Abstract
Physical activity (PA) is associated with numerous health-related benefits among adults with chronic diseases and the general population. As the benefits are dose-dependent, this review aims to establish the PA levels of adults with spondyloarthritis and to compare these to the general population. Electronic databases (Cochrane Central Register of Controlled Trials, EMBASE, MEDLINE/PubMed, PEDro, AMED, CINAHL) were systematically searched from inception to May 2014 using medical subject headings and keywords. This was supplemented by searching conference abstracts and hand-searching reference lists of included studies. Eligible studies were randomized controlled trials and observational studies of adults with SpA in which free-living PA or energy expenditure levels were measured. Subjects less than 18 years or with juvenile-onset SpA were excluded. Outcomes included objective and self-report measurements. Two reviewers independently screened studies for inclusion and assessed methodological quality using the Cochrane risk of bias tool and the RTI item bank. From the 2,431 records reviewed, nine studies involving 2,972 participants were included. This review focused on qualitative synthesis. Meta-analyses were not undertaken due to differences in study design, measurement tools, and participant characteristics. This heterogeneity, coupled with the risk of bias inherent in the included observational studies, limits the generalizability of findings. Objective measurements suggest PA levels may be lower among adults with spondyloarthritis than in healthy population controls. Self-reported PA and self-reported rates of adherence to PA recommendations varied largely across studies; higher disease activity was associated with lower self-reported PA levels. Physical activity levels may be lower in adults with axial spondyloarthritis, with higher disease activity associated with lower PA levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spondyloarthropathies (SpA) are a heterogeneous group of inflammatory conditions that include ankylosing spondylitis (AS), reactive arthritis (ReA), enteropathic spondylitis, or arthritis associated with inflammatory bowel disease (IBD), psoriatic arthritis (PsA), and undifferentiated spondyloarthropathy (uSpA) [1]. Depending on the clinical features and imaging, SpA can be classified as predominantly axial or predominantly peripheral [2, 3]. They are associated with decreased physical function, decreased work productivity, and lower health-related quality of life (QoL) [4–6]. Elevated cardiovascular risk factors and increased cardiovascular morbidity and mortality have been associated with AS and PsA [7, 8].
A combination of pharmacological and non-pharmacological treatment modalities is advocated for optimal management of patients with AS and PsA [9, 10]. Exercise-based interventions have been found to be safe and to yield beneficial physical and psychological effects in adults with SpA [11]. In this context, exercise is a planned, structured, and repetitive activity, with an objective to improve or maintain the elements of physical fitness [12]. Exercise is just one component of physical activity (PA), which includes any bodily movement produced by skeletal muscles that results in energy expenditure [12]. PA occurring in all aspects of daily free living contributes toward energy expenditure; this is often subdivided into work, transport, leisure time, and domestic activities. Global recommendations for health enhancement and prevention of non-communicable diseases are based on PA rather than exercise, and the health-related benefits are numerous.
In the general population, PA has been found to reduce the risk of cardiovascular disease, obesity, colon and breast cancers, type 2 diabetes, and osteoporosis [13, 14]. It also improves musculoskeletal health and reduces the symptoms of depression [15]. The World Health Organization (WHO) recommendations on PA state that adults should perform ≥150 min/week of moderate intensity aerobic physical activity (PAmod), or ≥75 min/week of vigorous intensity aerobic physical activity (PAvig), or an equivalent weekly combination of PAmod and PAvig. Furthermore, PA should be performed in bouts of at least 10 min and should be supplemented by muscle-strengthening activities on two or more days [15]. It is further recommended that older adults with medical conditions engage in PA in a manner that effectively and safely treats their condition and prevents the development of other chronic diseases [16].
Despite strong evidence that physical inactivity adversely affects many aspects of health, the recommendations for the management of SpA do not contain specific guidance on PA. Studies have traditionally focused specifically on flexibility-based interventions, with few investigating the effects of aerobic exercise. No study has systematically reviewed PA in adults with SpA. The aims of this systematic review were to (1) explore the PA levels of adults with SpA, (2) compare the PA levels of adults with SpA to healthy, equivalent controls, and (3) examine the effect of interventions on PA levels of adults with SpA.
Materials and methods
A protocol outlining the planned search strategy and methods of analysis for this review was registered online with a registry of systematic reviews (available at http://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42014007607#.Uy_r0vl_u0I). This review was guided by the recommendations for reporting of systematic review and meta-analysis of observational studies in epidemiology (MOOSE) [17].
Eligibility criteria
Adults diagnosed by a rheumatologist as having AS, ReA, PsA, uSpA, or enteropathic spondylitis were included in this study. Participants under 18 years of age or with juvenile-onset SpA were excluded. Randomized controlled trials (RCTs) and observational studies with or without controls were considered for inclusion. Review articles, case reports, commentaries, and studies with ≤5 participants were excluded.
The primary outcomes of interest were free-living PA levels or energy expenditure levels collected over ≥24 h. These included both self-report methods (e.g., questionnaires) and objective measures (e.g., accelerometry). Depending on the measurement method employed, outputs were expressed as continuous variables (e.g., kJ/day, MET-minutes per week) or categorical variables (e.g., meeting/not meeting national guidelines, high/moderate/low PA level) (see Supplement 1).
Information sources and study selection
Studies were retrieved by searching six electronic databases (MEDLINE/PubMed, EMBASE, PEDro, AMED, CINAHL, and The Cochrane Central Register of Controlled Trials) from their inception to May 2014. Search terms were adapted for use with each database. Common keywords and medical subject headings were related to two components: (1) the condition and (2) PA (see Supplement 2). No search restrictions were imposed. The electronic database search was supplemented by searching abstracts from the annual congresses of the World Confederation for Physical Therapy (2003–2011), the American College of Rheumatology (2006–2013), the European League Against Rheumatism (2002–2013), and the American Physical Therapy Association (2002–2013). When only abstracts were available in the published literature, authors were contacted seeking full-text manuscripts of relevant studies. Finally, a hand search of the reference lists of included studies was conducted.
Two reviewers (TOD and FW) independently screened titles and abstracts to identify studies that potentially met the eligibility criteria. Full-texts of these reports were retrieved and assessed for eligibility by the same two reviewers. Foreign-language articles were translated into English. Disagreements on inclusion were resolved by discussion to achieve consensus, and failing agreement, a third reviewer (FOS) was consulted.
Data collection and analysis
A data extraction template based on Cochrane recommendations was piloted on five randomly selected studies and modified accordingly for this review [18]. One reviewer (TOD) recorded (1) study characteristics, (2) participant characteristics, and (3) PA outcome data. In studies including a control group, the differences in group means (with 95 % confidence interval) were calculated at clinically relevant time points (i.e., baseline and post-intervention). In cases where elaboration on published material was needed or further data were required, study authors were contacted requesting the pertinent information. Meta-analyses were planned but ultimately deemed inappropriate due to the heterogeneity of study designs and interventions. Statistical analysis was conducted using SPSS version 21 (IBM, Armonk, NY, USA).
Risk of bias and levels of evidence
A risk of bias appraisal of included studies was performed independently by two reviewers (TOD and FW). Disagreements between the reviewers were resolved through discussion to achieve consensus. Failing agreement, a third reviewer (FOS) arbitrated. For randomized and quasi-randomized controlled trials, the Cochrane Collaboration’s risk of bias tool was used. This tool rated the risk of bias across six domains as low, high, or unclear (selection bias, performance bias, detection bias, attrition bias, reporting bias, and other sources of bias including confounding) [18]. For observational studies, the RTI item bank for assessing the risk of bias and confounding was used [19]. In accordance with the developers’ instructions, this tool was adapted to cater to the review topic and the design types of the included observational studies. Eight validated items assessing selection bias, detection bias, reporting bias, confounding, and overall bias of each study were selected. For each item, criteria relevant to determining the risk of bias were indicated to assist reviewers.
Results
Study selection
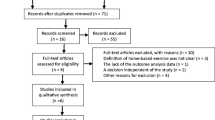
A total of nine studies, reported across 11 articles published between 2012 and 2014, were included in this review. The search strategy is summarized in Fig. 1. The electronic database search returned 2,410 records (after removal of duplicates), and an additional 21 reports were identified from the conferences abstracts search. Of the 2,431 records screened for eligibility by title and abstract, 47 titles were considered for full-text review. Studies were excluded for not meeting study design criteria (n = 1,454), not investigating an SpA cohort (n = 207), and not reporting free-living PA over at least 24 h (n = 723). When multiple articles reported different data from the same study, results were pooled under a primary study. As such, data reported by van Genderen et al. [20] are included with the data from Plasqui et al. [21], and results from the study by Halvorsen et al. [22] are combined with those from Fongen et al. [23]. Upon request, the authors of two studies provided additional relevant data not included in the published full-texts [24, 25].
Study characteristics
Seven of the included studies employed a cross-sectional design, while one RCT [26] and one validation/reliability study [27] were also included (baseline data are reported for RCT and reliability studies). Three studies included matched population controls [21, 23, 28], one study compared findings to existing normative data [29], and the RCT compared exercising and non-exercising groups of adults with AS [26].
Study characteristics are summarized in Table 1. A total of 2,972 participants with SpA were included (F/M; 1,373:1,585, 14 unspecified), and 198 population controls. The predominant subtype was AS. The median (IQR) study sample size was 106 (210). Subject characteristics varied in age, disease duration, disease severity, and medication use (Table 2).
Physical activity was measured by questionnaire in six studies [20, 23–25, 29, 30], by accelerometry in one study [28], and by both methods in three studies [21, 26, 27]. The diversity of measurement tools used to quantify PA generated a variety of outcome variables.
Risk of bias within studies
The Cochrane risk of bias form was used to assess the RCT by Niedermann et al. [26], and the risk of bias was low. The remaining observational studies were appraised using the RTI item bank, and the results are summarized in Table 3; taking study limitations into consideration, individual study results were deemed credible. All studies included participants with a formal diagnosis of SpA, although the criteria used in the study by Brophy et al. [30] are unclear, and two studies used patient-reported doctor diagnoses to classify patients attending rheumatology clinics [24, 25]. Although no study protocols were reported, reporting bias appears low as no primary outcomes appeared missing. Confounding was accounted for in four studies by statistical adjustment [29], or control matching for age and gender. Additionally, studies also matched controls for residential area [23], body mass index [21], and data collection period [28].
Synthesis of results
A meta-analysis was not undertaken due to the heterogeneity of study designs, participant characteristics, PA measurement tools, and outcome variables. Consequently, this review focused on a qualitative synthesis of the studies.
Accelerometry
Four studies objectively measured PA using accelerometry (Table 4). Niedermann et al. [26] used an ActiGraph to monitor PA before and after a 12-week aerobic exercise intervention. The waist-mounted monitor was worn for 7 days, including two weekend days. The authors reported mean (SD) output of 336.3 (184.9) counts/min prior to the intervention; this was comparable to baseline PA of a control group with AS who attended group discussions on coping strategies and mindfulness-based stress reduction (mean difference [95 % CI]; 34.2 counts/min [−29.1 to 97.5]). Post-intervention PA scores were not significantly changed in either group (MD [95 % CI]; 2.9 counts/min [−53.0 to 47.2]).
Arends et al. [27] explored the construct validity of two PA self-report questionnaires by comparing agreement with output from uniaxial ActiGraph accelerometers in an AS cohort. The devices were worn for a minimum of 10 waking hours (except during water-based activities) for 5–7 days (including two weekend days). Mean (SD) ActiGraph output of 236 (106) kilocounts/day was reported.
Swinnen et al. [28] used the SenseWear Pro3 Armband to measure PA. The device combines a two-axial accelerometer with thermal sensors and estimates minute-by-minute energy expenditure and PA intensity. The device was worn for 5–7 consecutive days, for a minimum of 90 % of each 24-h day. In a group of patients with axial SpA, the authors reported physical activity levels (PAL), energy expenditure, and moderate/vigorous physical activity (MVPA). These outcomes were significantly lower than healthy matched controls (p < 0.048, p < 0.045 and p < 0.029, respectively).
Plasqui et al. [21] used the triaxial Tracmor accelerometer to measure PA of adults with AS and matched controls. The device was worn around the waist during waking hours for seven consecutive days. Unlike Swinnen et al. [28], this was not statistically different to PA levels of the healthy control group.
Self-report questionnaires
Eight studies used self-report questionnaires to measure PA (Table 5). In their validation and reliability study, Arends et al. [27] assessed PA using both the long form of the International Physical Activity Questionnaire (IPAQ-LF) and the Short Questionnaire to Assess Health (SQUASH). Participants were divided into validity and reliability arms, and the authors reported median (IQR) group PA measured with the IPAQ-LF of 3,849 (1,470–8,132) MET-minutes per week and 5,937 (2,126–11,601) MET-minutes per week, respectively. Total activity scores on the SQUASH were 7,267 (3,453) [mean (SD)] and 5,760 (3,360–6,890) [median (IQR)], respectively.
Fongen et al. [23] compared PA of participants with low disease activity and those with high disease activity (high disease activity: ASDASCRP > 2.1 units), and compared both to healthy controls. The IPAQ-LF was used to measure PA. Participants with high disease activity reported significantly less weekly PA compared to participants with low disease activity and controls (p < 0.02 and p < 0.01, respectively).
In a questionnaire circulated to an AS cohort, Brophy et al. [30] measured PA on the short form of the IPAQ (IPAQ-SF) and reported a median (IQR) PA of 1,719 MET-minutes per week (396–3,892). The authors also reported that participants with a high level of disease activity (BASDAI > 60; scale 0–100) had lower PA levels than those with milder disease activity (p = 0.0028).
In the study by Manning et al. [24], 508 patients attending a rheumatology clinic completed the IPAQ-SF. Fourteen respondents had a diagnosis of AS and reported a mean score of 1,862 MET-minutes per week (95 % CI 873.0–2,851.9).
In addition to measuring PA by accelerometry, Niedermann et al. [26] employed the Office in Motion Questionnaire (OIMQ) and assigned METs to each reported activity. Mean baseline OIMQ in the intervention group was 71.8 (39.1) METs per week, which was comparable to controls (MD [95 % CI]; −6.6 MET per week [−25.5 to 12.3]). Post-intervention, there were no significant between-group differences reported. In keeping with these findings, Van Genderen et al. [20] found no significant differences between an AS group and matched controls in self-reported PA measured on the Baecke Physical Activity questionnaire [median (IQR); 8.4 (1.4) vs. 8.4 (1.6), respectively].
Comparisons to physical activity guidelines
Four studies examined compliance with PA guidelines. Fongen et al. [23] determined whether or not participants were meeting health-enhancing physical activity (HEPA) requirements based on IPAQ-LF results. They defined meeting HEPA as engaging in 30-min PAmod on ≥5 days/week, or engaging in 20-min PAvig on ≥3 days/week achieving ≥600 MET-minutes per week. Participants with low disease activity met the criteria more frequently than respondents with high disease activity (61 vs. 41 %, p < 0.02). Neither group was significantly different compared to the control group (49 % meeting HEPA criteria, p < 0.1 and p < 0.28, respectively).
Based on the results from the IPAQ-SF, Manning et al. [24] reported that 71 % of adults with AS exceeded weekly PA recommendations of >500MET-minutes per week; 21 % were classified as inactive. O’Dwyer et al. [25] administered a single-item measure to determine self-reported PA in a population attending rheumatology clinics. Of the SpA subset, 17.2 % met national guidelines of participating in ≥30 min of PA on ≥5 days per week (41 % reported no PA).
Haglund et al. [29] gathered information on PA in a large SpA cohort through a questionnaire enquiring about weekly PA frequency, duration, and intensity. They reported that 68 % of respondents with SpA met the WHO recommendation for PA, a rate slightly higher than that observed in the general Swedish population (RR 1.09, 95 % CI 1.04–1.15).
Discussion
Main findings
This is the first systematic review of PA in adults with SpA. The evidence from two studies comparing objectively measured PA levels of adults with axial SpA to matched population controls is conflicting; PA may be decreased [28] or no different [21]. In the AS subtype, participants with high disease activity reported engaging in significantly less PA than respondents with low disease activity [23, 30]. Arends et al. [27] also found PA scores to be related to disease activity, physical function, and quality of life; however, causality has not been established due to the cross-sectional design of these studies.
Estimates of compliance with PA recommendations to achieve health-related benefits vary across studies from 17 % [25] to 71 % [24]. Comparisons with healthy population controls are mixed. The large disparity may be in part due to differences in outcome measures, criteria for meeting recommendations, participant characteristics, and condition-related factors (e.g., disease duration, medications usage, and disease severity).
Interventions
Just one study measured the effect of an aerobic exercise intervention on PA [26]. Although the volume of PA performed by the exercise group during the intervention phase was substantial, at the conclusion, participants reverted to baseline PA levels and no follow-up period was performed to examine long-term effect on PA behavior. This is not surprising as increasing free-living PA was not the primary aim of this aerobic exercise and flexibility intervention. Interventions specifically targeting PA beliefs and motivation to engage in PA have shown efficacy in improving PA participation in adults with arthritis [31, 32].
Methodological considerations
Accurate measurement of PA is essential for the study of the relationship between PA and health in adults with rheumatic conditions. The practical benefits of using self-reported PA questionnaires include the low cost, low participant burden, and general acceptance among patient groups. However, they possess several limitations in reliability and validity [33]. Arends et al. [27] found only modest construct validity for the IPAQ-LF and SQUASH questionnaires compared to the ActiGraph accelerometer. The validity and reliability of the remaining questionnaires included in the review has not been established in an SpA population.
Direct measurement by accelerometry allows objective PA data to be gathered, although this method is not without limitations. Certain types of activities, such as upper body work or strengthening exercises, may be underestimated, while many devices cannot monitor water-based activities [34]. Self-reported PA measures can both under- and overestimate PA compared to accelerometry [35], with no clear trends in the degree to which PA estimates diverge across measures. For these reasons, direct comparison and pooling has been avoided in this review.
A priori sample size calculations were reported in the studies by Niedermann [26] and Halvorsen [22]; however, neither were calculated for PA outcomes. The remaining studies may also have been underpowered and at risk of type 2 error, or overestimating effect sizes. The lack of healthy population controls in some studies limits conclusions that may be drawn. Sampling methods varied, and there is a possibility of selection bias.
Clinical implications
Current practice guidelines advocate exercise as a key component of the management of axial SpA, but lack guidance on the amount of PA needed to derive condition-specific and general health benefits [9]. This is particularly pertinent as SpA have a higher incidence of comorbidities, such as cardiovascular disease and osteoporosis, that may be favorably impacted by increasing PA [7, 8, 36]. Although evidence of a higher prevalence among the SpA population of other chronic conditions such as obesity and diabetes is conflicting, PA could potentially also ameliorate these.
Advice on PA would be welcomed by the majority of patients attending rheumatology clinics, though in approximately half the cases, this is never discussed [24, 37]; adults with rheumatic conditions are largely unaware that PA guidelines exist [25]. There is a strong case for recommendations on PA among adults with SpA; however, it is yet to be determined whether PA guidelines for the general population are optimal for adults with rheumatic conditions.
Limitations of the study
The diversity of design and the inherent biases of observational studies make reviewing the methodological quality challenging. Numerous appraisal tools are described in the literature, yet no gold standard exists for evaluating risk of bias [38, 39]. In this review, a decision to use either the RTI item bank or the Cochrane Collaboration’s risk of bias tool was made on the basis of the methodological design of individual studies. Neither tool ascribes a score, as quality scoring of observational studies remains controversial; key components of design rather than aggregate scores were considered more important [17].
The heterogeneous nature and methodological limitations of the included studies prevent firm conclusions being drawn and restrict the generalizability of findings. The included studies included participants classified as predominantly axial-SpA; consequently, no inference can be made about predominantly peripheral-SpA, in which PA may be differently affected due to peripheral joint involvement [2, 3].
Areas for future research
Larger epidemiological studies objectively measuring free-living PA would build on existing studies that utilized self-report questionnaires. Longitudinal studies would allow investigation of cause–effect relationships between sociodemographic and condition-related factors and PA behavior. Studies exploring the effect of current management strategies on long-term PA habits are needed, as are studies investigating strategies to positively influence PA behavior in adults with SpA. Finally, working toward including specific PA recommendations in SpA practice guidelines would add clarity for patients and healthcare practitioners.
Conclusions
Higher disease activity was associated with lower self-reported PA levels compared to lower disease activity and controls. Objective measurement suggests PA levels may be lower among adults with SpA than among the general population. However, the heterogeneity of study designs and measurement tools, coupled with the inherent risk of bias of observational studies, limits the conclusions that may be drawn regarding the PA levels of adults with SpA.
References
Zochling J, Brandt J, Braun J (2005) The current concept of spondyloarthritis with special emphasis on undifferentiated spondyloarthritis. Rheumatology 44(12):1483–1491
Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M, Huang F, Gu J, Khan MA, Kirazli Y, Maksymowych WP, Mielants H, Sorensen IJ, Ozgocmen S, Roussou E, Valle-Onate R, Weber U, Wei J, Sieper J (2009) The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68(6):777–783. doi:10.1136/ard.2009.108233
Rudwaleit M, van der Heijde D, Landewe R, Akkoc N, Brandt J, Chou CT, Dougados M, Huang F, Gu J, Kirazli Y, Van den Bosch F, Olivieri I, Roussou E, Scarpato S, Sorensen IJ, Valle-Onate R, Weber U, Wei J, Sieper J (2011) The assessment of spondyloarthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70(1):25–31. doi:10.1136/ard.2010.133645
Singh JA, Strand V (2009) Spondyloarthritis is associated with poor function and physical health-related quality of life. J Clin Rheumatol Pract Rep Rheumat Musculoskelet Dis 36(5):1012–1020
Heikkila S, Viitanen JV, Kautiainen H, Kauppi M (2002) Functional long-term changes in patients with spondyloarthropathy. Clin Rheumatol 21(2):119–122
Haglund E, Bremander A, Bergman S, Jacobsson LTH, Petersson IF (2013) Work productivity in a population-based cohort of patients with spondyloarthritis. Rheumatology 52(9):1708–1714
Papagoras C, Voulgari PV, Drosos AA (2013) Atherosclerosis and cardiovascular disease in the spondyloarthritides, particularly ankylosing spondylitis and psoriatic arthritis. Clin Exp Rheumatol 31(4):612–620
Han C, Robinson DW Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV (2006) Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 33(11):2167–2172
Braun J, Van Den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, Dagfinrud H, Dijkmans B, Dougados M, Emery P, Geher P, Hammoudeh M, Inman RD, Jongkees M, Khan MA, Kiltz U, Kvien TK, Leirisalo-Repo M, Maksymowych WP, Olivieri I, Pavelka K, Sieper J, Stanislawska-Biernat E, Wendling D, Ozgocmen S, Van Drogen C, Van Royen BJ, Van Der Heijde D (2011) 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 70(6):896–904
Ritchlin CT, Kavanaugh A, Gladman DD, Mease PJ, Helliwell P, Boehncke WH, De Vlam K, Fiorentino D, FitzGerald O, Gottlieb AB, McHugh NJ, Nash P, Qureshi AA, Soriano ER, Taylor WJ (2009) Treatment recommendations for psoriatic arthritis. Ann Rheum Dis 68(9):1387–1394
O’Dwyer T, O’Shea F, Wilson F (2014) Exercise therapy for spondyloarthritis: a systematic review. Rheumatol Int 34(7):887–902
Caspersen CJ, Powell KE, Christenson GM (1985) Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep (Washington, DC: 1974) 100(2):126–131
Warburton DE, Nicol CW, Bredin SS (2006) Health benefits of physical activity: the evidence. CMAJ 174(6):801–809. doi:10.1503/cmaj.051351
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK (2009) American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 41(2):459–471. doi:10.1249/MSS.0b013e3181949333
Global recommendations on physical activity for health. World Health Organization (2010)
Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C (2007) Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39(8):1435–1445. doi:10.1249/mss.0b013e3180616aa2
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Higgins JP, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration. Available from http://www.cochrane-handbook.org. Accessed 7 March 2011
Viswanathan M, Berkman ND, Dryden DM, Hartling L (2013) Assessing risk of bias and confounding in observational studies of interventions or exposures: further development of the RTI Item Bank. Rockville
Van Genderen S, Van Den Borne C, Geusens P, Van Der Linden S, Boonen A, Plasqui G (2014) Physical functioning in patients with ankylosing spondylitis: comparing approaches of experienced ability with self-reported and objectively measured physical activity. J Clin Rheumatol Pract Rep Rheumat Musculoskelet Dis 20(3):133–137
Plasqui G, Boonen A, Geusens P, Kroot EJ, Starmans M, Van Der Linden S (2012) Physical activity and body composition in patients with ankylosing spondylitis. Arthritis Care Res (Hoboken) 64(1):101–107
Halvorsen S, Vollestad NK, Fongen C, Provan SA, Semb AG, Hagen KB, Dagfinrud H (2012) Physical fitness in patients with ankylosing spondylitis: comparison with population controls. Phys Ther 92(2):298–309. doi:10.2522/ptj.20110137
Fongen C, Halvorsen S, Dagfinrud H (2013) High disease activity is related to low levels of physical activity in patients with ankylosing spondylitis. Clin Rheumatol 32(12):1719–1725
Manning VL, Hurley MV, Scott DL, Bearne LM (2012) Are patients meeting the updated physical activity guidelines? Physical activity participation, recommendation, and preferences among inner-city adults with rheumatic diseases. J Clin Rheumatol Pract Rep Rheumat Musculoskelet Dis 18(8):399–404
O’Dwyer T, Rafferty T, O’Shea F, Gissane C, Wilson F (2014) Physical activity guidelines: is the message getting through to adults with rheumatic conditions? Rheumatology 53(10):1812–1817
Niedermann K, Sidelnikov E, Muggli C, Dagfinrud H, Hermann M, Tamborrini G, Ciurea A, Bischoff-Ferrari H (2013) Effect of cardiovascular training on fitness and perceived disease activity in people with ankylosing spondylitis. Arthrit Care Res 65(11):1844–1852
Arends S, Hofman M, Kamsma YPT, der Veer EV, Houtman PM, Kallenberg CGM, Spoorenberg A, Brouwer E (2013) Daily physical activity in ankylosing spondylitis: validity and reliability of the IPAQ and SQUASH and the relation with clinical assessments. Arthritis Res Ther 15(4):R99. doi:10.1186/ar4279
Swinnen TW, Scheers T, Lefevre J, Dankaerts W, Westhovens R, de Vlam K (2014) Physical activity assessment in patients with axial spondyloarthritis compared to healthy controls: a technology-based approach. PLoS ONE 9(2):e85309. doi:10.1371/journal.pone.0085309
Haglund E, Bergman S, Petersson IF, Jacobsson LT, Strombeck B, Bremander A (2012) Differences in physical activity patterns in patients with spondylarthritis. Arthritis Care Res (Hoboken) 64(12):1886–1894. doi:10.1002/acr.21780
Brophy S, Cooksey R, Davies H, Dennis MS, Zhou SM, Siebert S (2013) The effect of physical activity and motivation on function in ankylosing spondylitis: a cohort study. Semin Arthritis Rheu 42(6):619–626
Ehrlich-Jones L, Lee J, Semanik P, Cox C, Dunlop D, Chang RW (2011) Relationship between beliefs, motivation, and worries about physical activity and physical activity participation in persons with rheumatoid arthritis. Arthritis Care Res (Hoboken) 63(12):1700–1705. doi:10.1002/acr.20616
Ehrlich-Jones L, Mallinson T, Fischer H, Bateman J, Semanik PA, Spring B, Ruderman E, Chang RW (2010) Increasing physical activity in patients with arthritis: a tailored health promotion program. Chronic Illn 6(4):272–281. doi:10.1177/1742395309351243
Shephard RJ (2003) Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med 37(3):197–206
Matthews CE, Hagstromer M, Pober DM, Bowles HR (2012) Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc 44(Suppl 1):S68–S76. doi:10.1249/MSS.0b013e3182399e5b
Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M (2008) A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 5:56. doi:10.1186/1479-5868-5-56
van der Weijden MA, Claushuis TA, Nazari T, Lems WF, Dijkmans BA, van der Horst-Bruinsma IE (2012) High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis: a systematic review. Clin Rheumatol 31(11):1529–1535. doi:10.1007/s10067-012-2018-0
Fontaine KR, Bartlett SJ, Heo M (2005) Are health care professionals advising adults with arthritis to become more physically active? Arthritis Rheum 53(2):279–283. doi:10.1002/art.21073
Sanderson S, Tatt ID, Higgins JP (2007) Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 36(3):666–676. doi:10.1093/ije/dym018
Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG (2003) Evaluating non-randomised intervention studies. Health Technol Assess 7(27):iii-x, 1–173
Acknowledgments
We wish to thank David Mockler (John Stearne Library, Trinity Centre for Health Sciences, Dublin 8) for his assistance in devising the electronic search strategy. TOD reports receipt of a studentship grant from Trinity College Dublin, during the conduct of the study.
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
O’Dwyer, T., O’Shea, F. & Wilson, F. Physical activity in spondyloarthritis: a systematic review. Rheumatol Int 35, 393–404 (2015). https://doi.org/10.1007/s00296-014-3141-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-014-3141-9