Abstract
Numerous systematic reviews investigating the effects of mesenchymal stem cells (MSCs) in treating knee osteoarthritis (OA) have been published with controversial conclusion. The purpose of the overview was (1) to perform an overview of systematic reviews investigating MSCs for knee OA and (2) to synthesize evidence qualitatively to assess confidence in the review findings. A systematic search of systematic reviews published through Aug 2017 was conducted using the MEDLINE, EMBASE and Cochrane Library. The methodological quality and risk of bias of included systematic reviews was assessed by AMSTAR instrument and ROBIS tool, respectively. Best evidence choice procedure was conducted according to the Jadad decision algorithm. The systematic reviews with high methodological quality and low risk of bias were selected ultimately for further evidence synthesis based on the CERQual tool. Four systematic reviews were eligible for inclusion. According to the ROBIS tool, there was one systematic review with low risk of bias and three with high risk of bias. Thus, only one systematic review conducted by Pas et al. with highest AMSTAR score and low risk of bias was selected. For all outcomes after evidence synthesis via the CERQual tool, confidence for decision making was either low (self-reported measurement and MRI/histological outcome) or moderate (adverse events). The present study demonstrates that moderate confidence could be placed in safety of MSCs therapy for knee OA, but with low confidence in efficacy outcomes due to limitations of the current evidence. Further high-quality studies with high internal and external validity are still required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a common chronic disease, knee osteoarthritis (OA) is with rising prevalence [1,2,3]. OA is regarded to be the fourth leading cause of disability worldwide [4]. Knee OA affects the whole body and self, ultimately affecting the quality of life on many levels [5]. The socioeconomic burden in terms of medical care cost for both government and individuals was also increased with knee OA. Currently, the ultimate goal of knee OA treatment is to relieve symptoms and to improve joint function and quality of life. Although total knee replacement may serve as an effective alternative for patients with severe knee OA, the risk of surgical complications cannot be eliminated completely. Numerous conservative treatment options are currently available, including analgesic medication, physical therapy, unloaded bracing, and intra-articular injections etc, aiming to relieve the knee joint pain and delay the surgical intervention [6].
As an innovative therapy, researchers believe that cell therapy is the next logical generation in the progression of surgical intervention [7]. As one type of cells, mesenchymal stem cells (MSCs) are extraordinarily popular due to their ease of harvesting, safety and potential to differentiate into cartilage tissue [8,9,10]. Furthermore, paracrine mechanism and immunoregulation effects of growth factor and cytokines released by MSCs are beneficial to treating knee OA [11,12,13]. Recently, the number of clinical studies within this field of MSCs research is fast growing, while more clinics are offering MSCs treatments with lax medical regulations [14]. Although several case-series and clinical controlled trials have shown favorable results of MSCs injections in knee OA [15,16,17,18,19], variation in evidence level of these primary studies cannot give us confidence to regular care practices with questions about MSCs treatment. Moreover, Osborne et al [20] reported that MSCs therapy might be hallmarks of ‘quack’ medicine: desperate patients, pseudoscience and large amounts of money being charged for unproven therapies. Thus, we do not have confidence in recommendation based on the current evidence and debates.
Recently, several systematic reviews or meta-analyses concerning this topic have been published [21,22,23,24]. Yubo et al [21] reported that MSCs intervention has great potential with relative safety as an efficacious cell therapy for patients with knee OA. Xia et al [23] reported beneficial effects of MSCs therapy in knee OA, although insufficient evidence remains available to recommend its use. However, Pas et al [24] did not recommend MSCs therapy for knee OA for the absence of high-level evidence. Filardo et al [25] demonstrated that the effectiveness of MSCs in treating articular defects and OA was in conclusive, because we could not distinguish the observed effects of MSCs themselves from placebo effects and related factors. We cannot conclude the MSCs effectiveness exactly according to results of these published primary studies and systematic reviews. These controversial results could not inform decisions on health and social interventions, and definite conclusions about using MSCs treating knee OA cannot yet be made with absolute certainty. Therefore, it is required to assess how much confidence to be paid in findings from systematic reviews evaluating the effectiveness of MSCs therapy in knee OA.
The purpose of this study was (1) to perform an overview of overlapping systematic reviews that assessed the efficacy and safety of MSCs injections for knee OA; (2) to evaluate the methodological quality and risk of bias of relevant systematic reviews; (3) to synthesize the current evidence qualitatively to determine how much confidence to place when using MSCs for knee OA.
Materials and methods
The present study was conducted according to the guideline of Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) checklist (Supplemental Table 1) [26]. Based on the EPC guidance, existing systematic reviews have been integrated into a new review [27].
Search strategy
All systematic reviews that meet the following inclusions/exclusion criteria were searched in databases (MEDLINE, EMBASE, Scopus and Cochrane library) from database inception to 1 August 2017. The following MeSH words and free texts were used for search: knee, osteoarthritis, arthritis, stem cell, mesenchymal stem cell, MSC, meta-analysis and systematic review (Supplemental Table 2). In addition to electronic literature search, the references of searched studies were also screened to identify other systematic reviews.
Secondary investigation into unpublished literature and abstracts was performed by searching the following conference ACR, OARSI and APLAR.
Inclusion and exclusion criteria
Systematic reviews or meta-analyses evaluating MSCs for knee OA patients that met the following inclusive /exclusive criteria were eligible for inclusion:
-
1.
Type of studies: Meta-analysis or systematic review;
-
2.
Participants: knee OA patients;
-
3.
Interventions: The included systematic reviews had compared all types of MSCs from any origin injection with any other interventions in treating knee OA. MSCs injection combined with another intervention were included if this combined intervention was compare with an intervention without MSCs injection.
-
4.
Compare: Placebo, HA and other intervention.
-
5.
Outcomes: The included systematic reviews had to evaluate the effects of MSCs injection on pain, function, quality of life, radiological outcomes, histological analysis or adverse events.
Exclusion criteria included the following items:
-
1.
MSCs used in hand, hip, ankle and other joints OA;
-
2.
MSCs seeded into scaffold implantation for cartilage defects;
-
3.
Basic science review and systematic reviews based on in vitro, in vivo preclinical studies;
-
4.
Abstract without precise outcomes, commentary, methodology study, overview, narrative review, and clinical practice guidelines.
Studies selection and date extraction
Two reviewers independently screened the titles and abstracts of systematic reviews of choice for the eligibility criteria. They were uninformed of the journals/authors’ information and affiliations. Subsequently, systematic reviews which were regarded as potentially relevant by any of the reviewers were obtained in full text for further review by the same reviewers independently. Any disagreement was resolved by discussion for reaching a consensus.
The data from each included systematic reviews were extracted by two authors independently using a predefined data extraction form. The following data were extracted: title, year, journal, authors, study design, total number of primary studies, the pooled outcomes, methodology, type of MSCs and MSCs identification methods. Any disagreement concerning the extracted data was resolved by discussion.
Methodological quality assessment for systematic reviews
The methodological quality of included systematic reviews was evaluated by using Assessment of Multiple Systematic Reviews (AMSTAR) tool [28]. The assessment process was conducted by two authors independently. Any controversial viewpoint was resolved by discussion. The AMSTAR was a measurement tool with eleven items for evaluating methodological quality of systematic reviews [29].
Heterogeneity among systematic reviews
Heterogeneity of each outcome was summarized for each included systematic reviews when with pooling results. We also assessed the following two aspects: (1) whether sensitivity analysis was performed in systematic reviews and (2) whether the included reviews assessed potential sources of heterogeneity among primary studies. I 2 value was utilized to demonstrate the degree of heterogeneity quantitatively among primary studies.
Choice of best evidence
Best evidence choice procedure was conducted based on to the Jadad decision algorithm [30], which was adopted to help clinical decision maker select reliable ones among all the systematical reviews. The assessment criteria of Jadad decision algorithm include: clinical question development, study selection and inclusion process, data extraction, study quality assessment, feasibility to combine studies, and statistics for data synthesis [30]. This procedure was conducted by two independent authors. The consensus was reached through agreeing on which of the included systematic reviews can provide best evidence according to the current information.
Risk of bias assessment for systematic reviews
The risk of bias of included systematic reviews was assessed by two authors independently with the help of ROBIS tool [31]. Disagreements were resolved by discussion. According to the ROBIS tool, risk of bias was evaluated by assessing the following four domains: study eligibility criteria, identification and selection of studies, data collection and study appraisal, and synthesis and findings. These four domains covered the main review processes.
Each domain was evaluated for information that adopted to support the judgments, signaling questions, and judgement of concern about risk of bias. The answers for the signaling questions included “Yes”, “Probably Yes”, “No”, “Probably No” and “No Information”. The answer only with “Yes” indicates low concerns. Thus, each domain for risk of bias was classified as “Low”, “High”, or “Unclear”. If one domain was categorized as low level of concern, all signaling questions for the domain were Yes or Probably Yes. Concern about bias was elevated if any signaling questions were reported as “No” or “Probably No” [31].
Assessment of credibility in the review findings (CERQual)
Confidence in the Evidence from Reviews of Qualitative research (CERQual) [32] tool was utilized to assess our confidence in relying the outcomes of systematic review. The CERQual tool was developed under the supports of the Cochrane Methods Group, and draws on the principles used by the GRADE approach to quantitative literature systematic reviews [33]. CERQual tool assesses confidence in evidence based on the following four key components contributing to a review finding: (1) the methodological limitations; (2) the relevance; (3) the coherence; and (4) the adequacy of the data. Assessment of each of the four components allows for judgment about the overall confidence for each systematic review finding. Confidence ratings commence at ‘high confidence’ and are rated down by one or more levels if there are concerns regarding individual CERQual components [32,33,34]. The confident judgments were achieved through discussion between two reviewers.
Results
Literature search
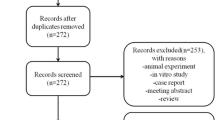
A total of 47 titles and abstracts were preliminarily reviewed, and four published systematic reviews [21,22,23,24] ultimately met the eligibility criteria (Fig. 1). After title and abstract screening, one was excluded because they aimed to review animal studies of MSCs in treating OA. Seven studies were omitted because of MSCs used for rheumatoid arthritis. Two was excluded due to investigating MSCs in patients with juvenile idiopathic arthritis. Twenty-three narrative reviews and mini-review without methodological evaluation were excluded after full-text reading. One literature was not included because it conducted systematic review involving mechanism of MSCs in treating OA. One was excluded due to primary studies involving other joints.
Characteristics of systematic reviews
The characteristics of systematic reviews have been presented in Table 1. These reviews were published from 2015 [23] to 2017 [21, 24]. The numbers of original studies included in systematic reviews varied from six in that study published in 2017 [24] to 18 that published in 2016 [22] (Table 2). One systematic review conducted qualitative synthesis without pooled data [24]. Only one systematic review reported whether or not phenotypic characterization was performed in the primary trials [24].
Search methodology
The comprehensive search source which was utilized by individual systematic reviews is presented in Table 3. Medline, Embase and Cochrane database are the most frequently used searching databases of the included systematic reviews.
Methodological quality of systematic reviews
Table 4 presented the methodological features of individual systematic reviews. Only one included systematic review [21] reported that only RCTs were included, while others [22,23,24] included randomized controlled trials (RCTs) and non-RCTs. The degree of evidence for each systematic review was Level II. REVMAN or STATA software was used in systematic reviews with pooling data [21,22,23]. Both sensitivity and subgroup analysis were conducted in two included studies [22, 23]. None of systematic review assessed quality of evidence body in their study.
The total AMSTAR score with each item of individual systematic reviews are presented in Table 5. The average AMSTAR score of individual literatures was 8.25, ranging from 7 [21, 22] to 11 [24]. Two of the included systematic reviews [21, 24] declared no conflict of interest in making investigation. The systematic review conducted by Pas et al [24] was regarded as the highest quality study.
Heterogeneity among primary studies
The heterogeneity of each outcome with pooled quantitatively in each systematic review have been presented in Table 6. The I2 parameter was shown to present the heterogeneity among primary clinical trials. The outcomes of almost all the pooled results had moderate or high heterogeneity.
Jadad decision algorithm
All the pooled quantitative outcomes reported in systematic reviews are shown in Fig. 2. Based on the procedure of jadad decision algorithm, the eligible systematic review was selected on account of the methodological quality of systematic review (Fig. 3). Therefore, only one study conducted by Pas and his colleagues [24] with highest AMSTAR score was selected ultimately.
Risk of bias of systematic reviews
The risk of bias of included reviews by ROBIS tool has been presented in Table 7, so do the assessment results of each item in phase 2 of ROBIS tool. The 3rd phase demonstrated whether the systematic reviews as a whole was at risk of bias. There was only one systematic review [24] with low risk of bias, while other three with high risk of bias. Judgments regarding each ROBIS item were presented as percentages across all the included SRs in Fig. 4. Based on the AMSTAR instrument and ROBIS tool, the above mentioned systematic review performed by Pas et al [24] with higher methodological quality and lower risk of bias was regarded to provide best evidence.
Risk of bias of the included systematic reviews with ROBIS tool. The ROBIS tool incorporates the assessment of study eligibility criteria, identification and selection of studies, data collection and study appraisal, and synthesis and findings. The overall risk of bias is determined based on the above four domains. Each risk of bias item is presented as the percentage across all the systematic reviews, which indicates the proportion of different levels of risk of bias for each item
Assessment of credibility in the review findings (CERQual)
Confidence ratings for the three main outcomes (self-reported measure, MRI/histological examination and adverse events) in the selected systematic review [24], assessed using the CERQual tool, are shown in Table 8. For all outcomes, confidence was either low (outcome: self-reported measure and MRI/histological examination) or moderate (outcome: adverse events). The general reasons for downgrading of ratings were the problems with internal validity of primary studies, the limited generalisability and transferability of some data, and the small number of articles and small sample sizes within available studies.
Discussion
Meta-analysis and systematic review are generally considered to be the best way to obtain evidence for healthcare decision making, thus can be used to resolve wide range of clinical problems. Decision-makers in medical institutions look forward to get consistent, stable and unbiased recommendations based on systematic reviews [35]. However, it is not uncommon to have several systematic reviews under the same topic published evaluating the same interventions, yet without consistent conclusions. This also occurred in the study of MSCs injection in treating knee OA. Although several systematic reviews or primary studies had supported MSCs injections, current evidence was unable to recommend for or against MSCs for knee OA.
The methodological quality and risk of bias among individual systematic reviews may account for the discrepancy in outcomes of systematic reviews. The following types of biases can be induced when systematic reviews are applied at all steps of review process, including study eligibility criteria, study selection, data collection and evidence synthesis. Thus, the decision-makers should put methodological quality and risk of bias in systematic reviews into consideration when pooled conclusions are used [28]. [31]. Therefore, the AMSTAR tool [28] was used in the present study to evaluate the methodological quality of systematic reviews about MSCs in treating knee OA, and Jadad decision algorithm [30] was utilized to select the best evidence. Furthermore, in purpose of collecting the systematic reviews and assessing the risk of bias, a newly developed tool, ROBIS (http://www.robis-tool.info) was used [31]. Ultimately, one systematic review [24] with lower risk of bias and offered the best evidence were selected in the present study. Although the systematic review with high methodological quality and with lower risk of bias was selected and determined, the quality for evidence body for each outcome was unknown. We also do not have total confidence to place in outcomes from systematic review of the effectiveness of MSCs therapy in knee OA. Thus, in the present study, we used CERQual tool [32] to assess our confidence in the selected systematic review [24] findings across primary studies.
As the first domain of CERQual tool, the risk of bias of primary studies, namely the internal validity, is known to influence results in important ways. The high risk of bias found in the reviewed studies must be taken into account when interpreting the synthesized findings. It should be noted that though all trials proposed to have used MSCs, within the identified trials, phenotypic characterization as described here was only performed in four trials. Two primary studies did not perform any specific immune-phenotypic characterization, making their claim of having used MSCs questionable. Although it has been suggested that MSCs should meet the criteria put forward by the International Society for Cellular Therapy, not all MSCs fall under these definitions such as a subpopulation of BMSCs and adipose-derived MSCs that are nonadherent to plastic but still exhibit all the other properties of MSCs [36]. Furthermore, none but one [18] primary studies reported patients blinding. The subject outcomes, including visual analogue scale (VAS), Lysholm score, Tegner activity scale, International Knee Documentation Committee (IKDC) clinical scores and Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index, can be influenced obviously by preference of patients and researchers. The pooled outcomes of forementioned self-reported score will be overestimated away from the real-world consequence. Unfortunately, the Delayed Gadolinium-Enhanced MRI of Cartilage (dGEMRIC) [37] that could assess the glycosaminoglycan content from the hyaline cartilage did not be used to evaluate the structural outcomes in primary studies. We, therefore, do not have sufficient confidence in getting good clinical results when treating knee OA by MSCs.
Although three primary studies [17,18,19] included in the selected systematic review reported assessors blinding in study design, no information was provided on how the assessors were blinded. Only one trial [18] reported investigators blinded to the patients’ data. Furthermore, high risk of selection bias was introduced to synthesized results due to the use of quasi-randomization procedures [15, 16, 19] or no allocation concealment [15, 16]. Therefore, detection bias can be introduced to cartilage evaluation using MRI or histological assay. Although assessors blinding reported in three trials, a high risk of selection bias was present before evaluating cartilage repair. Thus, these limitations of methodological quality in primary studies will lower our confidence when interpreting the results about cartilage healing.
For the aspect of generalisability to other clinical contexts, we should note that all but one trial [18] used a surgical cointervention. Performance bias could be introduced by surgical cointerventions introduces, as the personnel with varied surgical technique performing the surgical interventions could not be blinded. Four studies [15,16,17, 19] used PRP or HA as cointervention when evaluating the effects of MSC in symptoms change and cartilage healing. We do have confidence to not exclude the positive effects of PRP or HA in treating OA. Furthermore, the heterogeneity of varied inclusion criteria among the trials could limit the generalisability from current evidence to other clinical contexts. In the trials conducted by Koh et al. [16] and Wong et al [19], the patients with isolated medial knee compartment OA were recruited. One included study [17] included patients aged 18–50 years that was younger than that in other studies. Other heterogeneity may be caused by the preexisting conditions of patients, severity of OA, type of MSCs, dose of MSCs injection, follow-up duration and rehabilitation procedures after administration. These above mentioned sources of heterogeneity will also lower our confidence in transforming to other clinical contexts from current synthesized evidence.
Concerns about their safety remained among clinicians. Two systematic reviews [21, 22] reported no significant difference between MSCs treated and untreated groups by including RCTs and/or non-RCTs. The four studies [15, 17,18,19], included in the present selected systematic review, also reported no serious adverse events, although the follow-up was relatively short. Although the rate of adverse events could not be influenced largely by methodological limitations or generalisability, the relatively few studies with small participant numbers and short-term follow-up duration may low our confidence in drawing conclusion. Furthermore, we should bear in mind that their safety could be impacted by the dose, graft type (allogeneic, xenogeneic or autologous MSCs) and source of MSCs when application. The various procedures used in detaching, processing, storage and delivery of the MSCs could also influence their characteristics and reliability. Thus, we have moderate confidence to confirm that MSCs are relatively safe based on short-term adverse event. This conclusion is partly consistent with that drawn by Peeters et al. that application of cultured stem cells in joints appears to be safe [10]. Nonetheless, long-term adverse events are still poorly researched in further studies.
The strength of the present study is the combined utilization of ROBIS tool, AMSTAR instrument and Jadad decision algorithm and CERQual tool for evaluating the risk of bias and methodological quality of the systematic reviews and confidence in the systematic review findings across primary studies, simultaneously. The ultimate purpose was to help decision-makers select the best evidence with low risk of bias in terms of MSCs injection for knee OA from discordant systematic reviews and to give them confidence to place in specific review findings to help them judge how much emphasis they should give to these findings in their further decisions. Hence, based on the existing optimal evidence, we cannot take recommendations that intra-articular MSCs injection might be efficacious in treating knee OA.
The following items are the limitations of the present study: (1) English language systematic reviews were included. Non-english language literatures could have been omitted, leading to language bias. (2) The methodological quality of the primary studies may influence the results of included systematic reviews radically. Although we assessed risk of bias and quality of the included systematic reviews, the limitations of primary studies, especially conflict of interests, should be considered when the results are interpreted. (3) The primary studies that included in other systematic reviews which were not regarded as the best evidence with low risk of bias could be considered when ratings confidence for the outcomes.
In the present overview of systematic reviews investigating efficacy and safety of MSCs for knee OA, the best available evidence with low risk of bias suggested that there was no sufficient high-level evidence to recommend MSCs therapy for knee OA. Furthermore, we have moderate confidence to place in safety of MSCs therapy for knee OA but with low confidence in efficacy. High-quality clinical studies with rigorous standardized methodology are still required.
References
Sacks JJ, Luo YH, Helmick CG (2010) Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res (Hoboken) 62(4):460–464
Lawrence RC, Felson DT, Helmick CG et al (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 58(1):26–35
Helmick CG, Felson DT, Lawrence RC et al (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 58(1):15–25
Kane P, Frederick R, Tucker B et al (2013) Surgical restoration/repair of articular cartilage injuries in athletes. Phys Sportsmed 41:75–86
Nyvang J, Hedström M, Gleissman SA (2016) It’s not just a knee, but a whole life: a qualitative descriptive study on patients’ experiences of living with knee osteoarthritis and their expectations for knee arthroplasty. Int J Qual Stud Health Well-being 11:30193
Crawford DC, Miller LE, Block JE (2013) Conservative management of symptomatic knee osteoarthritis: a flawed strategy. Orthop Rev (Pavia) 5(1):e2
de Girolamo L, Kon E, Filardo G et al (2016) Regenerative approaches for the treatment of early OA. Knee Surg Sports Traumatol Arthrosc 24:1826–1835
Carstairs A, Genever P (2014) Stem cell treatment for musculoskeletal disease. Curr Opin Pharmacol 16:1–6
Freitag J, Bates D, Boyd R et al (2016) Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy—a review. BMC Musculoskelet Disord 17:230
Peeters CM, Leijs MJ, Reijman M et al (2013) Safety of intra-articular cell-therapy with culture-expanded stem cells in humans: a systematic literature review. Osteoarthritis Cartilage 21:1465–1473
Wei CC, Lin AB, Hung SC (2014) Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: bench, bedside, and industry. Cell Transplant 23:505–512
Pers YM, Ruiz M, Noël D et al (2015) Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage 23:2027–2035
Castro-Manrreza ME, Montesinos JJ (2015) Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res 2015:394917
Turner L, Knoepfler P (2016) Selling Stem Cells in the USA: assessing the Direct-to-Consumer Industry. Cell Stem Cell 19:154–157
Koh YG, Choi YJ (2012) Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 19:902–907
Koh YG, Kwon OR, Kim YS et al (2014) Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy 30:1453–1460
Saw KY, Anz A, Siew-Yoke JC et al (2013) Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy 29:684–694
Vega A, Martín-Ferrero MA, Del CF et al (2015) Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 99:1681–1690
Wong KL, Lee KB, Tai BC et al (2013) Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy 29:2020–2028
Osborne H, Castricum A (2016) Change to Australasian College of Sport and Exercise Physicians-position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med 50:1229
Yubo M, Yanyan L, Li L et al (2017) Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: a meta-analysis. PLoS One 12:e0175449
Cui GH, Wang YY, Li CJ et al (2016) Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: a meta-analysis. Exp Ther Med 12:3390–3400
Xia P, Wang X, Lin Q et al (2015) Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop 39:2363–2372
Pas HI, Winters M, Haisma HJ et al (2017) Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med 51:1125–1133
Filardo G, Perdisa F, Roffi A, Marcacci M, Kon E (2016) Stem cells in articular cartilage regeneration. J Orthop Surg Res 11:42
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Robinson KA, Chou R, Berkman ND et al (2016) Twelve recommendations for integrating existing systematic reviews into new reviews: EPC guidance. J Clin Epidemiol 70:38–44
Shea BJ, Grimshaw JM, Wells GA et al (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7:10
Shea BJ, Bouter LM, Peterson J et al (2007) External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS One 2(12):e1350
Jadad AR, Cook DJ, Browman GP (1997) A guide to interpreting discordant systematic reviews. CMAJ 156(10):1411–1416
Whiting P, Savović J, Higgins JP et al (2016) ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 69:225–234
Lewin S, Glenton C, Munthe-Kaas H et al (2015) Using qualitative evidence in decision making for health and social interventions: an approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual). PLoS Med 12:e1001895
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394
Carstensen K, Lou S, Groth JL et al (2017) Psychiatric service users’ experiences of emergency departments: a CERQual review of qualitative studies. Nord J Psychiatry 71:315–323
Young D (2005) Policymakers, experts review evidence-based medicine. Am J Health Syst Pharm 62(4):342–343
Kristjánsson B, Honsawek S (2014) Current perspectives in mesenchymal stem cell therapies for osteoarthritis. Stem Cells Int 2014:194318
Bekkers JE, Bartels LW, Benink RJ, Tsuchida AI, Vincken KL, Dhert WJ et al (2013) Delayed gadolinium enhanced MRI of cartilage (dGEMRIC) can be effectively applied for longitudinal cohort evaluation of articular cartilage regeneration. Osteoarthritis Cartil 21:943–949
Tan YH, Jiang MM, Yu HY et al (2013) Therapeutic effect of arthroscopy combined with autologous bone marrow stem cell grafting on knee osteoarthritis. J Trad 25(10):35–38
Nejadnik H, Hui JH, Feng CEP et al (2010) Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med 38(6):1110–1116
Vangsness CT, Farr J, Boyd J et al (2014) Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am 96(2):90–98
Akgun I, Unlu MC, Erdal OA et al (2015) Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg 135(2):251–263
Liang HS, Huang K, Lin L et al (2015) Arthroscopic microfracture surgery combined with autologous bone marrow mesenchymal stem cells transplant in the treatment of knee cartilage defect. Chin J Mod Drug App 9(9):1–3
Lv XX, Huang C, Yin Z et al (2015) Effectiveness of autologous bone marrow mesenchymal stem cell transplant for knee osteoarthritis. Chin J Cell Stem Cell 5(2):28–32
Varma HS, Dadarya B, Vidyarthi A (2010) The new avenues in the management of osteo-arthritis of knee--stem cells. J Indian Med Assoc 108(9):583–585
Orozco L, Munar A, Soler R et al (2014) Treatment of knee osteoarthritis with autologous mesenchymal stem cells: two-year follow-up results. Transplantation 97(11):e66–e68
Gan FY, Tang C, Guo DB et al (2014) The treatment of mesenchymal stem cell transplantation for knee osteoarthritis: a clinical observation study. Modern Diagn Treat 1(15):3512–3513
Davatchi F, Sadeghi AB, Mohyeddin M et al (2016) Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis 19:219–225
Emadedin M, Aghdami N, Taghiyar L et al (2012) Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med 15(7):422–428
Koh YG, Jo SB, Kwon OR et al (2013) Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 29(4):748–755
Buda R, Vannini F, Cavallo M et al (2010) Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am 92 Suppl 2:2–11
Gobbi A, Karnatzikos G, Scotti C et al (2011) One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage 2(3):286–299
Turajane T, Chaweewannakorn U, Larbpaiboonpong V et al (2013) Combination of intra-articular autologous activated peripheral blood stem cells with growth factor addition/ preservation and hyaluronic acid in conjunction with arthroscopic microdrilling mesenchymal cell stimulation Improves quality of life and regenerates articular cartilage in early osteoarthritic knee disease. J Med Assoc Thai 96(5):580–588
Orozco L, Munar A, Soler R et al (2013) Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation 95(12):1535–1541
Kim JD, Lee GW, Jung GH et al (2014) Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol 24(8):1505–1511
Koh YG, Choi YJ, Kwon SK et al (2015) Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 23(5):1308–1316
Gobbi A, Karnatzikos G, Sankineani SR (2014) One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med 42(3):648–657
Jo CH, Lee YG, Shin WH et al (2014) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 32(5):1254–1266
Kim YS, Choi YJ, Suh DS et al (2015) Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold. Am J Sports Med 43(1):176–185
Acknowledgements
The authors thank Professor Joey Kwong from Department of Health Policy, National Center for Child Health and Development, Tokyo, Japan for her great advice on methodology in interpreting the evidence. We also thank Professor Wei Liu from Department of Biomedical Engineering, School of Medicine, Tsinghua University for his sharing knowledge about basic research in mesenchymal stem cells.
Author information
Authors and Affiliations
Contributions
Project conceptualization: D Xing and JH Lin. Study design: D Xing, Q Wang and JH Lin. Data collection/validation: D Xing, Q Wang, ZY Yang and JH Lin. Data analysis: D Xing, Q Wang and YF Hou. Result interpretation: D Xing, Q Wang, Q Liu and YF Hou. Reporting & editing: D Xing, YF Hou and W Zhang. Final approval of the version to be submitted: D Xing, Q Wang and JH Lin. Project guarantor: YL Chen and JH Lin.
Corresponding author
Ethics declarations
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81501919) and Peking University People's Hospital Scientific Research Development Funds (No. RDH2017-05).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xing, D., Wang, Q., Yang, Z. et al. Mesenchymal stem cells injections for knee osteoarthritis: a systematic overview. Rheumatol Int 38, 1399–1411 (2018). https://doi.org/10.1007/s00296-017-3906-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3906-z