Abstract
Rheumatoid arthritis is a chronic inflammatory disease characterized by the destruction of articular cartilage and bone in a chronic phase. Pathology of rheumatoid arthritis suggests autoimmunity linked to inflammation. In our study, rheumatoid arthritis was induced in Wistar rats by intradermal injections of 100 μl of emulsion containing bovine type II collagen in complete Freund’s adjuvant at the base of the tail. Disease developed about 13 ± 1 days after immunization and treatment with hesperidin (HES) at a dose of 160 mg kg−1 body weight was given after onset of disease daily until 20th day. The effect of treatment in the rats was monitored by clinical scoring, biochemical parameters and histological evaluations in joints. A steady increase in the articular elastase, nitric oxide and lipid peroxidation was observed in joints of arthritic rats as compared to control, whereas a significant decrease in reduced glutathione, superoxide dismutase activity and catalase was observed in collagen-induced arthritis rats as compared to control group. The results from the present work indicate that the treatment with hesperidin was effective in bringing about significant changes on all the parameters studied in collagen-induced arthritis rats. These data confirm that erosive destruction of the joint cartilage in collagen-induced arthritis is due free radicals released by activated neutrophils and produced by other biochemical pathways. In the present study, an attempt has been made to amelioration of the disease process by a natural product. These results suggest that oral administration of HES could be effective for treating human RA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several models of rheumatoid arthritis have been established, each of these has advantages and disadvantages. Collagen-induced arthritis (CIA) is most widely used animal model of inflammatory polyarthritis with clinical and pathological features similar to human rheumatoid arthritis (RA) [1, 2]. Rheumatoid arthritis is a chronic inflammatory disease that leads to joint destruction mediated in part by the migration of inflammatory cells into the synovial tissue [3]. There has been progress in defining the aetiology, but overall pathogenesis of this disease is still not fully understood. It has been suggested that an autoimmune-mediated attack on the joints has a crucial role in the pathogenesis of RA, which start a cascade of inflammatory events that lead to degradation of tissues [4].
The generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is thought to be key modulators of joint inflammation and might have a role in the pathogenesis of rheumatoid arthritis [5–8]. These are highly reactive transient chemical species with the potential to initiate cellular damage in cartilage directly and damage components of the extracellular matrix either directly or indirectly by up-regulating mediators of matrix degradation [9]. These reactive molecules are formed during normal aerobic metabolism in human body. During infection or inflammation, phagocytes generate free radicals which damage biomolecules that lead to altered functions and disease [10]. It has been reported that ROS damage antioxidant systems and that RA patients are thus exposed to oxidant stress and lipid peroxidation because of the reduced endogenous antioxidant defence system [11].
Presently, there are several effective therapies to treat RA, but they are associated with number of serious side effects. These treatments include methotrexate, anti-TNF and non-steroidal anti-inflammatory drugs [12]. Therefore, to avoid these drugs, many patients opt for other substitutes like natural anti-inflammatory drugs to alleviate their arthritis pain [13].
Common dietary substances having anti-inflammatory activity may have important health implication, as they can afford protection or modulate the onset and severity of arthritis. These may be natural sources like flavonoids, terpenes, quinones, catechins and alkaloids [14]. Hesperidin is a flavanone glycoside composed of the flavanone hesperetin and the disaccharide rutinose. Normal intake of hesperidin or related compounds has been observed to show no signs of toxicity [15, 16]. Hesperidin possesses a wide range of pharmacological properties, such as antioxidant [17], anti-inflammatory [18, 19], hypolipidaemic [20] and anti-carcinogenic [21] actions. Hesperidin is also reported to have anti-rheumatic effects [22, 23]. The aim of the present project was therefore to assess the possible ability of hesperidin in limiting inflammation and joint cartilage erosion in CIA model of experimental animals.
Materials and methods
Chemicals
Hesperidin, Freund’s adjuvant complete (CFA), N-methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide and Griess Reagent system were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Collagen type II from bovine nasal septum was purchased from Elastin Products Co, INC, Owensville, MO, USA. Thiobarbituric acid (TBA), trichloroacetic acid (TCA), 5-5′dithio-bis-2-nitrobenzoicacid (DTNB), nitrobluetetrazolium (NBT), ethylene diamine tetra-acetic acid (EDTA), xanthine, xanthine oxidase and tris–hydrochloride were purchased from SD Fine chemicals India. All other routine chemicals used in this investigation were of research grade.
Animals
Male Wistar rats (150–170 g) were used. They were kept in the Central Animal House of Hamdard University in colony cages at an ambient temperature of 25 ± 2 °C and relative humidity 45–55 % with 12 h light/dark cycles after initial acclimatization for about 1 week. They had free access to standard rodent pellet diet and water ad libitum. The experimental study was conducted in accordance with the Institutional Animal Ethics Committee of the University.
Induction of collagen-induced arthritis (CIA)
Arthritis was induced in rats as described previously [24]. Collagen Type II from bovine nasal septum was dissolved in 0.05 M acetic acid at a concentration of 2 mg/ml and emulsified with an equal volume of complete Freund’s adjuvant (CFA) containing 1 mg/ml Mycobacterium tuberculosis H37 RA and stored on ice before use. Rats were immunized intradermally at about 1.5 cm distal from the base of the tail. All rats were randomly assigned to three groups of six animals each. The first group served as control and saline was given orally, the second was collagen-induced arthritis (CIA) and the third was administered hesperidin (160 mg/kg body weight) daily, starting from day 0 followed by CIA. The dose of hesperidin was selected from literature and in vivo studies demonstrating its antioxidant and anti-inflammatory efficacy [25] in other diseases without any resultant toxicity. 
Measurement of clinical severity of arthritis
For macroscopic assessment of arthritis, the thickness of each affected hind paw was measured every 3rd day with digital calliper (YAMAYO, Japan) and the diameter was expressed as an average for inflamed hind paws per rats. Evaluation of joint inflammation was performed by a blinded independent observer with no knowledge of the treatment protocol. The severity of the arthritis was quantified every 3rd day by a clinical score measurement [26] from 0 to 4.
Preparation of cell-free extract of the knee joints
At the end of experiment, animals were sacrificed by cervical dislocation. Arthritic and non-arthritic joints were removed and cut into small pieces and homogenized in 5 vol of 50 mM Tris–HCl buffer, pH 7.4 containing 0.1 M NaCl and 0.1 % Triton X-100 with 1 vol. of fine glass powder using a mortar and pestle. The extract was sonicated for 20 s. The homogenate was centrifuged at 3,000×g for 5 min, and the resulting supernatant was stored at −80 °C until further analysis.
Biochemical analyses
Biochemical parameters were carried out in articular joints. The supernatant was centrifuged at 3,000×g for 5 min to estimate LPO and GSH and at 12,000×g for 5 min, and resultant PMS was used to carry out elastase, catalase, SOD and NO assay. TBARS determination was done according to Utley et al. [27], and GSH was measured following the method of Sedlak and Lindsay [28]. ELA levels in the articular joints were evaluated as an index of polymorphonuclear leucocyte (PMNs) accumulation and activation in the inflamed tissue as described earlier [29]. Catalase activity was determined by the method of Sinha [30]. SOD activity was measured according to the method described by Beauchamp and Fridovich [31]. NO level was determined by Griess method [32], and protein concentration of the tissue was measured as per Bradford method [33].
Histological examinations
Rats were sacrificed at day 21 by cervical dislocation. Knee joints were removed and fixed in 4 % formaldehyde. After decalcification in 5 % formic acid, the samples were processed for paraffin embedding [34]. Tissue sections (5 μm thick) were stained with haematoxylin–eosin for light microscope examination.
Statistical analysis
Results are expressed as mean ± SEM. Statistical analysis of the data was done by applying the analysis of variance (ANOVA), followed by Tukey’s test for all parameters. The p value <0.05 was considered statistically significant.
Results
Clinical severity of disease
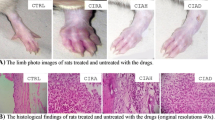
CIA developed rapidly in rats immunized with CII. Clinical signs of the disease were erythema of one or more ankle joints, followed by involvement of the metatarsal and interphalangeal joints, first appeared in the hind paws between 8 and 9 days after CII immunization, with a 100 % CIA incidence by day 13 ± 1 (Table 1). Hesperidin treatment suppressed the evolution of collagen-induced arthritis. There was no macroscopic evidence of either hind paw erythema or oedema in the control group.
Cartilage ELA activity
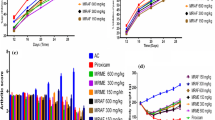
ELA activity was assayed on day 21 in the different groups. Very low ELA concentration was detected in the joints of control rats (55.9 ± 0.45 ng/g protein); however, elevated level of this enzyme was seen in CIA group (184.7 ± 0.39 ng/g protein). Administration of the Hesperidin showed a significant reduction (p < 0.05) in neutrophil activation and infiltration at a dosage of 160 mg (Fig. 1).
Assessment of TBARS
Determination of TBARS in the articular cartilage was performed to estimate free radical damage to biological membranes (Fig. 2). Low level of TBARS was seen in the control group at the end of the experiment (day 21), and these values were considered normal. In contrast, a significant increase (p < 0.05) in TBARS production was found in the joints of CIA rats. Treatment with hesperidin decreased TBARS concentrations by inhibiting lipid peroxidation in the cartilage tissue.
GSH assay
GSH content was evaluated to estimate endogenous defences against hydrogen peroxide formation. Table 2 represents the changes in GSH content evaluated in the joints (day 21) in the different groups. A marked decrease (p < 0.05) in GSH concentration was found in the joint of CIA rats. Treatment with a dose of 160 mg of hesperidin significantly inhibited the decrease in GSH levels (p < 0.05).
SOD activity
SOD activity was evaluated to estimate endogenous defences against superoxide anions. Table 2 summarizes the articular content of SOD in the different groups. In control animals, normal SOD activity was 9.16 ± 0.59 Units/mg of protein. In contrast, a significant decrease in this antioxidant level was seen in CIA rats (5.92 ± 0.29). Administration of 160 mg dose of hesperidin significantly (p < 0.01) restored the SOD level.
Catalase activity
Table 2 shows cartilage catalase activity evaluated on the day 21 in the joints of different groups. In the control group, catalase activity was 16.83 ± 0.49 μmol H2O2 consumed/min/mg protein. On the contrary, a substantial reduction in this enzyme was observed in the cartilage of CIA rats (9.45 ± 0.27). Also in this case, the treatment with 160 mg dose of hesperidin was significantly (p < 0.01) effective to replenish level of the enzyme.
Nitric oxide analysis
Analysis of nitrite is summarized in Fig. 3. In the control group, the nitrite concentration was 3.02 ± 0. 19 μmol/mg wet tissue while CIA group showed high nitrite content (9.36 ± 0.35). Treatment with hesperidin lowered the nitrite content significantly (p < 0.01) as compared to the CIA group.
Histology
Persistent with the biochemical alterations, the histological findings (Fig. 4) revealed massive cell infiltration in the CIA group. Bone suffered resorption and pannus formation, whereas synovial hyperplasia was consistent finding. The treatment with hesperidin ameliorated the changes at histological level and was able to restore these alterations to a greater extent.
Histological findings. Massive and diffuse polymorphonuclear cellular flux (white arrows) in the collagen-induced arthritic rats (b) in comparison with the control rats (a). Cellular infiltration leads to the cartilage erosion mainly by inflammatory necrosis (b). Reduction in cellular flux and cartilage erosion, which was evidenced by the minimum necrotic lesions (black arrows) in the rats fed with hesperidin (c). Original magnification ×40
Statistical analysis
All the data presented were mean ± SEM. The variation within the groups was analysed by ANOVA. Post hoc analysis was carried out by Tukey’s multiple comparisons. Any variations with p < 0.05 were considered to be significant.
Discussion
Our investigation was focused on the evaluation of the antioxidant and anti-arthritic activity of hesperidin by using collagen-induced arthritis model. This model is widely used to check anti-arthritic potential of drugs as it possesses many of the cellular and humoral immune events found in human rheumatoid arthritis [35]. Free radicals have long been implicated as mediators of tissue damage in rheumatoid arthritis [6]. They are released in response to activation of phagocytic cells such as macrophages and neutrophils, undergo an oxidative burst that produces highly toxic ROS that are designed to kill the invading pathogens [36] which are present in large amounts into the surrounding tissue [37, 38]. When the endogenous antioxidant defences are overcome, the resulting production of free radicals induces impairment and destruction of the affected joint constituents such as synovial fluid, cartilage and other articular constituents [24].
In collagen-induced arthritis, hesperidin demonstrated significant antioxidant potential. Its anti-arthritic efficacy was also evident from the reduction in joint swelling and severity throughout the observation period. To further validate the anti-arthritic activity of the hesperidin, we evaluated articular elastase activity, which is a marker for joint inflammation. Its activity is directly proportional to the accumulation and activation of polymorphonuclear leucocytes in the inflamed tissue as it is released from stimulated granulocytes at the site of injury. The inflammation so caused by the infiltrating cells leads to the release of ROS and RNS [30–32]. Lipid peroxidation is considered a decisive mechanism of the injury that occurs during rheumatoid arthritis. The large amount of TBARS found is consistent with the occurrence of damage mediated by free radicals. We suggest that the decrease in elastase activity observed in our study might be due to the inhibition of lipid peroxidation levels and the consequent decrease in the reduction of chemotactic peroxide [39].
Lipid peroxidation has been implicated in the pathogenesis of cancer, atherosclerosis, degenerative diseases, and inflammatory arthritis [40]. During lipid peroxidation, lipid peroxyl radicals are produced that can lead to cell membrane damage. Matrix degradation arising from cytokine-stimulated chondrocytes has been shown to be primarily due to lipid peroxidation [41]. The large amount of TBARS found in the arthritic group is consistent with the occurrence of damage mediated by free radicals. Treatment with hesperidin produced a significant decrease in TBARS.
Free radicals production that occurs during development of arthritis in the articular cartilage leads to decreased GSH and SOD levels as a result of their consumption during oxidative stress and cellular lysis [42–44]. This decrease contributes to increased cellular damage by favouring attack by free radicals. Hesperidin replenished GSH and SOD level significantly probably by scavenging free radicals and as a result helped to maintain the integrity of cellular membranes in the injured cartilage.
Nitric oxide (NO) is an important signalling molecule. It is synthesized from oxidative deimination of l-arginine by nitric oxide synthase (NOS) and produces the highly reactive peroxynitrite radical (ONOO−), nitrate and nitrite. Reactive nitrogen species, in particular NO, interfere with interactions between chondrocytes and the extracellular matrix [45–47]. NO can also increase chondrocyte apoptosis. In the present study, increased NO level has been detected in arthritic group as similar to those previously reported in synovial fluids of patients with rheumatoid arthritis [36]. Treatment with hesperidin produced a significant decrease in nitric oxide level.
The biochemical alterations were further supported by histopathological observations of the tissues. The arthritic group showed higher number of infiltrating cells, extensive bone degradation and synovial hyperplasia, which are hallmarks of RA. Bone degradation was characterized by absence of the trabecular structure in the bone where as synovial hyperplasia was noticed as proliferation of synoviocytes to the cartilages and bone. Treatment with hesperidin was able to restore the histological changes to almost normal.
In conclusion, present study highlights the role of inflammatory cells in conjunction with reactive oxygen and nitrogen species in the development of the pathology of rheumatoid arthritis. Moreover, increased level of the nitrite in the articulate tissue suggests further work to be done in order to understand peroxynitrite-mediated damage in rheumatoid arthritis. These observations suggest that hesperidin may be a clinically viable protective agent as it abolished a number of factors known to be involved in RA pathogenesis and against a variety of conditions where cellular damage is a consequence of oxidative stress.
References
Haqqi TM, David CS (1990) T-cell receptor V beta genes repertoire in mice. Possible role in resistance and susceptibility to type II collagen-induced arthritis. J Autoimmun 3(2):113–121
Myers LK, Rosloniec EF, Cremer MA, Kang AH (1997) Collagen-induced arthritis, an animal model of autoimmunity. Life Sci 61(19):1861–1878
Lee DM, Weinblatt ME (2001) Rheumatoid arthritis. Lancet 358(9285):903–911. doi:10.1016/S0140-6736(01)06075-5
Distler JH, Jungel A, Huber LC, Seemayer CA, Reich CF III, Gay RE, Michel BA, Fontana A, Gay S, Pisetsky DS, Distler O (2005) The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci USA 102(8):2892–2897. doi:10.1073/pnas.0409781102
Blake DR, Merry P, Unsworth J, Kidd BL, Outhwaite JM, Ballard R, Morris CJ, Gray L, Lunec J (1989) Hypoxic-reperfusion injury in the inflamed human joint. Lancet 1(8633):289–293
Bauerova K, Bezek A (1999) Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. Gen Physiol Biophys 18:15–20
Hagfors L, Leanderson P, Skoldstam L, Andersson J, Johansson G (2003) Antioxidant intake, plasma antioxidants and oxidative stress in a randomized, controlled, parallel, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr J 2:5. doi:10.1186/1475-2891-2-5
Walwadkar SD, Suryakar AN, Katkam RV, Kumbar KM, Ankush RD (2006) Oxidative stress and calcium-phosphorus levels in Rheumatoid arthritis. Indian J Clin Biochem 21(2):134–137
Hitchon CA, El-Gabalawy HS (2004) Oxidation in rheumatoid arthritis. Arthritis Res Ther 6(6):265–278
Lunec J (1990) Free radicals: their involvement in disease processes. Ann Clin Biochem 27(Pt 3):173–182
Campo GM, Avenoso A, Campo S, Ferlazzo AM, Altavilla D, Calatroni A (2003) Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res Ther 5(3):R122–R131
Shivaprasad H (2011) Immunomodulation of autoimmune arthritis by herbal CAM. Evid Based Complement Alternat Med 2011:986797
Ahmed S, Anuntiyo J, Malemud CJ, Haqqi TM (2005) Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evid Based Complement Alternat Med 2(3):301–308. doi:10.1093/ecam/neh117
Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakkara AB, Sung B, Aggarwal A, Aggarwal BB (2007) Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol 7(3):344–351. doi:10.1016/j.coph.2007.03.002
Erlund I, Meririnne E, Alfthan G, Aro A (2001) Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr 131(2):235–241
Garg A, Garg S, Zaneveld LJ, Singla AK (2001) Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res 15(8):655–669. doi:10.1002/ptr.1074
Tirkey N, Pilkhwal S, Kuhad A, Chopra K (2005) Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol 5:2. doi:10.1186/1471-2210-5-2
Emim JA, Oliveira AB, Lapa AJ (1994) Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice. J Pharm Pharmacol 46(2):118–122
Guardia T, Rotelli AE, Juarez AO, Pelzer LE (2001) Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 56(9):683–687
Monforte MT, Trovato A, Kirjavainen S, Forestieri AM, Galati EM, Lo Curto RB (1995) Biological effects of hesperidin, a citrus flavonoid (note II): hypolipidemic activity on experimental hypercholesterolemia in rat. Farmaco 50(9):595–599
Tanaka T, Makita H, Kawabata K, Mori H, Kakumoto M, Satoh K, Hara A, Sumida T, Ogawa H (1997) Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 18(5):957–965
Rotelli AE, Guardia T, Juarez AO, de la Rocha NE, Pelzer LE (2003) Comparative study of flavonoids in experimental models of inflammation. Pharmacol Res 48(6):601–606
Kawaguchi K, Maruyama H, Kometani T, Kumazawa Y (2006) Suppression of collagen-induced arthritis by oral administration of the citrus flavonoid hesperidin. Planta Med 72(5):477–479. doi:10.1055/s-2005-916254
Haqqi TM, Anthony DD, Gupta S, Ahmad N, Lee MS, Kumar GK, Mukhtar H (1999) Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci USA 96(8):4524–4529
Li R, Li J, Cai L, Hu CM, Zhang L (2008) Suppression of adjuvant arthritis by hesperidin in rats and its mechanisms. J Pharm Pharmacol 60(2):221–228. doi:10.1211/jpp.60.2.0011
Larsson P, Kleinau S, Holmdahl R, Klareskog L (1990) Homologous type II collagen-induced arthritis in rats. Characterization of the disease and demonstration of clinically distinct forms of arthritis in two strains of rats after immunization with the same collagen preparation. Arthritis Rheum 33(5):693–701
Utley HG, Bernheim F, Hochstein P (1967) Effect of sulfhydryl reagents on peroxidation in microsomes* 1. Arch Biochem Biophys 118(1):29–32
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25(1):192–205
Yoshimura K, Nakagawa S, Koyama S, Kobayashi T, Homma T (1994) Roles of neutrophil elastase and superoxide anion in leukotriene B4-induced lung injury in rabbit. J Appl Physiol 76(1):91–96
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Sajad M, Zargan J, Chawla R, Umar S, Sadaqat M, Khan HA (2009) Hippocampal neurodegeneration in experimental autoimmune encephalomyelitis (EAE): potential role of inflammation activated myeloperoxidase. Mol Cell Biochem 328(1–2):183–188. doi:10.1007/s11010-009-0088-3
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Durie FH, Fava RA, Foy TM, Aruffo A, Ledbetter JA, Noelle RJ (1993) Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science 261(5126):1328–1330
Brand DD, Latham KA, Rosloniec EF (2007) Collagen-induced arthritis. Nat Protoc 2(5):1269–1275. doi:10.1038/nprot.2007.173
van der Vliet A, Eiserich JP, Halliwell B, Cross CE (1997) Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem 272(12):7617–7625
Knight JA (2000) Review: free radicals, antioxidants, and the immune system. Ann Clin Lab Sci 30(2):145–158
Babior BM (2000) Phagocytes and oxidative stress. Am J Med 109(1):33–44
Wills ED (1969) Lipid peroxide formation in microsomes. Relationship of hydroxylation to lipid peroxide formation. Biochem J 113(2):333–341
Bauerova K, Ponist S, Mihalova D, Drafi F, Kuncirova V (2011) Utilization of adjuvant arthritis model for evaluation of new approaches in rheumatoid arthritis therapy focused on regulation of immune processes and oxidative stress. Interdiscip Toxicol 4(1):33–39. doi:10.2478/v10102-011-0007-9ITX-4-033
Narendhirakannan RT, Limmy TP (2011) Anti-inflammatory and anti-oxidant properties of Sida rhombifolia stems and roots in adjuvant induced arthritic rats. Immunopharmacol Immunotoxicol. doi:10.3109/08923973.2011.605142
Khan MM, Ishrat T, Ahmad A, Hoda MN, Khan MB, Khuwaja G, Srivastava P, Raza SS, Islam F, Ahmad S (2010) Sesamin attenuates behavioral, biochemical and histological alterations induced by reversible middle cerebral artery occlusion in the rats. Chem Biol Interact 183(1):255–263. doi:10.1016/j.cbi.2009.10.003
Kiziltunc A, Cogalgil S, Cerrahoglu L (1998) Carnitine and antioxidants levels in patients with rheumatoid arthritis. Scand J Rheumatol 27(6):441–445
Hassan MQ, Hadi RA, Al-Rawi ZS, Padron VA, Stohs SJ (2001) The glutathione defense system in the pathogenesis of rheumatoid arthritis. J Appl Toxicol 21(1):69–73. doi:10.1002/jat.736
Tsubata M, Takagaki K, Hirano S, Iwatani K, Abe C (2011) Effects of flavangenol, an extract of French maritime pine bark on collagen-induced arthritis in rats. J Nutr Sci Vitaminol (Tokyo) 57(3):251–257
Szabo C, Thiemermann C (1994) Invited opinion: role of nitric oxide in hemorrhagic, traumatic, and anaphylactic shock and thermal injury. Shock 2(2):145–155
Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM (2008) Bioavailable constituents/metabolites of pomegranate (Punica granatum L) preferentially inhibit COX2 activity ex vivo and IL-1beta-induced PGE2 production in human chondrocytes in vitro. J Inflamm (Lond) 5:9. doi:10.1186/1476-9255-5-9
Acknowledgments
Author is grateful to Indian Council of Medical Research, for senior research fellowship (SRF) and to Central Council of Research in Unani Medicine (CCRUM), Department of AYUSH, Government of India for providing the financial support for this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umar, S., Kumar, A., Sajad, M. et al. Hesperidin inhibits collagen-induced arthritis possibly through suppression of free radical load and reduction in neutrophil activation and infiltration. Rheumatol Int 33, 657–663 (2013). https://doi.org/10.1007/s00296-012-2430-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2430-4