Abstract
Joint proprioceptive deficit is documented in a variety of musculoskeletal conditions including osteoarthritis, ligament and meniscal injuries, and individuals with increased joint hypermobility, such as those with Ehlers–Danlos. No systematic reviews have assessed joint proprioception in people with benign joint hypermobility syndrome (BJHS). This study addresses this to determine whether people with BJHS exhibit reduced joint proprioception, and, if so, whether this is evident in all age groups. The search strategy was conducted on 31st January 2013. The published literature was assessed using the databases: AMED, CINAHL, MEDLINE, EMBASE, PubMed and the Cochrane Library. Unpublished literature and trial registries were assessed including: OpenGrey, the WHO International Clinical Trials Registry Platform, Current Controlled Trials, the UK National Research Register Archive. All studies comparing the proprioceptive capability of people with and without BJHS were included. Study methodological quality was assessed using the CASP appraisal tool. Meta-analysis techniques were used when study homogeneity permitted. Five studies including 254 people were identified. People with BJHS demonstrated statistically significantly poorer lower limb joint position sense (JPS) (p < 0.001) and threshold detection to movement (p < 0.001) than those without BJHS. The evidence for upper limb proprioceptive difference was less clear, with no statistically significant difference between the cohorts for shoulder JPS (p = 0.10), but a statistically significant difference in finger JPS (p < 0.001). One study which assessed childhood BJHS reported reduced knee proprioceptive capability in those with BJHS (p < 0.001). To conclude, lower limb joint proprioception is reduced in those with BJHS compared to non-BJHS cohorts, whilst unclear in the upper limb.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign joint hypermobility syndrome (BJHS) is one of the most common heritable connective tissue disorders [1]. It is associated with joint laxity, instability and pain [1]. It has been associated with abnormalities in genes coding for elastin, fibrillin, tenascin and collagen (principally type I), and manifests as decreased stiffness and stability from tendons, ligaments, joint capsules and skin [2, 3]. BJHS has also been associated with demineralised bone and nerve receptors [4, 5]. Its incidence ranges from one [6] to 31 % [7] of adults and is five times more prevalent in females [4]. The diagnosis of BJHS is currently based entirely on clinical grounds, with the Brighton criteria most commonly accepted for the diagnosis of adults [8]. This system consists of the Beighton score (a 9-point system, assessing multi-joint laxity) in addition to questions on symptoms such as pain and dislocation [8]. The literature about children and young people with BJHS is scarce. Indeed, there is significant doubt as to whether the Brighton score is applicable to children and young people, and no recent attempt to validate a more appropriate scoring system for this group [9].
BJHS presents with excessive joint motion, which can cause pain, reduced joint stability and motion coordination, and decreased joint position sense (JPS). Consequentially, these joints may be more vulnerable to damage and abnormal postures since abnormal weight-bearing on articular surfaces may lead to chondral damage and osteoarthritis [2, 10, 11].
Proprioception has been defined as a joint’s ability to determine its position (JPS), detect movement (kinaesthesia) and sense of resistance to force [12]. It derives from mechanoreceptors in the muscle, joint capsule, tendon, ligaments and cutaneous tactile receptors [12, 13]. Motion stimulates mechanoreceptors to provide proprioceptive sensation. Proprioception encompasses several different components including JPS, velocity, movement detection and force [14]. It is an essential sense to assist in the coordination of movement during normal activities of daily living as well as physically demanding tasks [15]. However, trauma and pathological processes can damage this feedback system, which may make the limb more susceptible to injury [16–18].
Proprioceptive deficit has been reported as evident in other cohorts with increased joint hypermobility, most notably those people diagnosed with Ehlers–Danlos [12, 19]. However, no systematic reviews have been undertaken to assess proprioception (JPS and kinaesthesia) in people diagnosed with BJHS. This systematic review aims to answer the following questions: Do people with BJHS have reduced joint proprioception? If so, is this evident in all age groups?
Materials and methods
The study methodology was conducted in accordance with the PRISMA guidelines [20].
Search strategy
Primary search strategy
The electronic databases: MEDLINE, EMBASE, CINAHL, AMED (via Ovid), Cochrane Library, PubMed and the PEDro databases were reviewed from their inceptions to January 2013. The MEDLINE MeSH, keyword search terms and Boolean operators adopted are presented in Table 1. These were modified to accommodate each search database.
Secondary search
Unpublished and trial registry databases including OpenGrey, the WHO International Clinical Trials Registry Platform, Current Controlled Trials and the UK National Research Register Archive were reviewed from inception to January 2013. Reference lists of all potentially eligible study were searched. Corresponding authors for each included study were contacted and asked to review the search results and to identify any omitted studies.
Eligibility criteria
Inclusion criteria
In order to answer the research question full publications of comparative observational studies assessing proprioception through any means between people with BJHS to an asymptomatic non-joint hypermobile cohort. Based on current convention within the literature, joint hypermobility was regarded as a score of ≥4 using the Beighton scoring system [6]. Symptomatic was regarded as the current report of pain, instability or any symptoms limiting the functional or perceived capabilities of an individual necessitating a healthcare consultation. There was no restriction of the age, gender, ethnicity, co-morbidities, previous injury rate or severity of joint hypermobility syndrome. No restrict was placed on the age, publication source or language of the included studies to ensure that all potentially eligible studies were included. Therefore, we included studies published in all languages including English, French, Spanish, German and Chinese, seeking translation services when required.
Exclusion criteria
Individuals with other genetic/hypermobility connective tissue disorders such as Marfans and Ehlers–Danlos were excluded (except type III as these participants were included). Single case studies were also excluded.
The results of the search strategy were screened independently by two reviewers (TS, EJ) by assessing each title and abstract. Full texts were obtained for potentially eligible papers. These were read to determine final eligibility. Disagreements were resolved by consensus through discussion between the two reviewers.
Outcome measures
To determine whether proprioception is affected, joint proprioceptive-based measurements were assessed in individuals with and without BJHS. No restriction was placed on the outcome measures used to assess this domain; however, expected measurements used within the literature included: JPS, postural sway, stabilometry and threshold detection methods. The upper or lower limbs could be used for this assessment. This was considered appropriate since there remains no 'gold standard' method of assessing proprioception [21].
Data extraction and critical appraisal
For each eligible paper, data were independently extracted by one reviewer (EJ) and verified by a second (VE). Data extracted included: characteristics of participants (both symptomatic and asymptomatic controls) including age, gender, duration of symptoms, method of diagnosis, degree of joint hypermobility (frequently assessed using the Beighton scoring system), co-morbidities, method of assessing proprioception, findings. Data were tabulated on a standard data extraction form.
The methods used in each study were appraised. This critical appraisal was based on the CASP ‘case control’ tool. This tool was considered appropriate since it has been widely utilised in the review of previous musculoskeletal studies [22–24].
Each paper was reviewed by one reviewer (EJ) and then independently verified by a second (VE). Any difference in appraisal score was discussed and agreed by consensus.
Data analysis
Data homogeneity was assessed by visually examining the data extraction findings and forest-plot trends. When methodological heterogeneity was evident, a qualitative narrative review of results was made. When methodological homogeneity was evident, pooling results as part of a meta-analysis were deemed appropriate, when participant characteristics, intervention, follow-up period and outcomes measurement assessed were similar.
Statistical heterogeneity was assessed using the chi-squared and I-squared statistical tests. When p ≤ 0.10 and I-squared ≥20 %, a random effects model was performed. When p > 0.10 and I-squared <20 %, a fixed effects model was used. Continuous outcomes such as actual angle error in JPS results, mean difference (MD) or standardised mean difference (SMD) was calculated. Standardised mean difference was used when there was variability in the method used to assess a specific type of proprioceptive domain. For each calculation, the mean effect estimate, 95 % confidence interval and p value were presented. Analyses were made to assess upper and lower limb proprioceptive capabilities separately.
All statistical analyses were conducted on RevMan version 5.0 (Review Manager (RevMan) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.)
Results
Search strategy
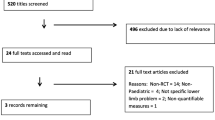
The search results are summarised in Fig. 1. A total of 116 citations were identified from the search strategy. Of these, 18 were thought potentially eligible. Of these, five were eligible after reviewing the full text. Two studies [25, 26] were excluded since it was not possible to confirm whether their hypermobile cohort could be termed as having BJHS and were symptomatic. Additional reasons for excluding studies included: not presenting results of JPS data (n = 4), editorials, commentary or review papers (n = 7), duplicate citations on the search strategy (n = 32) or irrelevant to the research questions (n = 66).
Methodological assessment
A summary of the CASP methodological appraisal results are presented in Table 2. This indicates that the methodological quality of the current evidence base was moderate.
The strengths to the literature include the provision of a clearly focused research question (100 % studies), appropriate use of a case–control study design (100 %) and clearly defining the recruitment processes adopted (100 %). All studies also appropriately recruited non-symptomatic control cohorts without hypermobility (100 %), matched their cases and controls to important characteristics such as age and gender (100 %), employed valid and reliable outcome measurements to assess proprioception and clinical outcomes (100 %), and correctly used inferential and descriptive statistical tests to analyse their data (100 %). However, only one study [27] based their sample on a power calculation, and only Mallik et al. [28] presented their results with 95 % CIs to provide an indication on the precision of their findings, and no studies blinded their assessors to whether participants were from BJHS or non-BJHS groups (100 %). All studies were recruited from hospital settings, suggesting clinically generalisablity.
Characteristics of included studies
A summary of the included study characteristics is presented in Table 3. All five studies were case–control designs. These studies consisted of 254 participants, 123 with JHS, 131 non-joint hypermobility controls. The JHS cohort consisted of 14 males and 109 females with a mean age of 19.7 years (SD = 10.0) ranging from 11.9 years [27] to 39.6 years [12]. The control cohort consisted of 34 males and 97 females, with a mean age of 19.6 (SD = 10.3) ranging from 11.5 years [27] to 40.2 years [12]. Only Fatoye et al. [27] exclusively assessed proprioception in a childhood population. Rombaut et al.’s [12] study recruited participants diagnosed with Ehlers–Danlos syndrome type III.
Joint hypermobility was classified as a Beighton score of equal or greater than 4 points, as recommended by Grahame and Hakim [8]. Mean Beighton score was presented in three studies [11, 12, 27]. This ranged from 5.9 [11] to 6.9 points [12] in the JHS group.
As Table 3 demonstrates, proprioception was assessed through a number of different measurements. Two studies assessed upper limb JPS, one study assessed shoulder JPS [12], whilst Mallik et al. [28] assessed finger JPS. Joint position sense in these studies was assessed using isokinetic dynamometry in Rombaut et al.’s [12] study, and passive-angle reproduction with a finger silhouette model [28].
Lower limb, specifically knee joint, proprioception was assessed in four studies [4, 11, 12, 27]. Lower limb JPS was assessed in three studies, all using an angle reproduction method on an isokinetic dynamometer [4, 12, 27]. Knee threshold detection of motion was assessed in Fatayo et al. [27] and Hall et al.’s [4] studies.
Outcomes: lower limb (knee)
Threshold detection
Two studies compared motion threshold detection measurement in the knee of people with BJHS compared to non-hypermobile control samples [4, 27]. As Fig. 2 demonstrates, there was a statistically significantly poorer threshold detection to movement in those with BJHS compared to the non-hypermobile cohort (MD:0.43; 95 % CI 0.29, 0.57; p < 0.001). Fatoye et al. [27] solely assessing children reported a similar trend with a significant difference in threshold detection measurement between their BJHS and control cohorts (p < 0.001).
Joint position sense
In those studies that compared JPS [4, 12, 27], those with BJHS were significantly poorer at detecting joint position than non-hypermobile controls (SMD = 0.90; 95 % CI 0.49, 1.31; p < 0.001; Fig. 3). This finding was reflected when Fatoye et al.’s [27] paediatric cohort were assessed individually (p < 0.001).
Outcomes: upper limb (shoulder)
One study assessed JPS in the shoulder [12]. On meta-analysis of their various measurements, there was no statistically significant difference between the two groups (MD = 0.96; 95 % CI −0.19, 2.10; p = 0.10; Fig. 4).
Upper limb (finger)
The assessment of right index finger proximal interphalangeal joint in Mallik et al.’s [28] active joint reproduction test reported statistically significantly greater angle error in the BJHS compared to the control tests (p < 0.0001). Mean actual errors in the BJHS group were 5.8° (SD = 1.3°) compared to 3.9° (SD = 1.2°) in the non-hypermobile control group.
Discussion
The results of this study indicate that people with BJHS demonstrate poorer lower limb JPS and threshold detection to movement with statistically different results from those without joint hypermobility. Few studies have assessed the upper limb. There was no statistically significant difference between the cohorts for shoulder JPS (p > 0.10). Only one study looked at finger JPS and found those with BJHS were less able to detect finger position [28].
The findings from this systematic review should be interpreted with some caution. A number of key methodological limitations were highlighted by the CASP appraisal process (Table 2). Most notably, this identified that only one study reviewed based their sample size on a power calculation. Thus, a type II statistical error may have occurred [29]. Further analysis of larger BJHS cohorts may provide differing results which should be investigated. Furthermore, none of the studies blinded their assessors as to whether participants were from non-BJHS control cohorts or from the BJHS cohort. This therefore permits the potential for assessor bias from impacting from the results. Both of these limitations should be considered when designing future research in this area.
A number of hypotheses have been developed to account for this reduced proprioception in those with BJHS. These have included reduced capability due to joint reception damage from excessive joint mobility [12, 27, 28]. This may also imply deterioration of proprioception with age. Secondly, general enhancement of number of activated mechanoreceptions in the joint may occur from excessive motion [12, 28]. Therefore, when smaller motion is provided, reduced capsular and ligament stretching occur resulting in reduced perception and detection of knee joint receptions [12, 28]. Finally, in those cohorts who presented with pain, this symptom is documented to reduce proprioceptive acuity [30, 31]. Jeremiah and Alexander [26] commented that the increased laxity associated with BJHS may affect the feedback mechanisms with alterations in sensitivity of reception organs, altering afferent input [11, 26, 28]. Reduction in proprioception may therefore be attributed to impaired feedback mechanisms, pain or a combination of both.
The results indicated no statistically significant difference between those with BJHS and non-hypermobile cohorts for JPS testing of the upper limb, This is contrary to previous authors who suggested that JPS is reduced globally in BJHS [4, 11, 28]. No explanation can be made for this, although, with a small and under-powered cohort, Mallik et al. [28] reported non-significant findings which could be attributed to type II statistical error [30]. Whether there is a difference in the risk of injury and therefore damage to the joint capsule and subsequently mechanoreceptors in shoulder pathologies, or a difference in anatomical function and stability to account for this remains unclear. Further study, using sufficiently powered cohorts, would be required to explore whether this finding is supported by more rigorous testing.
Whilst this study has demonstrated that joint proprioception is reduced in those with BJHS, only one study has begun to assess the role of proprioceptive-based exercises in the management of this deficit. Sahin et al. [4] reported that an eight-week exercise programme significantly improved pain and function in those with BJHS (p < 0.03). However, it remains unclear what the optimal type of exercises is, the dosage of such exercise and whether there is a difference in outcome between the prescription of specific-proprioceptive exercises compared to more global strengthening/aerobic exercise programmes. Further study is therefore warranted on this population, particularly given the lower limb deficits in proprioception described in this study.
Only one study specifically assessed proprioceptive capability in children [27]. It was impossible to say whether under 16 year olds were included in all but two of the other study cohorts [11, 28] since only these studies presented the age range values for their cohorts (Table 3). Consistent with the adult evidence base, the authors concluded that there was a statistically significant difference in knee proprioceptive acuity [27]. The results of this study support previous reports of children with BJHS being ‘clumsy’ and presenting with coordination difficulties during functional tasks [32, 33]. This has been attributed to poor motor development as well as impaired proprioception in childhood populations [32]. No studies have compared motor development to proprioceptive capability longitudinally in cohorts with BJHS. Finally, whilst the tests involved assessed laboratory-based proprioception, further assessment would be needed to explore how this physiological deficit manifests in functional and everyday tasks. These areas would provide further insight into the relationship between motor development and proprioception between children and adults, and would have value for assessing their importance in this population’s activities of daily living.
To conclude, the results of this study indicate that joint proprioception is reduced in those with BJHS compared to non-hypermobile cohorts, with possible implications for coordination during functional tasks. Further study is now required to investigate to what degree this is specifically the case in children and young people with BJHS. Study on interventions to address this deficit will then be required.
References
Gazit Y, Nahir AM, Grahame R, Jacob G (2003) Dysautonomia in the joint hypermobility syndrome. Am J Med 115:33–40
Booshanam DS, Cherian B, Joseph CP, Mathew J, Thomas R (2011) Evaluation of posture and pain in persons with benign joint hypermobility syndrome. Rheumatol Int 31:1561–1565
Seçkin U, Tur BS, Yilmaz O, Yağci I, Bodur H, Arasil T (2005) The prevalence of joint hypermobility among high school students. Rheumatol Int 25:260–263
Sahin N, Baskent A, Ugurlu H, Berker E (2008) Isokinetic evaluation of knee extensor/flexor muscle strength in patients with hypermobility syndrome. Rheumatol Int 28:643–648
Grahame R (2000) Hypermobility–not a circus act. Int J Clin Pract 54:314–315
Beighton P, Solomon L, Soskolne CL (1973) Articular mobility in an African population. Ann Rheum Dis 32:413–418
Al-Rawi ZS, Al-Aszawi AJ, Al-Chalabi T (1985) Joint mobility among university students in Iraq. Br J Rheumatol 24:326–331
Grahame R, Bird HA, Child A (2000) The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). J Rheumatol 27:1777–1779
Bulbena A, Duró JC, Porta M, Faus S, Vallescar R, Martín-Santos R (1992) Clinical assessment of hypermobility of joints: assembling criteria. J Rheumatol 19:115–122
Helliwell PS (1994) Impaired proprioceptive acuity at the proximal interphalangeal joint in patients with the hypermobility syndrome. Br J Rheumatol 33:1192–1193
Hall MG, Ferrell WR, Sturrock RD, Hamblen DL, Baxendale RH (1995) The effect of the hypermobility syndrome on knee joint proprioception. Br J Rheumatol 34:121–125
Rombaut L, De Paepe A, Malfait F, Cools A, Calders P (2010) Joint position sense and vibratory perception sense in patients with Ehlers-Danlos syndrome type III (hypermobility type). Clin Rheumatol 29:289–295
Olsson L, Lund H, Henriksen M, Rogind H, Bliddal H, Danneskiold-Samsoe B (2004) Test-retest reliability of a knee joint position sense measurement method in sitting and prone position. Adv Physiother 6:37–47
Stillman BC (2002) Making sense of proprioception. The meaning of proprioception, kinaesthesia and related terms. Physiother 88:667–676
Kinzey SJ, Armstrong CW (1998) The reliability of the star-excursion test in assessing dynamic balance. J Orthop Sports Phys Ther 27:356–360
Jerosch J, Prymka M (1996) Knee joint proprioception in patients with posttraumatic recurrent patella dislocation. Knee Surg Sports Traumatol Arthrosc 4:14–18
Jerosch J, Prymka M (1996) Proprioception and joint stability. Knee Surg Sports Traumatol Arthrosc 4:171–179
Baker V, Bennell K, Stillman B, Cowan S, Crossley K (2002) Abnormal knee joint position sense in individuals with patellofemoral pain syndrome. J Orthop Res 20:208–214
Galli M, Rigoldi C, Celletti C, Mainardi L, Tenore N, Albertini G, Camerota F (2011) Postural analysis in time and frequency domains in patients with Ehlers-Danlos syndrome. Res Dev Disabil 32:322–325
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Smith TO, Davies L, Hing CB (2013) A systematic review to determine the reliability of knee joint position sense assessment measures. Knee 20:162–169
Smith TO, Walker J, Russell N (2007) Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc 15:1301–1314
Reilly KA, Barker KL, Shamley D (2006) A systematic review of lateral wedge orthotics-how useful are they in the management of medial compartment osteoarthritis? Knee 13:177–183
Postle K, Pak D, Smith TO (2012) Effectiveness of proprioceptive exercises for ankle ligament injury in adults: a systematic literature and meta-analysis. Man Ther 17:285–291
Mebes C, Amstutz A, Luder G, Ziswiler HR, Stettler M, Villiger PM, Radlinger L (2008) Isometric rate of force development, maximum voluntary contraction, and balance in women with and without joint hypermobility. Arthritis Rheum 59:1665–1669
Jeremiah HM, Alexander CM (2010) Do hypermobile subjects without pain have alteration to the feedback mechanisms controlling the shoulder girdle? Musculoskelet Care 8:157–163
Fatoye F, Palmer S, Macmillan F, Rowe P, van der Linden M (2009) Proprioception and muscle torque deficits in children with hypermobility syndrome. Rheumatology (Oxford) 48:152–157
Mallik AK, Ferrell WR, McDonald AG, Sturrock RD (1994) Impaired proprioceptive acuity at the proximal interphalangeal joint in patients with the hypermobility syndrome. Br J Rheumatol 33:631–637
Bland M (2006) An introduction to medical statistics, 3rd edn. Oxford University Press, Oxford
Felson DT, Gross KD, Nevitt MC, Yang M, Lane NE, Torner JC, Lewis CE, Hurley MV (2009) The effects of impaired joint position sense on the development and progression of pain and structural damage in knee osteoarthritis. Arthritis Rheum 61:1070–1076
Hirata RP, Ervilha UF, Arendt-Nielsen L, Graven-Nielsen T (2011) Experimental muscle pain challenges the postural stability during quiet stance and unexpected posture perturbation. J Pain 12:911–919
Adib N, Davies K, Grahame R, Woo P, Murray KJ (2005) Joint hypermobility syndrome in childhood. A not so benign multisystem disorder? Rheumatology (Oxford) 44:744–750
Ferrell WR, Tennant N, Sturrock RD, Ashton L, Creed G, Brydson G, Rafferty D (2004) Amelioration of symptoms by enhancement of proprioception in patients with joint hypermobility syndrome. Arthritis Rheum 50:3323–3328
Acknowledgments
We would like to thank the Information Services at the University of East Anglia’s Library for their assistance in gathering the academic papers required for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, T.O., Jerman, E., Easton, V. et al. Do people with benign joint hypermobility syndrome (BJHS) have reduced joint proprioception? A systematic review and meta-analysis. Rheumatol Int 33, 2709–2716 (2013). https://doi.org/10.1007/s00296-013-2790-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-013-2790-4