Abstract
Retinoid, a derivative of vitamin A, is a general term used to describe compounds that bind to and activate retinoic acid receptors [RARs (RARα, RARβ, and RARγ)] and/or retinoid X receptors [RXRs (RXRα, RXRβ, and RXRγ)]. They have been shown to surpress the differentiation of Th1/Th17 cells and induce the development of Th1/regulatory T cells. They also affect the proliferation of B cells as both an inducer and suppressor. Furthermore, retinoids may induce the maturation of dendritic cells and production of interleukin-10 from monocytes/macrophages. We recently demonstrated that retinoids suppressed the production of reactive oxygen species, the release of elastase from neutrophils by inhibiting mitogen-activated protein kinase signals, and both the migration speed and chemotaxis directionality of neutrophils. Retinoids, such as all-trans retinoic acid and tamibarotene, were previously shown to have positive effects on animal models of several rheumatic diseases, including arthritis, myositis, and vasculitis in vivo. Moreover, retinoids have been used in a pilot study to effectively treat patients with lupus nephritis and systemic sclerosis. We herein reviewed the effects of retinoids on immune cells, animal models of rheumatic diseases, and rheumatic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological drugs, such as anti-tumor necrosis factor (TNF) monoclonal antibodies, were recently shown to markedly improve arthritis and inhibit bone destruction associated with rheumatoid arthritis (RA) [1, 2]. However, some patients do no respond to these treatments, and biological agents have been shown to increase the risk of severe infection [3, 4]. Other rheumatic diseases, such as myositis and vasculitis, are treated with prednisolone (PSL) monotherapy or PSL combined with immunosuppressive therapy, which can also increase the risk of infection. Previous studies reported that biological drugs may be effective for vasculitis and myositis [5, 6]; however, these treatments have not yet been established. Therefore, therapies urgently need to be developed that are more effective, cheaper, and safer than conventional treatments.
RA was previously treated with retinoids, but was unsuccessful because of severe adverse events and low efficacy [7, 8]. Mucida et al. demonstrated that retinoids regulated the differentiation of T helper (Th) cells in 2007 [9], which led to a marked increase in the number of studies examining the immunoregulatory effects of retinoids. We previously reported that the synthetic retinoid, Am80, attenuated arthritis, myositis, and vasculitis in the respective murine models [10–12]. In addition to all-trans retinoic acid (ATRA), tamibarotene (Am80) was approved for the treatment of acute promyelocytic leukemia (APL) in Japan in 2005. We herein reviewed the immunological function of retinoids, and their potential as therapeutic agents in the treatment of rheumatic diseases.
Retinoids
Retinoid, a derivative of vitamin A, is a general term used to describe compounds that bind to and activate retinoic acid receptors [RARs (RARα, RARβ, and RARγ)] and/or retinoid X receptors [RXRs (RXRα, RXRβ, and RXRγ)], members of the nuclear receptor superfamily [13]. RARs and RXRs are transcriptional regulators that bind to specific retinoic acid response elements present in the promoters of their target genes. Retinoids are critically involved in embryonic development, organogenesis, tissue homeostasis, cell proliferation, differentiation, and apoptosis [13]. A previous study showed that retinoids also contributed to immune regulation through RARs and RXRs [14], including Th differentiation and B cell activation [15]. Etretinate has been used clinically for the treatment of cutaneous inflammatory disorders such as psoriasis and acne [16, 17]. ATRA, which is a ligand for RARα, β, and γ, and Am80, which is a specific ligand for RARα and β, but not for RARγ [18], are also used to treat APL [19, 20].
Effects of retinoids on immune cells
T cells
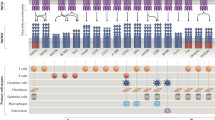
T cells play an important role in the immune system. Signals from dendritic cells (DCs), macrophages, and cytokines induce the differentiation of cells into Th1, Th2, Th17, or regulatory T (Treg) cells. ATRA has been shown to inhibit differentiation into Th1 cells by downregulating T-box expressed in T cells (T-bet) expression and promotes the differentiation of Th2 cells by inducing the expression of GATA-binding protein-3 (GATA3) and MAF as well as activating STAT6 in vitro [21] (Fig. 1). A deficiency in vitamin A was shown to result in an environment that was conducive to the differentiation of naive precursor CD4+ T cells into interferon (IFN) γ–secreting Th1 cells [22]. In addition, ATRA directly induced the differentiation of Th2 cells via RAR [21] and indirectly promoted that of Th2 cells by increasing the production of interleukin (IL)-4 and IL-5 from Th2 cells, which are important cytokines for Th2 differentiation [23]. ATRA can also inhibit differentiation into Th17 cells by downregulating the expression of retinoid-related orphan receptor-gamma t (RORγt) and induce forkhead box P3 (FOXP3)-positive Treg by upregulating the expression of FOXP3 in vitro [24].
Regulation of Th differentiation by retinoic acid. Retinoic acid enhances the differentiation of Th2 by inducing the expression of GATA3, MAF, STAT6, IL-4, and IL-5, and also Treg differentiation through the expression of FOXP3. In contrast, retinoic acid suppresses Th1 and Th17 differentiation by downregulating the expression of T-bet and RORγt, respectively
All-trans retinoic acid strongly enhances the production of IL-2 from T cells, which, in turn, induces the proliferation of T cells [25, 26]. A previous study demonstrated that ATRA regulated the migration of T cells into the gut by inducing the expression of α4β7-integrin and CC chemokine receptor 9 (CCR9) on T cells [27]. These findings indicated that retinoids could regulate Th differentiation as well as the proliferation and migration of T cells.
B cells
The proliferation of B cells is induced by stimulating the B cell receptor (BCR), CD38, CD40, CD19, Toll-like receptor (TLR) 4, and TLR9 [28–30]. ATRA can also regulate B cell proliferation as both an inducer and suppressor. The incubation of B cells with ATRA inhibited their proliferation due to the stimulation of BCR and TLR4 [31, 32]. In contrast, ATRA enhanced the proliferation of memory B cells by stimulating TLR9 [33]. The effects of retinoic acid on B cell proliferation may depend on the B cell subpopulation and co-stimulations.
Activation-induced cytidine deaminase (AID) is expressed in germinal center B cells and leads to the somatic hypermutation and class switch recombination of immunoglobulin genes. The expression of AID in B cells is induced by stimulations with lipopolysaccharide (LPS), IL-4, transforming growth factor-β (TGF-β), IFN-γ, and the CD40 ligand [34]. ATRA also increased the expression of AID in BCR-stimulated B cells, which suggested that it plays a positive role in regulating somatic hypermutation and class switch recombination [31]. A previous study showed that retinoic acid increased TGF-β-promoted IgA-class switch recombination [35] and the CD40 ligand and IL-4-induced IgG-class switch recombination, but inhibited the CD40 ligand and IL-4-induced IgE-class switch recombination [31].
Regarding the effects of retinoids on total immunoglobulin production, there are no reports that treatment with ATRA altered serum immunoglobulin level in patients with APL. However, serum IgG2a and IgG2b anti-myosin antibody levels, as well as IgG1, IgG2a, and IgG2b anti-collagen antibody levels, were decreased by Am80 in murine myosin-induced myositis and collagen-induced arthritis, respectively [10, 11].
Dendritic cells
RARα and RXRα are highly expressed in human monocyte-derived DCs, whereas murine splenic DCs express all RAR receptors [36]. ATRA was shown to increase the number of DCs in the spleen and promoted the expression of HLA-DR, CD11c, and CD1c on epidermal DCs [36]. In the presence of inflammation, ATRA also induced DC maturation and upregulated the capacity of antigen presentation through RXR signaling [36], but elicited programmed cell death in DCs in the absence of an inflammatory stimulation [36]. ATRA also suppressed the production of IL-12, but enhanced that of TGF-β and IL-6 from monocytes derived from DCs [36]. These effects may contribute to the regulation of Th differentiation.
On the other hand, ATRA has been shown to increase the expression of matrix metalloproteinases in endothelial cells, which have the potential to boost tumor-specific T cell responses by increasing the migration of tumor-infiltrating DCs to draining lymph nodes [37]. Gut-associated DCs also enhance the differentiation of Treg cells and production of IgA in an ATRA dose-dependent manner in vitro [38, 39]. IgA was decreased in the lamina propria of the small bowel in vitamin A-deficient mice, and the oral administration of an RAR agonist significantly increased serum IgA levels [40]. These findings suggested that gut-associated DCs stimulated with retinoic acid may induce the production of IgA from B cells. Taken together, these findings indicate that retinoic acid has several effects, such as cytokine production, maturation, and B cell stimulation, on DCs.
Monocytes/macrophages
All-trans retinoic acid was previously shown to induce the expression of CC chemokine ligand 2 (CCL2) in human monocytes derived from leukemia patients [41]. ATRA also induced the production of IL-10 from monocytes/macrophages, while ATRA suppressed TNF-α and IL-12 from monocytes/macrophages via interactions between RXR and NF-κB [42–44]. ATRA could also attenuate inflammation-induced tissue damage by inducing the production of plasminogen activator inhibitor-2 in peripheral blood mononuclear cells [45]. In addition, ATRA increased the number of T cells, natural killer cells, and macrophages in the lungs and spleen, which attenuated severe infections, such as tuberculosis [46]. RARγ-deficient macrophages exhibited the impaired production of inflammatory cytokines when stimulated with TLR as well as a defective immune response to Listeria monocytogenes [47]. Therefore, retinoids play important roles in the activation of monocytes/macrophages with inflammation, including infection.
Neutrophils
Retinoids inhibit the activation of neutrophils by suppressing the production of the superoxide anion and release of protease [48–51]. In addition, we recently reported that Am80 could suppress the production of reactive oxygen species (ROS) and release of elastase from human neutrophils by inhibiting mitogen-activated protein kinase (MAPK) signals in vitro [12]. Am80 could also inhibit the migration speed and chemotaxis directionality of human neutrophils in vitro [12]. Neutrophil extracellular traps (NETs) also play an important role in innate immunity [52]. However, the role of retinoids in the formation of NETs remains unknown.
Effects of retinoids on animal models of rheumatic diseases
Several studies demonstrated the efficacy of retinoids in animal models of autoimmune diseases. Treatments with 13-cis-retinoic acid, ATRA, and Am80 attenuated murine and rat collagen-induced arthritis [10, 40, 53, 54]. Am80 inhibited Th17 and enhanced Treg differentiation and decreased anti-collagen antibodies in vivo [10]. ATRA decreased the infiltration of macrophages into the glomeruli, suppressed the expression of CCL2 in the kidney in vivo, and inhibited proteinuria and renal involvement, such as fibrin deposits, necrosis, and crescents in NZB/WF1 mice, which were used as a lupus nephritis model [55]. A treatment with Am80 also ameliorated murine experimental autoimmune myositis [11]. We recently reported that Am80 significantly attenuated Candida albicans water-soluble fraction (CAWS)-induced vasculitis, which is characterized by the infiltration of neutrophils into inflamed vessels. Moreover, Am80 inhibited the migration of transferred neutrophils into the site of vasculitis in vivo [12]. Thus, retinoids could be a promising therapeutic target for rheumatic disease.
Current status of retinoid therapy for rheumatic diseases
Retinoids have regulatory effects on immune cells and have been shown to improve rheumatic diseases in animal models. These findings suggest that retinoids may be a new therapy for rheumatic diseases. To date, four clinical trials have been conducted on retinoid therapy for rheumatic diseases, including RA, lupus nephritis, and systemic sclerosis (Table 1).
In the first trial, RA patients were treated with etretinate, a synthetic retinoid, for 24 weeks. One mg/kg/day etretinate was administered to 15 RA patients for the first 4 weeks, and then, the dosage was reduced to 0.5 mg/kg/day. However, 8 of 15 patients discontinued the treatment by week 12 because of severe liver involvement, and arthritis only improved in three patients [7].
The efficacy of 4-HPR (300 mg/day), a synthetic retinoid, was then evaluated in 12 severe and long-standing RA patients for 24 weeks [8]. Six of the 12 patients withdrew before the completion of the study because 2 exhibited toxic effects (visual problems), 2 flare, and 2 gastrointestinal bleeding. Histological changes and metalloproteinase gene expression were evaluated in synovial tissues pre- and post-medication using biopsy samples, and no patient met the predetermined Paulus criteria treatment response. In addition, no improvements were observed in the laboratory parameters, except for a modest decrease in C-reactive protein and no decrease in the mRNAs of metalloproteinases or collagenase in the synovial tissue.
Retinoids, such as etretinate and 4-HRP, were not effective in the treatment of RA patients in these studies. However, Am80, a ligand for RARα and β, but not for RARγ (Table 2 [56]), was used to effectively treat murine CIA [10]. Therefore, the different structures and binding abilities of retinoids to RAR or RXR may have affected the efficacy of these treatments. Am80 also induces fewer side effects than ATRA [12]. Therefore, Am80 may represent a possible retinoid treatment for RA. The effects of Am80 need to be examined in a large number of patients at several clinical stages of RA.
Seven patients with active lupus nephritis were treated with ATRA (10 mg/day) for 6 months in an open clinical trial. Clinical symptoms, proteinuria, and hematuria as well as serum albumin, creatinine, anti-DNA antibody, and CH50 levels were evaluated. Improvements were observed in the clinical symptoms, such as fever and skin rash, and laboratory findings, including proteinuria and anti-DNA antibody levels of four patients. Moreover, they reached the complete remission criteria of nephrotic syndrome. ATRA was not effective in the other three patients and was discontinued after 3 months. No patient had adverse effects to the ATRA therapy [57].
Thirty-one patients with systemic sclerosis (7 were treated with etretinate monotherapy, 5 with etretinate plus immunosuppressive therapies, 13 with immunosuppressive therapy only, and 6 with no treatment) were evaluated using the modified Rodnan total skin thickness score [58]. A significant improvement was defined as a 75 % reduction in the score. The skin thickness scores in 6 of the 7 patients treated with etretinate monotherapy, 3 of 5 with etretinate plus immunosuppressive therapy, 1 of 13 with immunosuppressive therapy only, and 0 of 6 with none therapy significantly improved. These findings suggested that etretinate may be a useful treatment for skin involvement associated with systemic sclerosis [58].
Retinoid trials for other rheumatic diseases, including vasculitis and myositis, have not yet been conducted. Am80 was effective for the treatment of myositis and vasculitis in animal models [11, 12]. Retinoids may also be used to treat these diseases.
There are currently no ongoing clinical trials on retinoids for rheumatic diseases. However, further clinical trials are expected for rheumatic diseases.
Conclusion
Retinoids have immunoregulatory functions, and treatments with retinoids were shown to be effective for arthritis, nephritis, myositis, and vasculitis in experimental animal models. Some clinical studies confirmed the efficacy of retinoids for lupus nephritis and systemic sclerosis. Therefore, retinoids may be a new therapy for rheumatic diseases; however, evidence for the positive impact of retinoids on rheumatic patients is scarce. Further clinical trials are needed to elucidate the efficacy of retinoids for the treatment of rheumatic diseases.
References
Tanaka Y (2012) Intensive treatment and treatment holiday of TNF-inhibitors in rheumatoid arthritis. Curr Opin Rheumatol 24:319–326
Takeuchi T, Miyasaka N, Inoue K, Abe T, Koike T, RISING study (2009) Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod Rheumatol 19:478–487
Harigai M, Koike R, Miyasaka N, PPuA-TNFTPS Group (2007) Pneumocystis pneumonia associated with infliximab in Japan. N Engl J Med 357:1874–1876
Komano Y, Tanaka M, Nanki T, Koike R, Sakai R, Kameda H et al (2011) Incidence and risk factors for serious infection in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a report from the Registry of Japanese Rheumatoid Arthritis Patients for Longterm Safety. J Rheumatol 38:1258–1264
Tombetti E, Franchini S, Papa M, Sabbadini MG, Baldissera E (2013) Treatment of refractory Takayasu arteritis with tocilizumab: 7 Italian patients from a single referral center. J Rheumatol 40:2047–2051
Rouster-Stevens KA, Ferguson L, Morgan G, Huang CC, Pachman LM (2014) Pilot study of etanercept in patients with refractory juvenile dermatomyositis. Arthritis Care Res (Hoboken) 66:783–787
Bird HA, Hill J, Sitton NG, Dixon JS, Wright V (1988) A clinical and biochemical evaluation of etretinate in rheumatoid arthritis. Rheumatol Int 8:55–59
Gravallese EM, Handel ML, Coblyn J, Anderson RJ, Sperling RI, Karlson EW et al (1996) N-[4-hydroxyphenyl] retinamide in rheumatoid arthritis: a pilot study. Arthritis Rheum 39:1021–1026
Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M et al (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317:256–260
Sato A, Watanabe K, Kaneko K, Murakami Y, Ishido M, Miyasaka N et al (2010) The effect of synthetic retinoid, Am 80, on T helper cell development and antibody production in murine collagen-induced arthritis. Mod Rheumatol 20:244–251
Ohyanagi N, Ishido M, Suzuki F, Kaneko K, Kubota T, Miyasaka N et al (2009) Retinoid ameliorates experimental autoimmune myositis, with modulation of Th cell differentiation and antibody production in vivo. Arthritis Rheum 60:3118–3127
Miyabe C, Miyabe Y, Miura NN, Takahashi K, Terashima Y, Toda E et al (2013) Am 80, a retinoic acid receptor agonist, ameliorates murine vasculitis through the suppression of neutrophil migration and activation. Arthritis Rheum 65:503–512
Chambon P (1996) A decade of molecular biology of retinoic acid receptors. FASEB J 10:940–954
Blomhoff R, Blomhoff HK (2006) Overview of retinoid metabolism and function. J Neurobiol 66:606–630
Mora JR, Iwata M, von Andrian UH (2008) Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 8(9):685–698
van de Kerkhof PC (2006) Update on retinoid therapy of psoriasis in: an update on the use of retinoids in dermatology. Dermatol Ther 19(5):252–263
Smith EV, Grindlay DJ, Williams HC (2011) What’s new in acne? An analysis of systematic reviews published in 2009–2010. Clin Exp Dermatol 36:119–122 quiz 23
Kagechika H (2002) Novel synthetic retinoids and separation of the pleiotropic retinoidal activities. Curr Med Chem 9:591–608
Ohnishi K (2007) PML-RARalpha inhibitors (ATRA, tamibaroten, arsenic troxide) for acute promyelocytic leukemia. Int J Clin Oncol 12:313–317
Miwako I, Kagechika H (2007) Tamibarotene. Drugs Today 43:563–568
Iwata M, Eshima Y, Kagechika H (2003) Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol 15:1017–1025
Cantorna MT, Nashold FE, Hayes CE (1995) Vitamin A deficiency results in a priming environment conducive for Th1 cell development. Eur J Immunol 25:1673–1679
Hoag KA, Nashold FE, Goverman J, Hayes CE (2002) Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J Nutr 132:3736–3739
Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B et al (2008) Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol 181:2277–2284
Engedal N, Ertesvag A, Blomhoff HK (2004) Survival of activated human T lymphocytes is promoted by retinoic acid via induction of IL-2. Int Immunol 16:443–453
Ertesvag A, Engedal N, Naderi S, Blomhoff HK (2002) Retinoic acid stimulates the cell cycle machinery in normal T cells: involvement of retinoic acid receptor-mediated IL-2 secretion. J Immunol 169:5555–5563
Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY (2004) Retinoic acid imprints gut-homing specificity on T cells. Immunity 21:527–538
Barrington RA, Schneider TJ, Pitcher LA, Mempel TR, Ma M, Barteneva NS et al (2009) Uncoupling CD21 and CD19 of the B-cell coreceptor. Proc Natl Acad Sci USA 106:14490–14495
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y et al (1999) Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162:3749–3752
Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R et al (1995) CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546–549
Chen Q, Ross AC (2005) Vitamin A and immune function: retinoic acid modulates population dynamics in antigen receptor and CD38-stimulated splenic B cells. Proc Natl Acad Sci USA 102:14142–14149
Chen Q, Ross AC (2007) Retinoic acid promotes mouse splenic B cell surface IgG expression and maturation stimulated by CD40 and IL-4. Cell Immunol 249:37–45
Ertesvag A, Aasheim HC, Naderi S, Blomhoff HK (2007) Vitamin A potentiates CpG-mediated memory B-cell proliferation and differentiation: involvement of early activation of p38MAPK. Blood 109:3865–3872
Xu Z, Pone EJ, Al-Qahtani A, Park SR, Zan H, Casali P (2007) Regulation of aicda expression and AID activity: relevance to somatic hypermutation and class switch DNA recombination. Crit Rev Immunol 27:367–397
Watanabe K, Sugai M, Nambu Y, Osato M, Hayashi T, Kawaguchi M et al (2010) Requirement for Runx proteins in IgA class switching acting downstream of TGF-beta 1 and retinoic acid signaling. J Immunol 184:2785–2792
Nagy L, Szanto A, Szatmari I, Széles L (2012) Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol Rev 92:739–789
Darmanin S, Chen J, Zhao S, Cui H, Shirkoohi R, Kubo N et al (2007) All-trans retinoic acid enhances murine dendritic cell migration to draining lymph nodes via the balance of matrix metalloproteinases and their inhibitors. J Immunol 179:4616–4625
Saurer L, McCullough KC, Summerfield A (2007) In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J Immunol 179:3504–3514
Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR et al (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204:1775–1785
Kuwabara K, Shudo K, Hori Y (1996) Novel synthetic retinoic acid inhibits rat collagen arthritis and differentially affects serum immunoglobulin subclass levels. FEBS Lett 378:153–156
Zhu L, Bisgaier CL, Aviram M, Newton RS (1999) 9-cis retinoic acid induces monocyte chemoattractant protein-1 secretion in human monocytic THP-1 cells. Arterioscler Thromb Vasc Biol 19:2105–2111
Mehta K, McQueen T, Tucker S, Pandita R, Aggarwal BB (1994) Inhibition by all-trans-retinoic acid of tumor necrosis factor and nitric oxide production by peritoneal macrophages. J Leukoc Biol 55:336–342
Na SY, Kang BY, Chung SW, Han SJ, Ma X, Trinchieri G et al (1999) Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB. J Biol Chem 274:7674–7680
Wang X, Allen C, Ballow M (2007) Retinoic acid enhances the production of IL-10 while reducing the synthesis of IL-12 and TNF-alpha from LPS-stimulated monocytes/macrophages. J Clin Immunol 27:193–200
Montemurro P, Barbuti G, Conese M, Gabriele S, Petio M, Colucci M et al (1999) Retinoic acid stimulates plasminogen activator inhibitor 2 production by blood mononuclear cells and inhibits urokinase-induced extracellular proteolysis. Br J Haematol 107:294–299
Yamada H, Mizuno S, Ross AC, Sugawara I (2007) Retinoic acid therapy attenuates the severity of tuberculosis while altering lymphocyte and macrophage numbers and cytokine expression in rats infected with Mycobacterium tuberculosis. J Nutr 137:2696–2700
Dzhagalov I, Chambon P, He YW (2007) Regulation of CD8+ T lymphocyte effector function and macrophage inflammatory cytokine production by retinoic acid receptor gamma. J Immunol 178:2113–2121
Gu B, Zhu Y, Zhu W, Miao J, Deng Y, Zou S (2009) Retinoid protects rats against neutrophil-induced oxidative stress in acute experimental mastitis. Int Immunopharmacol 9(2):223–229
Camisa C, Eisenstat B, Ragaz A, Weissmann G (1982) The effects of retinoids on neutrophil functions in vitro. J Am Acad Dermatol 6(4 pt 2 Suppl):620–629
Bohne M, Struy H, Gerber A, Gollnick H (1997) Effects of retinoids on the generation of neutrophil-derived reactive oxygen species studied by EPR spin trapping techniques. Inflamm Res 46:423–424
Coble BI, Dahlgren C, Molin L, Stendahl O (1987) Neutrophil function in psoriasis: effects of retinoids. Acta Derm Venereol 67:481–490
Yipp BG, Kubes P (2013) NETosis: how vital is it? Blood 122:2784–2794
Brinckerhoff CE, Coffey JW, Sullivan AC (1983) Inflammation and collagenase production in rats with adjuvant arthritis reduced with 13-cis-retinoic acid. Science 221:756–758
Trentham DE, Brinckerhoff CE (1982) Augmentation of collagen arthritis by synthetic analogues of retinoic acid. J Immunol 129:2668–2672
Nozaki Y, Yamagata T, Yoo BS, Sugiyama M, Ikoma S, Kinoshita K et al (2005) The beneficial effects of treatment with all-trans-retinoic acid plus corticosteroid on autoimmune nephritis in NZB/WF mice. Clin Exp Immunol 139:74–83
Kagechika H, Shudo K (2005) Synthetic retinoids: recent developments concerning structure and clinical utility. J Med Chem 48:5875–5883
Kinoshita K, Kishimoto K, Shimazu H, Nozaki Y, Sugiyama M, Ikoma S et al (2010) Successful treatment with retinoids in patients with lupus nephritis. Am J Kidney Dis 55:344–347
Ikeda T, Uede K, Hashizume H, Furukawa F (2004) The vitamin A derivative etretinate improves skin sclerosis in patients with systemic sclerosis. J Dermatol Sci 34:62–66
Conflict of interest
Dr. Nanki reports grants and personal fees from Chugai Pharmaceutical Co., LTD., grants and personal fees from Eisai Co., LTD., grants from Ono Pharmaceutical Co., LTD., grants and personal fees from Mitsubishi Tanabe Pharma Corporation., grants and personal fees from Takeda Pharmaceutical Co., LTD, grants and personal fees from AbbVie Inc., personal fees from UCB Japan Co. Ltd., personal fees from Astellas Pharma Inc., grants from Pfizer Japan Inc., personal fees from Bristol-Myers Squibb, personal fees from Santen Pharmaceutical Co., Ltd., personal fees from Taisho Toyama Pharmaceutical Co., Ltd., and grants from Asahi Kasei Pharma Corporation outside the submitted work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyabe, Y., Miyabe, C. & Nanki, T. Could retinoids be a potential treatment for rheumatic diseases?. Rheumatol Int 35, 35–41 (2015). https://doi.org/10.1007/s00296-014-3067-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-014-3067-2