Ramos-Casals M, Brito-Zerón P (2007) Emerging biological therapies in primary Sjogren’s syndrome. Rheumatology (Oxford) 46:1389–1396. https://doi.org/10.1093/rheumatology/kem078
Acknowledgements
All authors contributed to the study design. The authors would like to thank Dr. Bo Qin (Shenyang Research Institute of Chemical Industry) for his helpful assistance during the preparation of this manuscript. This study was funded by the National Nature Science Foundation (grant number 81300243), so we thank them for the support shown to our program.
Funding
This study was funded by the National Nature Science Foundation (grant number 81300243).
Author information
Authors and Affiliations
Contributions
LQ—conceptualization, writing-original draft, review, and editing; YZ, XY,—literature search, data curation, review, and editing; QL- formal analysis, review and editing; HW—conceptualization, review, and editing.
Corresponding author
Ethics declarations
Conflicts of interest
Li Qin, Yiwen Zhang, Xiaoqian Yang, Qiang Luo, and Han Wang declare that they have no conflict of interest.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, L., Zhang, Y., Yang, X. et al. Cardiac involvement in primary Sjӧgren’s syndrome. Rheumatol Int 42, 179–189 (2022). https://doi.org/10.1007/s00296-021-04970-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-021-04970-9