Abstract
We assessed the effectiveness of interferential current (IFC) and transcutaneous electrical nerve stimulation (TENS) therapies in the management of carpal tunnel syndrome (CTS) compared with splint therapy, a standard treatment modality for CTS. This was a prospective, single-blinded, single-center, randomized, three-group parallel intervention study of 3 weeks duration. Efficacy was examined in the third week after the end of treatments. Subjects were assigned randomly to one of three groups: group I patients received splint therapy, group II patients received TENS applied on the palmar surface of the hand and the carpal tunnel, and group III patients underwent IFC therapy applied on the palmar surface of the hand and the volar surface of the forearm. TENS and ICF treatments were applied five times weekly for a total of 15 sessions. Group 1 patients were stabilized with volar wrist splints for 3 weeks. The efficacy of the therapies was assessed before initiation of therapy and at 3 weeks after completion of therapy using a visual analog scale (VAS), a symptom severity scale, the functional capacity scale of the BCTQ, and measurement of median nerve motor distal latency (mMDL) and median sensory nerve conduction velocity (mSNCV). Groups were compared pairwise using the Mann–Whitney U test to identify the source of differences between groups. The Wilcoxon test was used to analyze changes in variables over time within a group. In the VAS, BCTQ, MDL, and mSNCV, no significant difference was observed between the groups (p > 0.05). In the VAS, BCTQ, and mSNCV, statistically significant improvements were detected in all groups (p < 0.05). There was no statistically significant difference between TENS and splint therapy with respect to improvement in clinical scores, whereas IFC therapy provided a significantly greater improvement in VAS, mMDL, and mSNCV values than splint therapy (VAS: 4.80 ± 1.18 and 6.37 ± 1.18; p = 0.001, mMDL: 3.89 ± 0.88 and 4.06 ± 0.61; p = 0.001, mSNCV: 41.80 ± 1.76 and 40.75 ± 1.48; p = 0.010). IFC therapy provided a significantly greater improvement in VAS, symptom severity, functional capacity, and mMDL and mSNCV values than TENS therapy (VAS: 4.80 ± 1.18 and 6.68 ± 1.42; p < 0.001, symptom severity: 2.70 ± 1.03 and 3.37 ± 1.21; p = 0.015, functional capacity: 1.90 ± 1.21 and 2.50 ± 0.78; p = 0.039, mMDL: 3.89 ± 0.88 and 4.06 ± 0.88; p = 0.003, and mSNCV: 41.80 ± 1.76 and 41.38 ± 1.78; p = 0.021). IFC may be considered a new and safe therapeutic option for the treatment of CTS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carpal tunnel syndrome (CTS) occurs as a result of compression of the median nerve, traveling through the carpal tunnel. Initial clinical symptoms of CTS include nocturnal pain and paresthesias. Some patients may experience dry skin, sweating, and nutritional problems associated with autonomic (sympathetic) nervous system involvement. In advanced stages, weakness and atrophy of the thenar muscles may ensue [1, 2].
Various treatment approaches, individually or in combination, have been recommended in the literature for the conservative treatment of CTS. They include splint use, steroid injections, nonsteroidal anti-inflammatory drugs, diuretics, vitamin B6, physical therapy agents, activity modification, tendon/nerve gliding exercises, and change in occupation [3, 4].
Transcutaneous electrical nerve stimulation (TENS) therapy, a modality used commonly in physical therapy, is considered to be effective through a number of mechanisms, including inhibition of nociceptors, blockade of pain transmission via afferent nerves, sympathetic blockade, gate control, and release of endogenous opiates. A few studies have investigated the effectiveness of TENS in the treatment of CTS, but contradictory results have been reported [5, 6].
Interferential current (IFC) therapy is a safe physical therapy method that has been used for many years in the treatment of musculoskeletal system disorders. IFC has analgesic, anti-inflammatory, sympatholytic, local vasodilatory, and muscle stimulatory actions [7, 8]. There is no reported study on the effectiveness of IFC therapy in CTS. IFC therapy might be anticipated to have favorable effects on pain scores and potential vasomotor changes in CTS.
There is a continuing need for more effective therapeutic options for the treatment of CTS. In the present study, we assessed the effectiveness of IFC and TENS therapy in the management of CTS compared with splint therapy, a standard therapy for CTS.
Methods
This was a prospective, single-blinded, single-center, randomized three-group parallel intervention study of 3 weeks duration. Efficacy was examined in the third week after the end of the treatments.
Patients
Patients who were diagnosed with idiopathic CTS were enrolled in the study after admission to the physical therapy and rehabilitation outpatient clinic of from April 2013 to November 2013.
Ethics committee approval was obtained before initiation of the study. All patients were informed about the study before enrollment and gave written informed consent.
Inclusion criteria were the presence of paresthesia, pain, and/or vasomotor symptoms of the hand through the distribution of the median nerve, with persistence of symptoms for longer than 6 weeks; a positive result for Phalen’s maneuver and/or Tinel’s sign and/or the carpal compression test during physical examination of the wrist; and a mild-to-moderate intensity median nerve lesion during a neurophysiological examination (mild: sensory nerve conduction velocity in the third digit-wrist segment <44 ms and distal motor latency ≤4 ms; moderate: sensory nerve conduction velocity in the third digit-wrist segment <44 ms and distal motor latency >4 ms) [9].
Exclusion criteria were the presence of predisposing etiological factors for CTS (e.g., diabetes mellitus, acute trauma, rheumatic diseases, renal failure, pregnancy, hypothyroidism, and hyperthyroidism); the presence of conditions that might cause numbness in the hand, including cervical radiculopathy, cervical ribs, plexopathy, and polyneuropathy; pharmacological treatment with oral steroids or nonsteroidal anti-inflammatory drugs within the previous month; and participation in a physical therapy program with administration of steroid injection(s) within the previous 6 months.
Pretreatment evaluation
During the physical examination, Spurling’s test, Adson’s test, Tinel’s sign for median and ulnar nerves, Phalen’s maneuver, and carpal compression tests were evaluated. Motor and sensory examinations were conducted, and deep tendon reflexes and pathological reflexes were checked. Complete blood counts, erythrocyte sedimentation rate, fasting blood glucose, uric acid, creatinine, electrolytes, C-reactive protein, rheumatoid factor, liver function tests, and thyroid function tests were conducted. Patients were questioned about pain, paresthesia, loss of strength, and vasomotor symptoms.
Median nerve motor distal latency (mMDL) and median sensory nerve conduction velocity (SNCV) measurements were used for electroneurophysiological examination. Pain intensity was rated using a visual analog scale (VAS 0–10 cm) in all patients. Symptomatic and functional assessments were performed using the Boston carpal tunnel syndrome questionnaire (BCTQ).
Therapies
Patients were randomized into three groups; randomization was performed by a second investigator using a simple randomization method based on the sequential admission of patients. For patients with bilateral CTS, both hands were included in the same group but examinations were made for each hand individually. Group I patients received splint therapy, group II received TENS, and group III had IFC therapy. TENS and IFC therapies were provided by the same physiotherapist.
Splint therapy (group I)
Patients were asked to wear a wrist-hand resting splint during night sleep for 3 weeks. The splints were made of elastic cotton fabric with a Velcro bandage on the dorsal side and supported by an aluminum bar. They were applied on the symptomatic hands of the patients to keep the wrist in its neutral position during 0–15° extension. The splint was designed to cover the proximal half of the palmar area and the distal one-third of the forearm, allowing movement of the metacarpophalangeal joints in all directions.
TENS therapy (group II)
Conventional TENS (Chattanooga Intellect Legend XT 2 Channel Combination System, USA) electrodes (35 × 45 mm) were applied as described previously [5]. Negative electrodes were placed on the carpal ligament, and positive electrodes were placed on the palmar area of the hand, with a layer of conductive gel applied to the area. The device was set at a pulse rate of 100-Hz frequency and a stimulation period of 80 ms. Each TENS session lasted 20 min, and a total of 15 sessions (5 per week) were completed [10].
IFC therapy (group III)
The IFC device was adjusted to a base frequency of 4,000 Hz, with a modulation frequency range of 20 Hz, ΔF of 10 Hz, and slope of 1/1 in quadripolar mode [11]. Two electrodes were placed at the 1/3 mid portion of the volar area of the forearm; of the second pair, one was placed on the palmar area of the hand and the other was placed on the thenar area while the patient was lying in a supine position with the elbow and wrist extended and the volar surfaces pointing upwards. Electrode pairs were placed ensuring that they were at least 2.5 cm apart. IFC therapy was conducted for a total of 15 sessions (five sessions per week), each lasting 20 min.
Medical treatment
Throughout the study, patients were allowed to use paracetamol (1 g/day) as needed for treatment of pain, except on the assessment days.
Efficacy measures
Electroneurophysiological examinations were conducted: the mMDL and mSNCV. Pain was assessed using a VAS. The symptom severity scale and the functional capacity scales of the BCTQ were also used.
Electroneurophysiological examinations
Patients with a clinical diagnosis of CTS were evaluated electrophysiologically to confirm the diagnosis. All electrophysiological examinations were performed by the same physician. Prior to the examination, the hands of the patients were prepared for the procedure by asking the patients to rest for 10 min at room temperature, 22–24 °C. A Dantec Keypoint (Medtronic, Skovlunde, Denmark) electroneurophysiological examination unit was used.
For mMDL, recordings were made using supramaximal stimulation by placing the actively recording electrode on the abductor pollicis brevis muscle and the reference electrode on the distal area of the first digit of the hand, ensuring that the stimulator electrode cathode and active electrode were 8 cm apart.
For mSNCV, recordings were made at the wrist using supramaximal stimulation by placing ring-type electrodes (active and reference electrodes) on the metacarpophalangeal and distal interphalangeal joints of the second digit, ensuring that the stimulator electrode and active electrode were 14 cm apart.
For the VAS, a visual scale (0–10 cm) was used for assessment of average pain levels at the hands and fingers in the patients during the previous week. A rating of “0” indicated no pain and “10” indicated the most severe, intolerable pain [12].
The BCTQ (symptom severity scale) contains 11 questions about symptoms. There are five answers for each item, rated on a five-point scale. The mean score is calculated by dividing the total score by the number of items. A higher score indicates more severe symptoms [13].
The BCTQ (functional capacity scale) contains eight questions. There are five answers for each item, rated on a five-point scale. The mean score is calculated by dividing the total score by the number of items. A higher score indicates lower functional capacity [13].
All study patients were assessed before treatment and at 3 weeks after completion of treatment using the efficacy measures described. Baseline and final controls were performed by a physician who was blinded to the patient group assignments.
Statistical analyses
The SPSS software (ver. 16.0 for Windows, SPSS Inc., Chicago, IL) was used for statistical analyses. The nonparametric Kruskal–Wallis test was used to compare means of independent variables between the groups because the sample size in each group failed to meet normal distribution criteria. Groups were compared pairwise using the Mann–Whitney U test to identify the source of differences between the groups. The Wilcoxon test was used for analyses of changes in variables over time within a group. The χ 2 test was used to compare categorical variables. Parametric values are expressed as means ± SDs. A p value < 0.05 was considered to indicate statistical significance in all comparisons.
Results
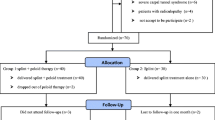
In total, 108 patients with CTS were considered; 87 of them met the criteria of the study. Of them, 12 patients declined to undergo the treatment. Thus, 75 patients were enrolled. Twelve patients who then failed to take part in the treatment regimen fully or to attend follow-up visits regularly were excluded. Thus, in total, 63 patients completed the study (43 females, 20 males; Fig. 1). The splint group consisted of 22 patients (33 wrists; 11 bilateral), the TENS group consisted of 20 patients (31 wrists; 11 bilateral), and the IFC group consisted of 21 patients (30 wrists; 9 bilateral). Figure 1 presents a study flow diagram.
The mean age of the study patients was 35.4 ± 4.2 years for group I, 34.2 ± 5.2 years for group II, and 34.9 ± 4.8 years for group III. There was no statistically significant difference between the groups in the duration of symptoms or the average body mass index (p > 0.05; Table 1). Between-groups comparisons for the same scores and values showed that there was no significant difference between the three groups in pretreatment VAS, symptom severity, functional capacity scores, or mMDL or mSNCV values (p > 0.05).
There was no significant difference between TENS and splint therapy with respect to improvement in clinical scores (p > 0.05). IFC therapy provided a significantly greater improvement in VAS, mMDL, and mSNCV values than splint therapy (VAS scores: 4.80 ± 1.18 and 6.37 ± 1.18; p = 0.001, mMDL scores: 3.89 ± 0.88 and 4.06 ± 0.61; p = 0.001, mSNCV scores: 41.80 ± 1.76 and 40.75 ± 1.48; p = 0.010). IFC therapy provided a significantly greater improvement in VAS, symptom severity, functional capacity, and mMDL and mSNCV values than TENS therapy (VAS scores: 4.80 ± 1.18 and 6.68 ± 1.42; p < 0.001, symptom severity scores: 2.70 ± 1.03 and 3.37 ± 1.21; p = 0.015, functional capacity scores: 1.90 ± 1.21 and 2.50 ± 0.78; p = 0.039, mMDL scores: 3.89 ± 0.88 and 4.06 ± 0.88; p = 0.003, and mSNCV scores: 41.80 ± 1.76 and 41.38 ± 1.78; p = 0.021) (Table 2).
No serious complication was associated with the treatments in any group, and all patients generally tolerated the treatments well. Only two patients in the TENS group experienced mild tenderness at the application site.
Discussion
We evaluated the effectiveness of TENS and IFC versus splint therapy in CTS patients. While there was not a significant difference between TENS and splint therapy with respect to improvement in clinical scores, IFC therapy generally provided greater improvement in clinical scores than TENS or splint therapy.
CTS occurs most commonly in the third to fifth decades of life and women suffer more than men, with a ratio of 3:1. Previous studies have reported that CTS affects 0.1–0.5 % of the general population [1]. Consistent with the literature, our study sample included patients with a mean age of 35 years and ~70 % were women.
Manente et al. [14] reported a significant improvement in pain scores in patients undergoing splint therapy for 4 months versus a control group. Akalin et al. [15] showed early, significant, and comparable clinical improvements in groups receiving splint therapy alone and splint therapy in combination with tendon- and nerve-stretching exercises for CTS. In the present study, which included an assessment of early outcomes of splinting, pain and function were improved significantly in splint users. However, although a significant improvement was observed in the median nerve conduction velocity, there was no significant difference in distal latency measurements during the electrophysiological examinations. It is possible that a longer duration of both therapy and examination is needed to observe sufficient improvement in all of the electrophysiological parameters.
TENS has long been used for the treatment of pain and has been used to treat pain associated with reflex sympathetic dystrophy, phantom limbs, and peripheral nerve injuries [16]. The efficacy of TENS has been demonstrated mainly for the relief of acute pain conditions and episodes of postoperative incisional pain [17]. C-fibers that carry the pain signal are known to inhibit transmitting neurons (the spinothalamic tract neuron pool) by stimulating an inhibitory intermediate neuron. This action of C-fibers is abolished, and sensory impulses carried by A fibers are able to excite transmission neurons more readily when TENS is applied [18]. Other proposed mechanisms of action for TENS include transient elevations in serotonin levels, increased cellular ATP, and decreased inflammation [16]. Naeser et al. [19] reported that TENS provided regression of pain scores, sensory latencies of the median nerve, and Phalen’s and Tinel’s signs in CTS patients, whereas placebo patients did not show differences in any parameter. A study by Casale et al. [20] evaluating the comparative effectiveness of a laser (combined 830–1,064 nm, radiating dose: 250 J/cm2) and TENS (100 Hz TENS, rectangular waves; 80 ms width) for the treatment of CTS reported that while the laser provided significant improvements in both pain and neurophysiological parameters, only TENS improved pain scores to a significant degree. Similarly, we observed significant improvements in pain and functioning scores in patients who received TENS therapy in the present study. However, in contrast to Casale et al.’s study, we also observed significant mSNCVs. This improvement might have been associated with one of the proposed mechanisms of TENS, which is the ability to increase excitation potential of the nerve impulses carried by A fibers. However, given that there was no significant difference between the effectiveness of TENS and splint therapy for CTS, splinting alone may be a more practical and cost-effective therapeutic approach.
There are reports on the effectiveness of IFC therapy for the management of lumbar disc hernia, fibromyalgia, knee pain, and shoulder discomfort; generally, they have indicated improvements in clinical scores. IFC therapy is believed to be effective for the management of musculoskeletal disorders through several mechanisms, including gate control, release of endogenous opiates, local pumping action, increased local circulation via autonomic nerves, and the removal of chemicals that stimulate pain receptors [21]. We believe that it is significant that we observed improvements in pain, functioning, and neurophysiological scores in patients who received IFC therapy, being the first study to evaluate the effectiveness of IFC in the treatment of CTS. The clinical improvements observed may involve the mechanisms of action of IFC described here.
Another important finding of the present study was that IFC therapy provided greater improvement in VAS and neurophysiological scores than TENS or splint therapy. In this context, we believe that IFC may provide an increase in local circulation and a decrease in interstitial edema as a result of its stimulatory and pumping effects on the forearm and hand muscles. This proposed mechanism may contribute to improved transmission along the median nerve.
Limitations of our study include the small sample size, especially for evaluating the findings of electroneurophysiological exams, and the failure to evaluate the clinical conditions of patients immediately after administration of study treatments and over the longer term. We were also unable to assess the effectiveness of other TENS and IFC regimens using different modes and frequencies.
In conclusion, our results indicate the potential for the use of IFC as a new and safe therapeutic option for the management of CTS. However, further studies on larger numbers of patients, using different IFC therapy regimens and longer follow-up periods, are needed to confirm our results.
References
Tanaka S, Wild DK, Seligman PJ et al (1994) The US prevalence of self-reported carpal tunnel syndrome: 1988 national health interview survey data. Am J Public Health 84(11):1846–1848
Greer BG, Jenkins WM, Roberts R (1992) Carpal tunnel syndrome: a challenge for rehabilitation. J Rehabil 24(2):43–46
Piazzini DB, Aprile I, Ferrara PE et al (2007) A systematic review of conservative treatment of carpal tunnel syndrome. Clin Rehabil 21(4):299–314
Kostopoulos D (2004) Treatment of carpal tunnel syndrome: a review of the non-surgical approaches with emphasis in neural mobilization. J Bodyw Mov Ther 8:2–8
Naeser MA, Hahn KA, Lieberman BE, Branco KF (2002) Carpal tunnel syndrome pain treated with low-level laser and microamperes transcutaneous electric nerve stimulation: a controlled study. Arch Phys Med Rehabil 83(7):978–988
Casale R, Damiani C, Maestri R, Wells CD (2013) Pain and electrophysiological parameters are improved by combined 830-1064 high-intensity LASER in symptomatic carpal tunnel syndrome versus transcutaneous electrical nerve stimulation. A randomized controlled study. Eur J Phys Rehabil Med 49(2):205–211
Jarit GJ, Mohr KJ, Waller R, Glousman RE (2003) The effects of home interferential therapy on post-operative pain, edema, and range of motion of the knee. Clin J Sport Med 3(1):16–20
Facci LM, Nowotny JP, Tormem F, Trevisani VF (2011) Effects of transcutaneous electrical nerve stimulation (TENS) and interferential currents (IFC) in patients with nonspecific chronic low back pain: randomized clinical trial. Sao Paulo Med J 129(4):206–216
DeLisa JA, Mackenzie K, Baran EM (1994) Manual of nerve conduction velocity and clinical neurophysiology. Raven Press, New York
Casale R, Damiani C, Maestri R, Wells CD (2013) Pain and electrophysiological parameters are improved by combined 830-1064 high-intensity LASER in symptomatic carpal tunnel syndrome versus Transcutaneous Electrical Nerve Stimulation A randomized controlled study. Eur J Phys Rehabil Med 49:205–211
Facci LM, Nowotny JP, Tormem F, Trevisani VF (2011) Effects of transcutaneous electrical nerve stimulation (TENS) and interferential currents (IFC) in patients with nonspecific chronic low back pain: randomized clinical trial. Sao Paulo Med J 129(4):206–216
Price DD, McGrath PA, Rafii A, Buckingham B (1983) The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 17:45–56
Levine DW, Simmons BP, Koris MJ et al (1993) A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Jt Surg Am 75(11):1585–1592
Manente G, Torrieri F, Di Balassio F, Staniscia T, Romano F, Uncini A (2001) An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve 24:1020–1025
Akalin E, El O, Peker O (2002) Treatment of carpal tunnel syndrome with nerve and tendon gliding exercises. Am J Phys Med Rehabil 81(2):108–113
Canthen JC, Renner EJ (1975) Transcutaneus and peripheral nevre for chronic pain states. Surg Neurol 11:102–104
Meyler WJ, de Jongste MJ, Rolf CA (1994) Clinical evaluation of pain treatment with different pain syndromes. Clin J Pain 10:22–27
Chen ZQ, Xu W, Lin Y (1986) Identification of cortico-thalamic neurons: involvement of cortical descending modulation in acupuncture analgesia. J Tradit Chin Med 6(3):195–200
Naeser MA, Hahn K-AK, Lieberman BE et al (2002) Carpal tunnel syndrome pain treated with low-level laser and microamperes transcutaneous electric nerve stimulation: a controlled study. Arch Phys Med Rehabil 83:978–988
Casale R, Damiani C, Maestri R, Wells CD (2013) Pain and electrophysiological parameters are improved by combined 830-1064 high-intensity LASER in symptomatic carpal tunnel syndrome versus Transcutaneous Electrical Nerve Stimulation. A randomized controlled study. Eur J Phys Rehabil Med 49(2):205–211
Fuentes JP, Olivo SA, Magee DJ, Gross DP (2010) Effectiveness of interferential current therapy in the management of musculoskeletal pain: a systematic review and meta-analysis. Phys Ther 90(9):1219–1238
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koca, I., Boyaci, A., Tutoglu, A. et al. Assessment of the effectiveness of interferential current therapy and TENS in the management of carpal tunnel syndrome: a randomized controlled study. Rheumatol Int 34, 1639–1645 (2014). https://doi.org/10.1007/s00296-014-3005-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-014-3005-3