Abstract
Medication adherence is believed to be a major contributor to treatment outcomes yet studies quantifying this relationship as rare in rheumatoid arthritis (RA). To determine the association of adherence to DMARD therapy with treatment outcomes among new and existing DMARD users over 2 years. Relevant clinical parameters were obtained from a longitudinal cohort of RA patients, most of who were treated with combination therapy. Patients were classified as adherent if the proportion of days covered for each DMARD was ≥80%. Outcome measures were the change in the disease activity score in 28 joints (DAS28), simplified disease activity index (SDAI), modified health assessment questionnaires (mHAQ) and proportion of patients who achieved response criteria. An inverse propensity-score weighting method was used to estimate the association of adherence with each outcome. Of 194 patients invited, a total of 111 patients (new = 45 and existing = 66 DMARD users) met study eligibility. DMARD-naive patients demonstrated relatively higher rates of adherence compared to existing users. After controlling for confounding variables, adherence was significantly associated with reduction in DAS28 (β = −1.5, 95% CI of β = − 2.17 to −0.83, p < 0.0001), SDAI (β = −9.44, 95% CI of β = −15.53 to −3.35, p = 0.002) and mHAQ (β = −0.269, 95% CI of β, −0.462 to −0.077, p = 0.017) over 2 years among new patients and adherent patients were more likely to achieve most response criteria compared to non-adherent patients. Such associations were not replicated among existing DMARD users. Adherence to combination DMARD therapy was associated with improvements in disease activity and functional outcomes in the first 2 years of therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug therapy is the cornerstone of disease management and it is likely that good adherence is required for the full benefit of therapy to be realised [1]. However, inadequate medication adherence is common in clinical practice. In rheumatoid arthritis (RA), rates of adherence to disease-modifying antirheumatic drugs (DMARDs) are highly variable, ranging from 30 to 107% for conventional DMARDs and 41 to 90% for biological agents [2–4].
The effect of medication adherence on treatment outcomes has been studied extensively in a number of chronic illnesses [5]. In RA, the literature regarding the relationship between adherence to DMARD and treatment endpoints is very limited [6–8]. In addition, the findings of prior studies may be limited in their applicability to the contemporary treat-to-target (T2T) strategy as they were focused on exploring the effect of single medication adherence, ignoring the dynamic nature of the T2T strategy. T2T strategy is a goal-driven process, whereby treatment is intensified until a pre-defined target disease activity is achieved [9]. In the setting of the T2T strategy, wherein use of multiple medications, treatment intensifications and switching drugs is very common, information regarding adherence to the entire treatment package, not just a single drug, is likely to be more useful.
We have described previously the physician adherence to a T2T strategy and found a strong association between adherence to T2T and long-term treatment outcomes [10–12]. Likewise, patients’ adherence to medications may have a role in determining the outcome of treatment following a T2T strategy. Furthermore, the relationship between adherence and treatment outcomes may be dependent on whether patients are new to treatment or established users. Therefore, in a cohort of DMARD-naïve and experienced patients receiving combination DMARDs according to an intensive T2T strategy, we aimed to determine the rate of medication adherence and its association with various treatment endpoints over 2 years.
Patient and methods
Study cohort and data source
The study population was drawn from a longitudinal observational cohort of RA patients at the Royal Adelaide Hospital Early Arthritis Clinic, a tertiary referral hospital in Adelaide, Australia. All consecutive patients attending the clinic during the study period (between May 2014 and June 2015) were invited to participate. Patients gave written informed consent for Pharmaceutical Benefits Scheme claims data to be obtained from the Australian Government Department of Human Services to determine medication adherence. The corresponding treatment outcomes and patient data were extracted from the custom-built Early Arthritis Clinic database at study hospital. Patients were excluded if they did not give consent; had incomplete or unsigned forms or their clinical and treatment data were unavailable. Ethics approval was obtained from the University of South Australia (protocol no.0000031928) and the Royal Adelaide Hospital (protocol no.140303) for the longitudinal observational cohort and for the linkage of prescribing data.
Treatment strategy
The characteristics of the study cohort, treatment approaches, and other relevant clinic logistic have been described elsewhere [10, 12, 13]. Briefly, at baseline, patients underwent a full clinical examination and a variety of laboratory investigations were performed, including rheumatoid factor (RF) and anti-cyclic citrullinated peptide (ACPA) titers, erythrocyte sedimentation rate (ESR), C-reactive protein, and shared epitope status. At each visit, patients rated their level of pain, fatigue and disease activity on 100-mm visual analogue scales (VAS) with higher scores representing greater symptoms and completed a questionnaire that assessed physical function (assessed by modified health assessment questionnaires, mHAQ, rated on 0–3 scale, lower score representing better functioning) [14] and helplessness (assessed by the Rheumatology Attitudes Index, scores range from 5 to 25, lower scores indicating lesser degree of helplessness) [15]. Physician global assessment (PhGA) (100 mm VAS, 100 mm = worst rating) was obtained. Disease activity according to the disease activity score in 28 joints (DAS28) based on the ESR (rate 0-9.4 scale, lower scores indicate less severe disease) [16] and simplified disease activity index (SDAI) (rated on a 0–86 scale, lower scores indicates less severe disease) [17] was calculated.
All patients were treated according to a protocolized T2T strategy with an initial therapy comprising methotrexate (MTX) (dose range 10–25 mg/week), hydroxychloroquine (HCQ) (400 mg/d) and sulfasalazine (SSZ) (dose range 500 mg/d–3g/d). Patients were reviewed every 3 weeks in the first 3 months, then every 6 weeks. If a pre-defined goal of therapy representing lower disease activity (DAS28 <2 0.6) was not achieved, further DMARDs including leflunomide, gold by injection, cyclosporine A and/or biological DMARDs were added sequentially. Single doses of corticosteroids (intra-articular if a specific joint was troublesome or intra-muscular if symptoms were more generalized) were used if needed to reduce disease activity, while oral corticosteroids and non-steroidal anti-inflammatory drugs were actively discouraged. Visit frequency was reduced to every 3–6 months once the target level of disease activity was achieved.
Medication adherence
Adherence was assessed according to the proportion of days covered (PDC) method using data from Australian Pharmaceutical Benefits Scheme dispensing claims. PDC is the proportion of days covered by prescription claims during a given measurement period. Accordingly, dispensing claims data between November 2010 and July 2015 were obtained and the data over the first 2 years since the index date were analysed in this study.
PDC approach is preferred over other methods when multiple medications are prescribed concurrently [18]. Given the pattern of medication consumption was different for each patient, we first created a medication usage pattern using a similar approach proposed by Choudhry et al. [18]. As a result, three distinct patterns of use were identified: treatment with only one drug without adding or switching drug (monotherapy), concurrent therapy without addition or switching drug or concurrent therapy with adding or switching drug (Online appendix 1). Then, for each patient, time arrays were created for each prescription and the number of days covered by the prescription fills was determined.
Next, medication adherence was measured as outlined below. Continuous measures of adherence were calculated using two approaches: (1) Adherence with at least one DMARD (PDC-1): the rate of adherence was the proportion of days in the follow-up period that a patient was in possession of at least one DMARD. (2) PDC-average: for patients taking more than one medication, adherence was calculated for individual DMARDs separately and then the average PDC was determined as described in Online appendix 1. In the case of discontinuation or addition of a new drug, assessment of adherence was limited to the time period during which that medication was intended to be in use. In the case of switching, the original and the new drug were considered interchangeable (i.e. the new drug was considered as the continuation of the prior drug).
Traditionally, consumption of 80% of prescribed medications has been considered an acceptable level of adherence in many chronic diseases. Accordingly, adherence status were created using an 80% cut-off point by three different methods: using PDC-1 (adherent if PDC-1 was ≥80%, non-adherent if PDC-1was <80%), using PDC-average (adherent if PDC-average was ≥80%, non-adherent if PDC-average was <80%) and using PDC of all DMARDs (PDC-all) (adherent if PDC for each DMARD was ≥80% and non-adherent if PDC of any DMARD was <80%).
Outcome measures
The primary endpoints were an absolute change in DAS28, SDAI and mHAQ scores over 2 years from the index date. The secondary endpoints were the proportion of patients who achieved a European League Against Rheumatism (EULAR) moderate (change in DAS28 of >0.6) or good response (change in DAS28 of >1.2) [19], SDAI minor (50% improvement), moderate (70% improvement) and major responses (85% improvement) [1] and minimally clinically important differences (MCID) in mHAQ (improvement of ≥0.22) [20].
Statistical analysis
Descriptive statistics were reported as appropriate. Baseline variables were compared using Chi-squared test for categorical variables and Mann–Whitney test for continuous variables. For analytical convenience and interpretation purposes, we used the categorized version of adherence. We used the PDC-all as it is the most stringent adherence measure. Given that baseline characteristics of adherent patients may differ systematically from non-adherent patients, the effect of adherence cannot be estimated by directly comparing outcomes between the adherent and non-adherent patients. To get an unbiased estimate, it’s important to ensure that the assignment to adherence groups is independent of measured baseline covariates [21]. We used the inverse propensity score weighting (IPSW) method to create a new sample where the distribution of baseline covariates is comparable between adherent and non-adherent patients [22].
This method comprises two steps. The first step involves estimating the propensity scores, which is the probability that a patient would be assigned to a specific adherence group conditional on measured baseline covariates [21]. It is determined using binary logistic regression considering adherence status as an outcome variable and all baseline covariates as independent variables. We used an iterative process whereby the specification of the propensity model was modified until the best balancing score was obtained [22]. Once the accurate propensity score was obtained, the entire study sample was weighted based on the inverse of the propensity score where the weight for the adherent patient was equal to the inverse of the propensity score while the weight for the non-adherent patient was equal to the inverse of 1 minus the propensity scores. As a diagnostic test to confirm the balance of covariates, we used a graphical illustration using box plots and a statistical test for comparing the distribution of baseline covariates before and after applying weighting.
Finally, once the balance of the baseline covariates was achieved, we determined the relationship between adherence and each primary and secondary outcome using inverse propensity score weighted generalized estimating equations (GEE) modelling with robust variance estimator and logit (for the dichotomous outcome) or linear link function (for the continuous outcomes). Follow-up variables, including the pattern of DMARD use and the number of DMARDs prescribed, were also included in the model. A separate analysis was done to determine the effect of adherence amongst new and existing DMARD users. The analysis was performed using Statistical Package for Social Sciences (SPSS) for Windows version 23.0 (SPSS Inc., Chicago, IL, USA).
Result
Patient cohort
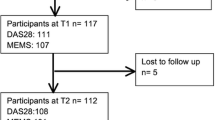
From a total of 194 patients, 111 patients (45 new and 66 existing DMARD users) were included. Fifty-eight patients did not return the informed consent forms, 11 returned incomplete consent forms and 14 had insufficient clinical and treatment data to be included (Online appendix 2). There were no significant differences in patient demographic, clinical characteristics and patient-reported outcomes between included and excluded patients at baseline (Table 1). The included patients were mostly female (64.0%) and RF (64.9%) and ACPA positive (60.4%). Existing DMARD users had median treatment duration of more than 5.5 years before the index date, lower disease activity, and patient-reported outcomes at index date compared to DMARD-naive patients (Table 1).
After the index date, only 5.4% of the patients added or switched drugs while the remaining patients continued taking their initial DMARDs throughout the follow-up period (88.3% with concurrent therapy containing ≥2 drugs and 6.3% with a single drug). The most frequent drug regimen used was MTX/SSZ/HCQ with or without leflunomide (59% of patients) followed by a regimen containing any two of MTX, SSZ, HCQ, LFN or gold injection (11.7% of patients). Only 8.1% of the cohorts used biological agents during the study period.
Medication adherence
The level of adherence depended on the definition used. Adherence according to PDC-1 classified most patients as adherent while adherence status according to PDC-all resulted in a lower estimate of adherence. In the DMARD-naïve patients, the mean adherence ranged from 88.4 to 98.9%, respectively, for PDC-average and PDC-1, and the proportion of patients who were classified as adherent according to PDC-all, PDC-average and PDC-1 were 62.2, 80.0, and 100.0%, respectively. On the other hand, the proportion of adherent patient ranged from 34.8 to 83.3% for existing DMARD users depending on the definition used (Online appendix 3).
Unweighted and inverse propensity score weighted baseline characteristics according to adherence status (PDC-all ≥80 vs. <80%) are shown in Table 2. The mean (SD) weight of propensity score was 2.0 (1.1), and the minimum and maximum weights were 1.10 and 8.12, respectively. Before weighting was applied, several variables were significantly higher in adherent patients. However, after weighting, all baseline variables were balanced between adherent and non-adherent patients (Table 2). Further details on balance diagnostic using boxplot for comparing the distribution of baseline covariates before and after weighting are available from the authors on request.
Medication adherence and treatment outcomes
Patients starting therapy will have a major change in disease activity but patients on established therapy will have little change as their improvement happened earlier. Consistent with this, DMARD-naïve patients demonstrated larger improvements compared to existing users: the mean (SE) changes in DAS28, SDAI and mHAQ were −1.79 (0.22), −13.65(1.81) and −0.336 (0.07), respectively, vs. −0.16 (0.07), −0.94(0.59) and 0.001 (0.02), respectively.
A significantly larger proportion of DMARD-naïve patients fulfilled response criteria compared to experienced patients. Moderate and good EULAR responses were achieved by 75.6 and 60.0% of the naïve patients, respectively, while the minor, moderate and major SDAI responses were fulfilled by 64.4, 57.8 and 44.4% of patients, respectively, while the MCID for mHAQ was achieved by 48.9%. In existing patients, the proportion of patient who achieved response criteria ranged from 34.8 to 83.3% depending on the criteria used (Online appendix 4).
The effect of medication adherence on treatment outcomes after controlling for differences in baseline variables using IPSW and adjustment for follow-up variables are shown in Table 3. The effect of adherence differed depending on treatment status. In DMARD-naive patients, adherence had a positive and significant effect on treatment outcomes. Accordingly, adherence was associated with significant reductions in DAS28 (β = −1.5, p < 0.0001), SDAI (β = −9.44, p = 0.002) and mHAQ (β = −0.269, p = 017). Furthermore, adherent patients were significantly more likely to achieve EULAR moderate (OR = 9.37, p = 0.013), EULAR good (OR = 4.78, p = 0.033), SDAI minor (OR = 6.50, p = 0.024) and SDAI moderate (OR = 5.82, p = 0.031) responses compared to non-adherent patients. In existing patients, however, adherence was neither associated with the absolute change scores nor achievement of response criteria (Table 3).
Discussion
The major finding of this study is that the relationship between adherence and treatment endpoints was dependent on the stage of therapy. After adjusting for confounding factors, adherence was significantly associated with improvements in disease activity and physical functional outcomes among DMARD-naïve patients but not among existing users. Furthermore, new DMARD users demonstrated relatively higher rates of adherence over 2 years compared to existing users. Physician adherence to T2T strategy has also been associated with disease activity and functional outcomes [23]. Although a cause and effect relationship cannot be inferred conclusively from this analysis because of the observational nature of the study, the findings suggest that adherence is a critical factor contributing to the outcome of treatment, especially in the early stages of contemporary RA therapy.
Adherence is believed to be the main contributor to treatment outcome in many clinical settings [5]. In RA, the relationship between medication adherence and treatment outcomes has not been widely explored [6–8]. As in our study, Cannon et al. found that adherence to MTX was positively associated with the change in disease activity measured by the DAS28. However, unlike our findings, adherence was not associated with improvements in physical function and its effect was not dependent on whether patients were new to treatment or existing users [6]. This could be attributed to differences in the treatment strategy used or the way adherence was measured. In other studies, non-adherence was also associated with a poorer disease activity outcome [7, 8].
Possible explanations for the lower rate of medication adherence and lack of association between adherence and treatment outcomes in existing DMARD users include the following. Over the course of treatment, it is likely that response to therapy decreases [24, 25] particularly as disease activity lessens. Consistent with this, the mean duration of therapy before the time period in which adherence was assessed in existing DMARD users was more than 6 years, by which time, drug therapy (and therefore increasing adherence) has a less pronounced effect on outcome. In addition, the disease in the later years may be influenced by non-inflammatory causes of pain such as secondary fibromyalgia and osteoarthritis so these are less responsive to DMARDs. In addition, at later stages of therapy, the duration between clinic visits was longer which might provide greater opportunity for non-adherence to medication. On the contrary, in the first few years of therapy, our patients were reviewed frequently to allow adjustment of therapy in response to disease activity which might have encouraged better adherence. For most patients, much of the improvements in disease activity usually occurred in the early stage of therapy, and therefore the effect of adherence was more likely to be apparent. Finally, for analytical convenience when applying the propensity score method, we used 80% cut-point to define adherence. Among existing DMARD users, a higher adherence threshold than the 80% cut-point might be needed to observe a significant beneficial effect of adherence.
The clinical significance of this study is the strong evidence linking medication adherence to treatment outcome, particularly immediately after diagnosis, which has been recognised as a critical period of RA management. This finding supports the ‘window of opportunity’ hypothesis, wherein effective disease control in the early stages of disease is a good indicator of long-term sustained benefits [26]. Therefore, efforts to increase medication adherence could improve the outcomes of treatment in RA. One such strategy could be more objective measures monitoring medication adherence along with routine assessments of disease activity and patient outcomes at follow-up clinic visits [27]. Future studies exploring suitable options for measuring adherence and its effectiveness when following a T2T strategy are needed.
The strengths of our study include its longitudinal design, the well-characterised cohort with comprehensive assessment of treatment outcomes and potential confounders available for analysis, measurement of adherence to the multiple medications used in the T2T strategy rather than to a single medicine, analysis according to the experience with therapy and the long follow-up period.
This study should be interpreted in the context of the following limitations. First, while dispensing claims data are believed to provide a valid estimate of medication adherence, it does not prove that the prescribed medication was actually consumed. The estimate is based on the assumption that a failure to collect prescriptions indicates the patient will not have taken their medication [18]. Second, one of the challenges in estimating the effect of adherence on outcomes is the difficulty in untangling the effect of adherence and the effect of confounding variables that might affect adherence status or the outcome itself [28]. To account for this, we have applied the propensity score weighting method. Propensity score-based methods have been used extensively for estimating causal effects in observational studies [21, 22]. However, due to lack of randomisation, this method still does not confirm a definitive cause-effect relationship. Third, our measurement of adherence and treatment outcome were at two-time points (i.e. at index date and 2 years later), and as such the dynamic interactive effects between time-varying adherence, confounding variables and treatment outcomes over time has not been accounted for [29]. Fourth, our current analysis did not take into account different patterns of adherence that might have existed in this cohort. Future work identifying the trajectories of adherence over time may provide better insight into the relationship between patterns of adherence and treatment outcomes. Furthermore, as the sample size in this study was small and they were all recruited from a single hospital, it is difficult to draw a valid generalization.
Conclusion
In conclusion, the rate and effect of adherence varied depending on whether patients were new or existing DMARD users. We found that adherence was positively associated with improvements in disease activity and functional outcomes among DMARD-naïve patients. Among existing DMARD users, a higher adherence threshold than the 80% cut-point we used to define adherence might be needed to observe a similar beneficial effect of adherence.
References
Wabe N, Wiese MD (2016) Treating rheumatoid arthritis to target: physician and patient adherence issues in contemporary rheumatoid arthritis therapy. J Eval Clin Pract. doi:10.1111/jep.12620 (Epub ahead of print)
Salt E, Frazier SK (2010) Adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a narrative review of the literature. Orthop Nurs 29:260–275
Blum MA, Koo D, Doshi JA (2011) Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther 33:901–913
Tamás K (2010) Clinical and pharmacoeconomic impact of patient medication adherence. Dissertation, Semmelweis University
DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW (2002) Patient adherence and medical treatment outcomes: a meta-analysis. Med Care 40:794–811
Cannon GW, Mikuls TR, Hayden CL et al (2011) Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken) 63:1680–1690
Pasma A, Schenk CV, Timman R et al (2015) Non-adherence to disease-modifying antirheumatic drugs is associated with higher disease activity in early arthritis patients in the first year of the disease. Arthritis Res Ther 17:281
Bluett J, Morgan C, Thurston L et al (2015) Impact of inadequate adherence on response to subcutaneously administered anti-tumour necrosis factor drugs: results from the Biologics in rheumatoid arthritis genetics and genomics study syndicate cohort. Rheumatology (Oxford) 54:494–499
Smolen JS, Aletaha D, Bijlsma JW et al (2010) Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 69:631–637
Wabe N, Sorich M, Wechalekar M et al (2015) Characterising deviation from treat-to-target strategies for early rheumatoid arthritis: the first three years. Arthritis Res Ther 17:48
Wabe N, Sorich MJ, Wechalekar MD et al (2016) Drug-induced toxicity and patient reported outcomes in rheumatoid arthritis patients following intensive treated-to-target strategy: does ceasing therapy due to toxicity worsen outcomes in long term? Int J Clin Pract 70:340–350
Wabe N, Sorich MJ, Wechalekar MD et al (2015) Determining the acceptable level of physician compliance with a treat-to-target strategy in early rheumatoid arthritis. Int J Rheum Dis. doi:10.1111/1756-185X.12816 (Epub ahead of print)
Proudman SM, Keen HI, Stamp LK et al (2007) Response-driven combination therapy with conventional disease-modifying antirheumatic drugs can achieve high response rates in early rheumatoid arthritis with minimal glucocorticoid and nonsteroidal anti-inflammatory drug use. Semin Arthritis Rheum 37:99–111
Pincus T, Summey JA, Soraci SA Jr et al (1983) Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum 26:1346–1353
DeVellis RF, Callahan LF (1993) A brief measure of helplessness in rheumatic disease: the helplessness subscale of the Rheumatology Attitudes Index. J Rheumatol 20:866–869
Wells G, Becker JC, Teng J et al (2009) Validation of the 28-joint disease activity score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 68:954–960
Smolen J, Breedveld F, Schiff M et al (2003) A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 42:244–257
Choudhry NK, Shrank WH, Levin RL et al (2009) Measuring concurrent adherence to multiple related medications. Am J Manag Care 15:457–464
Van Gestel A, Prevoo M, Van’t Hof M et al (1996) Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis: comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism criteria. Arthritis Rheum 39:34–40
Kosinski M, Zhao SZ, Dedhiya S et al (2000) Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum 43:1478–1487
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46: 399–424
Austin PC, Stuart EA (2015) Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34:3661–3679
Wabe NT, Sorich MJ, Wechalekar MD et al (2016) Effect of adherence to protocolized targeted intensifications of disease-modifying antirheumatic drugs on treatment outcomes in rheumatoid arthritis: results from an australian early arthritis cohort. J Rheumatol 43:1643–1649
Barnabe C, Sun Y, Boire G et al (2015) Heterogeneous disease trajectories explain variable radiographic, function and quality of life outcomes in the Canadian early arthritis cohort (CATCH). PloS one 10:e0135327
Siemons L, Ten Klooster PM, Vonkeman HE et al (2014) Distinct trajectories of disease activity over the first year in early rheumatoid arthritis patients following a treat-to-target strategy. Arthritis Care Res (Hoboken) 66:625–630
Quinn MA, Emery P (2003) Window of opportunity in early rheumatoid arthritis: possibility of altering the disease process with early intervention. Clin Exp Rheumatol 21:S154–S157
Jimmy B, Jose J (2011) Patient medication adherence: measures in daily practice. Oman Med J 26:155–159
McCaffrey DF, Griffin BA, Almirall D et al (2013) A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 32:3388–3414
Yu AP, Yu YF, Nichol MB (2010) Estimating the effect of medication adherence on health outcomes among patients with type 2 diabetes—an application of marginal structural models. Value Health 13:1038–1045
Acknowledgements
The authors are grateful to all rheumatologists and rheumatology nurses involved in the treatment and care of patients as well as patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors had no financial support or other benefits from commercial sources for the work reported in the manuscript, or any other financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
Conflict of interest
The authors have no competing interests to report.
Ethical approval
Ethics approval was obtained from the University of South Australia (protocol no.0000031928) and the Royal Adelaide Hospital (protocol no.140303) for the longitudinal observational cohort and for the linkage of prescribing data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
296_2017_3655_MOESM3_ESM.tif
The rate of medication adherence according to different definitions of adherence:*P<0.05 for DMARD-naïve vs experienced patients; PDC, proportion of days covered; PDC-1, adherence of ≥80% with at least one DMARD; PDC-average, average PDC≥80%; PDC-all, PDC≥80% for all DMARDs (TIF 168 KB)
296_2017_3655_MOESM4_ESM.tif
Proportion of patients who achieved EULAR and SDAI response criteria and MCID for mHAQ stratified according to treatment status:*P<0.0001 for DMARD-naïve vs experienced patients; EULAR, European League Against Rheumatism; SDAI, Simplified disease activity index; MCID, minimally clinically important differences, mHAQ, modified health assessment questionaries (TIF 204 KB)
Rights and permissions
About this article
Cite this article
Wabe, N., Lee, A., Wechalekar, M. et al. Adherence to combination DMARD therapy and treatment outcomes in rheumatoid arthritis: a longitudinal study of new and existing DMARD users. Rheumatol Int 37, 897–904 (2017). https://doi.org/10.1007/s00296-017-3655-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3655-z