Abstract
Despite the frequent co-ocurrence of hypermobile Ehler–Danlos syndrome (hEDS) and pathological anxiety, little is known about the psychosocial and health implications of such comorbidity. Our aim was to explore the association between high levels of anxiety and psychosocial (catastrophizing, kinesiophobia, somatosensory amplification, social support and functioning), health (pain, fatigue, BMI, tobacco/alcohol use, depression, diagnosis delay, general health), and sociodemographic factors in people with hEDS. In this cross-sectional study, 80 hEDS patients were divided into two groups according to self-reported anxiety levels: low and high. Psychosocial, sociodemographic and health variables were compared between the groups. Forty-one participants reported a high level of anxiety (51.2%). No differences were found in the sociodemographic variables between high-anxious and low-anxious patients. The percentage of participants with severe fatigue and high depressive symptomatology was significantly higher in the high-anxious group (80.5 vs 56.4; 26.8 vs 12.8%, respectively). High-anxious hEDS patients also showed significantly higher levels of pain catastrophizing, somatosensory amplification as well as a poorer social functioning and general health. Multivariate analyses showed that somatosensory amplification, pain catastrophizing and poor social functioning are variables that increase the probability of belonging to the high-anxious group. Despite limitations, this first study comparing high-anxious versus low-anxious hEDS patients with respect to health aspects, highlight the importance of considering the psychosocial factors (many susceptible to modification), to improve the adjustment to this chronic condition and provide support to those affected through a biopsychosocial approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Ehlers–Danlos syndromes (EDS) are a heterogeneous group of hereditary disorders affecting connective tissue matrix proteins. Joint hypermobility (JH; i.e., increased distensibility of joints), skin hyperextensibility and tissue fragility are their core characteristics [1]. Different subtypes of EDS have been described, certain of which are mild although highly disabling (e.g. hypermobile EDS; hEDS) whereas others are life threatening (e.g. vascular EDS). Six forms of EDS were described in the 1998 Villefranche classification [2], which are the criteria used in the investigations in recent years. A minimum prevalence of 1/5000 for all types of EDS has been reported [3]. Recently, a revised EDS classification was published [1] in which 13 EDS forms are recognized, based on the latest advances in clinical and genetic research.
hEDS is the most common form of EDS representing 80–90% of EDS cases [4]. This clinical entity overlaps with the Joint Hypermobility Syndrome described by rheumatologists [5]. Both conditions have been considered clinically indistinguishable [6].
hEDS is characterized by multisystem manifestations, varying according to age and gender [1], and which can be musculoskeletal (e.g. arthralgia, recurrent joint dislocations, mild scoliosis, temporomandibular joint dysfunction, fibromyalgia, epicondylitis) and non-musculoskeletal such as cardiovascular (e.g. pseudo-Raynaud’s phenomenon, mitral valve prolapse), gastrointestinal (e.g. abdominal pain, dysphagia, reflux gastroesophageal), dental (e.g. dental crowding, neuralgia), mucocutaneous (e.g. easy bruising, soft skin texture), urogynaecological (e.g. dysmenorrhea, dyspareunia, vaginal and uterine prolapses), ocular (e.g. myopia, strabismus, palpebral ptosis) and neuropsychiatric (e.g. proprioception dysfunction, dysautonomia, anxiety) [4, 7, 8]. Even though there is not life-risk in this EDS subtype, the variety and accumulation of symptoms and their long duration make hEDS a chronic, painful and highly disabling condition. All EDS subtypes can be confirmed by biological tests except the hEDS, the diagnosis of which remains clinical [4]. This aspect negatively affects the recognition of this pathology, which is currently underdiagnosed. Consequently, those affected are often confronted with the disbelief and incomprehension of the medical community and society in general [9].
Chronic painful conditions have been associated with the experience of negatives emotions and psychopathology [10] and hEDS is not the exception. Depressive feelings are common in hEDS [11,12,13] and can be understood as secondary to the difficulties linked to the disease such as pain, disability, frustration with the medical system, living a restricted life, etc. [11, 13,14,15,16,17]. High levels of anxiety, explored using dimensional scales and anxiety disorders (especially panic disorder and phobias) assessed by clinical interviews, have been reported as overrepresented in people with JH and hEDS in several studies [11]. Like depression, pathological anxiety could be seen as a response to the burden of the disease. As an adaptive response to threat, anxiety can be reactive to the constant danger of injury experienced by people with hEDS, in whom tissue fragility leads to an inherent propensity for trauma. Nevertheless, beyond these plausible hypotheses, the available data suggest a primary connection. Thus, biological hypotheses have been put forward to explain such an association [11, 18]. Specifically, a genetic link (duplication of human chromosome 15, named DUP 25) [19], alterations in the body awareness [20,21,22,23], dysautonomia [11,12,13, 18], and structural brain differences in areas related to emotion regulation [21] are clues to understanding the vulnerability of anxiety in hEDS patients [11,12,13,14, 18].
Despite the high prevalence of pathological anxiety in people with JH and hEDS, little is known about the psychosocial and health implications of such comorbidity in this pathology.
Thus, the aim of this observational study was to explore the association between high anxiety and psychosocial (coping styles, catastrophizing, fear of movement, somatosensory amplification, social support and functioning), health (pain, fatigue, BMI, tobacco/alcohol use, depression, diagnosis delay, general health), and sociodemographic factors in patients with hEDS.
Methods
Participants
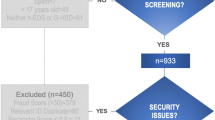
Participants included 80 outpatients (72 women; aged between 18 and 61 years old), recruited from the Ehlers–Danlos unit at the Hôtel Dieu Hospital (Paris, France). The diagnosis of hEDS is clinical and there are current no biological confirmatory tests. Thus, patients were all clinically assessed by a single national expert practitioner in EDS (CH) and all were diagnosed as having hEDS according to the Villefranche criteria [2] as published in previous reports [31, 32]. Patients with a serious somatic disease co-morbidity (i.e. cancer, degenerative), a medical history of head injury, not fluent in French or having cognitive problems preventing them from responding to the evaluation instruments were excluded.
Procedure
Patients were asked to participate in the study by the research assistants in the waiting room of the unit. They were given oral and written information. Those who agreed were asked to complete a series of self-questionnaires about demographic, psychosocial and health aspects. The study was conducted in accordance with the latest version of the Declaration of Helsinki. Ethical approval was obtained from the CERES (Comité d’évaluation éthique des projets de recherche; ID: 2015-51) of the University Paris Descartes. An informed consent was obtained from all participants before they began the study.
Instruments
Anxiety and depression were assessed with the French version of the Hospital Anxiety and Depression Scale (HADS) [33, 34]. The HADS is a self-questionnaire divided into 14 questions with scores ranging from 0 to 3. Half the questions address general anxiety and the other half address depression, particularly anhedonia symptoms. Two scores are derived from these (the highest score being 21 for each set of questions). For both subscales, a score equal to or higher than 11 points is considered a “high” level of anxiety/depression.
The perception of availability and satisfaction regarding social support were assessed with the Social Support Questionnaire (SSQ6) [35], adapted and validated for a French population by Rascle et al. [36]. This is a 6-item questionnaire and for each item, participants are asked to (1) indicate the name or initials of the people who support them. A list containing a network of people is then established and participants (2) indicate their degree of satisfaction regarding the support they receive.
Fear of pain and movement was evaluated using the Tampa Scale of Kinesiophobia (TSK) in its French version [37, 38]. The TSK is a 17-item self-administered Likert-type questionnaire on a 5-point scale with the end points 1 (“strongly disagree”) and 4 (“strongly agree”) developed to measure fear of pain and movement in chronic pain patients.
The French version of the Somatosensory Amplification Scale [39, 40] was used to assesses hypervigilance to mild somatic and visceral sensations, and the tendency to interpret them as pathological (perceptual and cognitive-affective components of symptom development and symptom report) [41]. The 10 items of this scale are rated from 1 to 5. The higher the score is, the greater the amplification of symptoms is.
The Pain Catastrophizing Scale (PCS) was also administered in its French version [42]. The PCS is a 13-item self-assessment scale used in clinical and non-clinical populations to evaluate the degree to which they catastrophize regarding pain. Participants are asked to indicate the extent to which they experience different feelings or thoughts when in pain. The PCS uses a Likert-type scale going from 0 (“not at all”) to 4 (“all the time”).
Social functioning (i.e. the extent to which ill health interferes with social activities) and perceptions of general health were assessed with the respective subscales of the SF-36 Survey [43]. This is a generic 36-item multidimensional measure derived from the Medical Outcome Study questionnaire and adapted and validated for a French population by Leplège et al. [44].
Usual pain and fatigue were assessed with a verbal rating scale. This instrument proposes a set of hierarchized qualifiers for pain and fatigue experienced in a general manner. It ranges from “absent” to “very severe”. These variables were dichotomized into “mild/moderate” vs. “severe/very severe”.
A questionnaire requesting sociodemographic and health information was also administered. It included questions concerning age, educational level, employment status, family situation, age at onset of symptoms and at diagnosis (from which the diagnosis delay was calculated), psychiatric antecedents, use of tobacco and alcohol, and height and weight (used to calculated the body mass index: BMI).
Analysis
For data analysis, participants were divided into two groups: Low- and high-anxious. Since none participant scored 0 to the anxiety subscale of the HADS, and the lower score was 2, the low-anxiety group included those with normal/mild anxiety or score 2–11. The high-anxious group included all the rest (score ≥ 11).
Statistical treatment consisted of a descriptive analysis of the data (percentage and average), the Mann–Whitney U test, the Chi squared method, and binary logistic regression. Data analysis was performed on a computer with the IBM SPSS 22 software package and the significance level was taken as 0.05 for all statistical tests.
Results
One hundred and twenty-eight patients agreed to participate in the study. Data from 81 participants were exploitable since all the self-questionnaires needed for this study were completed. One participant was withdrawn from the study at his request. Thus, the final sample (n = 80) was composed mostly by women (90%). The average age of patients was 37.1 years old (SD = 11.5). Fifty percent of participants are in a couple and 50.7% were active professionally. The average age at onset of hEDS symptoms was 12.1 years old (SD = 11.7). The average age at diagnosis was 34.5 years old (SD = 11.2) and the diagnosis delay was 22 years on average (SD = 13.3).
More than half of patients (67.1%) reported experiencing usual pain of severe/very severe intensity, and 68.8% reported usual fatigue with a severe/very severe intensity. More than half of participants presented an altered BMI. Overweight/obesity was found in 42.5% of the sample, while underweight was observed in 15%. In addition, a high level of depression symptomatology was reported by 20% of the sample, while 51.2% had a high level of anxiety (the high-anxious group).
When high-anxious and low-anxious hEDS subjects were compared with respect to the sociodemographic data, no differences were found. However, differences in some health variables were observed between the groups: the percentage of participants with severe fatigue and high depressive symptomatology was significantly greater in the anxious group. In addition, the perception of general health was significantly poorer among high-anxious patients.
Concerning the psychosocial measures, high-anxious hEDS patients scored significantly higher in pain catastrophizing and somatosensory amplification, and lower in social functioning than those without high anxiety (Table 1).
Binary logistic regressions were performed with “presence/absence of high anxiety” as the dependent variable (Table 2). Two models were tested using significant variables obtained in bivariate analyses and sociodemographic data. The first model included depression, somatosensory amplification, social functioning, sex, age and years of education as covariables. The results showed that the odds of being assessed with high anxiety were significantly increased in patients with somatosensory amplification and poor social functioning. The second model used pain catastrophizing, usual fatigue, general health, sex, age and years of education as covariables. According to these results, the odds of belonging to the high-anxious hEDS group were increased in those with pain catastrophizing.
Discussion
This study aimed to explore the association between high anxiety and psychosocial, health and sociodemographic factors in people with hEDS. First, our findings add weight to the cumulated evidence obtained in the last three decades concerning the high prevalence of pathological levels of anxiety among people with hEDS [45], as more than half of the patients (51.2%) presented high anxiety in our sample. The postal survey of Murray et al. [55] reported that 73% of people with hEDS present anxiety. Similar results were observed by Berglund et al. [47] in their study also conducted with a postal survey methodology, in which 74.8% of EDS patients (mostly hEDS) suffered from high anxiety as assessed with the HADS. The fact that these authors found a higher percentage of highly anxious patients using the same tool as that we employed, can be related to the characteristics of the population. Unlike our sample, which came from a clinical setting, the sample of Berglund et al. [47] was recruited through patient associations and it is possible that people experiencing more distress seek support in these associations. The Berglund et al.’s study also reported that 22.4% of the sample had high depression, which is concordant with our findings (20%), showing that anxiety is particularly prevalent in these patients, especially since the literature states that “anxiety disorders are second only to depression in psychological comorbidity in chronic pain populations” [48, p. 10]. Thus, our results support the hypothesis of a special vulnerability to anxiety states in those with this collagen condition. In this sense, Bulbena et al. [45] proposed the so-called “neuroconnective phenotype” to describe this mixed somatic-mental clinical situation (collagen laxity and anxiety), which currently lacks of nosological status.
In general, a mental disorder associated with a somatic disease worsens the clinical picture and the psychosocial scenario of those affected [60] but this assumption needed to be evidenced in hEDS. Besides, anxiety has a negative impact on thoughts and behaviors and limits the possibilities of rehabilitation [48]. Accordingly, untreated clinical anxiety has been associated with an increased use of health service resources, increased disability and diminished quality of life [54]. Nevertheless, according to our experience, it is not uncommon to find a resistance to recognizing a comorbid mental disorder, such as anxiety disorders, among a subgroup of hEDS patients and specialists. This is probably due to trivialization, a fear of stigmatization, and the idea that its recognition might reinforce the widespread disbelief in hEDS as a somatic pathology in the medical community, arguing instead for a psychological cause (“it’s in all in your head”). Thus, concerned patients and physicians may not be prepared to refer or to accept referral to mental health professionals [54]. Although understandable, these representations reinforce the outdated mind–body dualism, which goes against the holistic approach needed to address this complex pathology.
Moreover, physicians often believe that it is normal to respond anxiously to a medical illness [54], which is correct to some extent. It is necessary to distinguish between normal and clinical or pathological anxiety, whose intensity and duration interfere significantly with normal functioning. Although pathological anxiety is more common in people with a chronic illness than in those without, this concerns a small percentage of patients and its duration is limited [54]. Thus, a high and persistent level of anxiety should not be trivialized or overlooked to avoid a detrimental effect of anxiety on the course of the disease and the quality of life of those affected.
According to Bulbena et al. [11], there is preliminary evidence of high levels of depression symptomatology in hEDS subjects, especially when anxiety is present. This is the case in our study, in which the percentage of patients with high depression was significantly higher among high-anxious than in low-anxious patients. The comorbidity of anxiety-depression is common. As Woo [48, p. 10] stated “the presence of one (anxiety or depression) should alert rather than deter the diagnosis of the other”. According to Bair et al. [49] when anxiety and depression are present with chronic pain, there is greater pain severity and disability and poorer health related to quality of life than with pain only, pain with depression, or pain with anxiety.
In the present study, high anxiety appears to be associated with severe fatigue. Autonomic dysfunction might underlie the co-occurrence of both symptoms. There is evidence that symptoms of autonomic dysfunction are common in hEDS (70%) [50]. Dysautonomic conditions, such as orthostatic intolerance, and orthostatic dizziness related to heat and exercise, have been reported as predictors of fatigue severity in hEDS, as well as cardiovascular dysregulation [29, 30, 51]. The manifestations of autonomic dysfunction overlap with those of anxiety disorders (e.g. syncope, palpitations). Besides, it is well known that anxious people have frequently sleep disturbances [52] which might lead to fatigue. In view of these results, it is not surprising that high-anxious hEDS patients judge their general health to be poorer than low-anxious patients do.
As expected, the high-anxious group showed higher levels of pain catastrophizing and somatosensory amplification than low-anxious, and these variables were retained by multivariate analysis to distinguish between both groups of hEDS subjects. According to Kendall and Ingram [53], people suffering from anxiety-related psychopathologies have an automatic questioning style characterized by an internal dialogue that takes the form of “what if…?” questions. These generate maladaptive cognitions, which in turn perpetuate anxiety. This cognitive style in anxious people reflects a sense of personal incompetence [53]. Thus, those with high anxiety might catastrophize about their pain and body sensations and become hypervigilant and over-aware of threatening information as well as to overemphasizing the possibility of catastrophic outcomes concerning the body [54, 55].
Moreover, high anxiety was associated with lower social functioning. No doubt the burden of a disease has an effect on the social life of those affected. Recently, De Baets et al. [56] reported that for women with hEDS, the disease imposes a major impact on social behavior. Berglund et al. [17] found that EDS patients described their life like as restricted and with limited possibilities of self-actualization in social life. In addition, participation in personal relationships and community has been found to be a predictor of fatigue severity in this pathology [51].
Patients with hEDS often describe feelings of isolation and stigmatization. Feeling ignored, rejected and/or misunderstood by doctors and their families is common among them [15,16,17, 57]. In this sense, since anxiety is associated with poor communication [54], social withdrawal and behavioral inhibition [58], high anxiety in hEDS patients might increase their difficulties in interacting with the environment, thus contributing to maintaining/amplifying their feelings of isolation. This would help to explain their poorer social functioning compared to low-anxious patients. In the same vein, and considering that the high-anxious groups catastrophize more, diminished social functioning may also be related to the fact that high catastrophizing can reduce positive social interactions since it triggers distress in others [59].
Finally, it is worth pointing out the important diagnosis delay found in this sample (22 years on average). Unfortunately, delayed diagnosis and misdiagnosis in hEDS is more the rule than the exception [9, 16]. This is probably due to factors such as the lack of confirmatory biological test, the overlap with other pathologies, the lack of familiarity (or disbelief) of health professionals with the hEDS clinical picture, etc. In this sense, it is important to disseminate knowledge about hEDS to promote early recognition, and thus minimize negative health outcomes.
The main limitations to mention are the cross-sectional design, which precludes any inference about the directionality of relationships. The lack of control group for comparison. The fact that the variables are all self-report data. However, all the instruments chosen are validated self-questionnaires widely used in clinical and research settings. In this sense, it is worth highlighting the usefulness of the HADS to quickly and simply screen high levels of anxiety. The limited generalization of results, since the sample size is small, the participants were recruited from a unique center, and the missing data was not missing at random, which restricts the conclusions one may draw from them. Despite these limitations, this first study comparing high-anxious versus low-anxious hEDS patients with respect to health outcomes, highlight the importance of considering the psychosocial factors (many susceptible to modification), to improve the adjustment to this chronic condition and provide support to those affected through a biopsychosocial approach.
References
Malfait F, Belmont J, Berglund B, Black J, Bloom L, Bowen JM, Brady AF, Burrows NP, Castori M, Cohen H, Colombi M, Demirdas S, de Backer J, de Paepe A, Fournel-Gigleux, Frank M, Ghali N, Giunta C, Grahame R, Hakim A, Jeunemaitre X, Johnson D, Juul-Kristensen B, Kapferer-Seebacher I, Kazkaz H, Kosho T, Lavalle ME, Levy H, Mendoza-Londono R, Pepin M, Pope M, Reinstein E, Robert L, Rohrbach M, Sanders L, Sobey GL, Van Damme T, Vandersteen A, Van Mourik C, Voermans N, Wheeldon N, Zschocke J, Tinkle B (2017) The 2017 international classification on the Ehlers–Danlos syndromes. Am J Med Genet 175C:148–157
Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ (1998) Ehlers–Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers–Danlos National Foundation (USA) and Ehlers–Danlos Support Group (UK). Am J Med Genet 77:31–37
Steinmann B, Royce PM, Superti-Furga A (2002) The Ehlers–Danlos syndrome. In: Royce PM, Steinmann B (eds) Connective tissue and its heritable disorders. Wiley-Liss, New York, pp 431–524
Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H, Levy H (2017) Hypermobile Ehlers–Danlos Syndrome (a.k.a. Ehlers–Danlos syndrome type III and Ehlers–Danlos syndrome hypermobility type): clinical description and natural history. Am J Med Genet 175C:48–69
Grahame R, Bird HA, Child A (2000) The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). J Rheumatol 27:1777–1779
Tinkle BT, Bird HA Grahame R, Lavallee M, Levy HP, Sillence D (2009) The lack of clinical distinction between the hypermobility type of Ehlers–Danlos syndrome and the joint hypermobility syndrome (a.k.a. hypermobility syndrome). Am J Med Genet 149A:2368–2370
Colombi M, Dordoni C, Chiarelli N, Ritelli M (2015) Differential diagnosis and diagnostic flow chart of joint hypermobility syndrome/Ehlers–Danlos syndrome hypermobility type compared to other heritable connective tissue disorders. Am J Med Genet 169C:6–22
Hamonet C, Gompel A, Raffray Y, Zeitoun JD, Delarue M, Vlamynck E, Haidar R, Mazaltarine G (2014) Multiple pains in Ehlers–Danlos syndrome. Description and proposal of a therapy protocol. Douleurs 15:264–277. https://doi.org/10.1016/j.douler
Grahame R (2008) Hypermobility: an important but often neglected area within rheumatology. Nat Clin Pract Rheumatol 4:522–524
Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M (2009) Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 9:883–891. https://doi.org/10.1016/j.jpain.2008.05.005
Bulbena A, Baeza-Velasco C, Bulbena-Cabré A, Pailhez G, Critchley H, Chopra P, Mallorqui-Bagué N, Frank Ch, Porges S (2017) Psychiatric and psychological aspects in the Ehlers–Danlos syndrome. Am J Med Genet 175C:237–245
Smith TO, Easton V, Bacon H, Jerman E, Armon K, Poland F, Macgregor AJ (2014) The relationship between benign joint hypermobility syndrome and psychological distress: a systematic review and meta-analysis. Rheumatology 53:114–122. https://doi.org/10.1093/rheumatology/ket317
Sinibaldi L, Ursini G, Castori M (2015) Psychopathological manifestations of joint hypermobility and joint hypermobility syndrome/Ehlers–Danlos syndrome, hypermobility type: the link between connective tissue and psychological distress revised. Am J Med Genet 169C:97–106. https://doi.org/10.1002/ajmg.c.31430
Lumley MA, Jordan M, Rubenstein R, Tsipouras P, Evans MI (1994) Psychosocial functioning in the Ehlers–Danlos syndrome. Am J Med Genet 53:149–152. https://doi.org/10.1002/ajmg.1320530206
Baeza-Velasco C, Gély-Nargeot MC, Bulbena A, Bravo JF (2011) Joint hypermobility syndrome: problems that require psychological intervention. Rheumatol Int 31:1131–1136
Berglund B, Anne-Cathrine M, Randers I (2010) Dignity not fully upheld when seeking health care: experiences expressed by individuals suffering from Ehlers–Danlos syndrome. Disabil Rehabil 32:1–7
Berglund B, Nordström G, Lützén K (2000) Living a restricted life with Ehlers–Danlos syndrome (EDS). Int J Nurs Stud 37:111–118
Baeza-Velasco C, Pailhez G, Bulbena A, Baghdadli A (2015) Joint hypermobility and the heritable disorders of connective tissue: clinical and empirical evidence of links with psychiatry. Gen Hosp Psychiatry 37:24–30
Gratacos M, Nadal M, Martin-Santos R, Pujana MA, Gago J, Peral B, Armengol L, Ponsa I, Miro R, Bulbena A, Estivill X (2001) A polymorphic genomic duplication on human chromosome 15 is a susceptibility factor for panic and phobic disorders. Cell 106:367–379
Baeza-Velasco C, Gély-Nargeot MC, Bulbena A, Fénétrier C, Bravo JF (2011) Association between psychopathological factors and joint hypermobility syndrome in a group of undergraduates from a French university. Int J Psychiatry Med 41:187–201
Eccles JA, Beacher FD, Gray MA, Jones CL, Minati L, Harrison NA, Critchley HD (2012) Brain structure and joint hypermobility: relevance to the expression of psychiatric symptoms. B J Psychiatry 200:508Y9. https://doi.org/10.1192/bjp.bp.111.092460
Mallorqui-Bagué N, Bulbena A, Roe-Vellve N, Hoekzema E, Carmona S, Barba-Muller E, Fauquet J, Pailhez G, Vilarroya O (2015) Emotion processing in joint hypermobility: a potential link to the neural bases of anxiety and related somatic symptoms in collagen anomalies. Eur Psychiatry 30:454–458. https://doi.org/10.1016/j.eurpsy.2015.01.004
Mallorqui-Bagué N, Garfinkel SN, Engels M, Eccles JA, Pailhez G, Bulbena A, Critchley HD (2014) Neuroimaging and psychophysiological investigation of the link between anxiety, enhanced affective reactivity and interoception in people with joint hypermobility. Front Psychol 5:1162
Bart O, Bar-Haim Y, Weizman E, Levin M, Sadeh A, Mintz M (2009) Balance treatment ameliorates anxiety and increases self-esteem in children with comorbid anxiety and balance disorder. Res Dev Disabil 30:486–495
Balaban CD, Thayer JF (2001) Neurological bases for balance-anxiety links. J Anxiety Dis 15:53–79
Jacob RG, Furman JM, Durrant JD, Turner SM (1996) Panic, agoraphobia, and vestibular dysfunction. Am J Psychiatry 153:503–512. https://doi.org/10.1176/ajp.153.4.503
Stins FJ, Ledebt A, Emck C, van Dokkum EH, Beek PJ (2009) Patterns of postural sway in high anxious children. Behav Brain Funct 5:42
Rowe PC, Barron DF, Calkins H, Maumenee IH, Tong PY, Geraghty MT (1999) Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers–Danlos syndrome. J Pediatr 135:494–499
De Wandele I, Rombaut L, De Backer T, Peersman W, Da Silva H, De Mits S, De Paepe A, Calders P, Malfait F (2016) Orthostatic intolerance and fatigue in the hypermobile type of Ehlers–Danlos syndrome. Rheumatology 55(8):1412–1420
Hakim A, De Wandele I, O’Callaghan C, Pocinki A, Rowe P (2017) Chronic fatigue in Ehlers–Danlos syndrome-hypermobility type. Am J Med Genet 175C:175–180
Hugon-Rodin J, Lebègue G, Becourt S, Hamonet C, Gompel A (2016) Gynecologic symptoms and the influence on reproductive life in 368 women with hypermobility type Ehlers–Danlos syndrome: a cohort study. Orphanet J Rare Dis 11:124. https://doi.org/10.1186/s13023-016-0511-2
Zeitoun J-D, Lefèvre JH, de Parades V, Séjourné C, Sobhani I, Coffin B, Hamonet C (2013) Functional digestive symptoms and quality of life in patients with Ehlers– Danlos syndromes: results of a national cohort study on 134 patients. PLoS One 8:e80321
Zigmond A. Snaith R (1983) The hospital anxiety and depression scale. Acta Psiquiatr Scand 67:361–370
Lépine JP, Godchau M, Brun P, Lemperière T (1985) Evaluation de l’anxiété et de la dépression chez des patients hospitalisés dans un service de médecine interne. Annales Médico Psychologiques 43:175–189
Sarason IG, Levine HM, Basham RB, Sarason BR (1983) Assessing social support: the social support questionnaire. J Pers Soc Psychol 44:127–139
Rascle N, Bruchon-Schweitzer M, Sarason IG (2005) Short form of Saranson’s Social Support Questionnaire: French adaptation and validation. Psychol Reports 97:195–202
Miller R, Kori S, Todd D (1991) The Tampa Scale for Kinesiophobia, Tampa, FL. (Unpublished report)
French DJ, Roach PJ, Mayes S (2002) Peur du movement chez les accidentés du travail: l’Echelle de Kinesiophobie de Tampa (EKT). Can J Behav Sci 34:28–33
Barsky AJ, Wyshak G, Klerman GL (1990) The somatosensory amplification scale and its relationship to hypochondriasis. J Psychiatr Res 24:323–324
Bridou M, Aguerre C (2013) Validity of the French form of the Somatosensory Amplification Scale in a non-clinical sample. Health Psychol Res 2:1(1):e11. https://doi.org/10.4081/hpr.2013.e11.
Köteles F, Doering BK (2015) The many faces of somatosensory amplification: the relative contribution of body awareness, symptom labeling, and anxiety. J health Psychol 21:2903–2911
French DJ, Noël M, Vigneau F (2005) L’echelle de dramatization face à la douleur PCS-CF: adaptation canadienne en langue française de l’echelle “Pain Catastrohpizing Scale”. Can J Behav Sci 37:181–192
McHorney CA, Ware JE, Raczek AE (1993) The MOS 36-item Short Form Health Survey (SF-36): II. Psychometric and clinical test of validity in measuring physical and mental health constructs. Med Care 31:247–263
Leplège A, Ecosse E, Verdier A, Perneger TV (1998) The French SF-36 Health Survey: translation, cultural adaptation and preliminary psychometric evaluation. J Clin Epidemiol 51:1013–1023
Bulbena A, Pailhez G, Bulbena-Cabré A, Mallorqui-Bagué N, Baeza-Velasco C (2015) Joint hypermobility, anxiety and psychosomatics: two and a half decades of progress toward a bew phenotype. Adv Psychosom Med 34:143–157
Murray B, Yashar BM, Uhlmann WR, Clauw DJ, Petty EM (2013) Ehlers–Danlos syndrome, hypermobility type: a characterization of the patients’ lived experience. Am J Med Genet 161A:2981–2988
Berglund B, Petterson C, Pigg M, Kristiansson (2015) Self-reported quality of life, anxiety and depression in individuals with Ehlers–Danlos syndrome (EDS): a questionnaire study. BMC Musculoskeletal Disord 16:89
Woo AKM (2010) Depression and anxiety in pain. Rev Pain 4:8–12
Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K (2008) Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med 70:890–897
Gazit Y, Nahir AM, Grahame R, Jacob G (2003) Dysautonomia in the joint hypermobility syndrome. Am J Med 115:33–40
Krahe AM, Adams RD, Nicholson LL (2017) Features that exacerbates fatigue severity in joint hypermobility syndrome/Ehlers–Danlos syndrome hypermobility type. Disabil Rehabil 9:1–8
Roger RR, Bonnet MH, Kramer M (1983) The relationship of sleep and anxiety in anxious subjects. Biol Psychol 16:119–126
Kendall PC, Ingram RE (1987) The future for cognitive assessment of anxiety: Let’s get specific. In: Michelson L, Ascher LM (eds) Anxiety and stress disorders: Cognitive-behavioral assessment and treatment. Guilford Press, New York
House A, Stark D (2002) Anxiety in medical patients. BMJ 325:207–209
Turner JA, Aaron LA (2001) Pain-related catastrophizing: what it is? Clin J Pain 17:65–71
De Baets S, Vanhalst M, Coussens M, Rombaut L, Malfait F, Van Hove G, Calders P, Vanderstraeten G, van de Velde D (2017) The influence of Ehlers–Danlos syndrome-hypermobility type, on motherhood: a phenomenological, hermeneutical study. Res Dev Disabil 60:135–144
Pino-Ramirez G (2016) Empowerment, life experience and Ehlers–Danlos syndrome patient associations. Cuad Neuropsicol 10:78–94
Rubin KH, Burgess KB (2001) Social withdrawal and anxiety. In: Vasey MW, Dadds MR (eds) The developmental psychopathogy of anxiety. Oxford University Press, New York
Edwards RR, Bingham CO III, Bathon J, Haythirnthwaite JA (2006) Catastrophizing and pain in Arthritis, Fibromyalgia, and other rheumatic diseases. Arthritis Rheum 55:325–332
Baumeister H, Balke K, Härter M (2005) Psychiatric and somatic comorbidities are negatively associated with quality of life in physically ill patients. J Clin Epidemiol 58:1090–1100
Acknowledgements
The authors wish to thank all the participants of this study, Luis Cunha, Anne Gompel and Sabine Pommeret for their time and valuable collaboration.
Funding
This research did not receive any Specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
C. Baeza-Velasco designed and directed the study, collected the data, performed the statistical analysis and wrote the manuscript. C. Bourdon, L. Montalescot and C. de Cazotte collected the data, and agreed with the last version of the manuscript. G. Pailhez and A. Bulbena were involved in the interpretation of the data and agreed with the last version of the manuscript. C. Hamonet saw all the patients for physical assessment and diagnosis, and agreed with the last version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Rights and permissions
About this article
Cite this article
Baeza-Velasco, C., Bourdon, C., Montalescot, L. et al. Low- and high-anxious hypermobile Ehlers–Danlos syndrome patients: comparison of psychosocial and health variables. Rheumatol Int 38, 871–878 (2018). https://doi.org/10.1007/s00296-018-4003-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-4003-7