Abstract
Accumulating evidence suggests that defects in the function of CD4+CD25+ regulatory T cells (Tregs) are important in immune-mediated diseases such as rheumatoid arthritis. Here, we investigated the effects of various disease-modifying anti-rheumatic drugs (DMARDs) on Treg function. Tregs and CD4+CD25− effector T cells (Teffs) were isolated from peripheral blood mononuclear cells obtained from healthy adults. Isolated Tregs were cultured with the DMARDs methotrexate (MTX), sulfasalazine (SSZ), leflunomide (LEF), or infliximab (INF). We found that each DMARD had a different effect on Treg function. SSZ and LEF inhibited the anti-proliferative function of Tregs on cocultured Teffs and reduced Treg expression of Foxp3 mRNA, whereas MTX and INF did not.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CD4+CD25+ regulatory T cells (Tregs) play an important role in peripheral immune tolerance, serving to prevent autoimmune diseases such as rheumatoid arthritis (RA), which is characterized by chronic synovial inflammation that results in progressive cartilage and bone destruction. Tregs are a subset of CD4+ T cells that express high levels of CD25 and exhibit immunosuppressive activity. These cells are generated in the thymus and possibly in the periphery as well [1, 2]. It has been shown that Tregs are capable of suppressing CD4+CD25− effector T-cell (Teff) proliferation and cytokine production and directly suppress monocytes, macrophages, and dendritic cells, suggesting a role in both innate and adaptive immune responses [3, 4]. Although the exact mechanism of immune suppression by Tregs is controversial, it is thought to be mediated by a variety of cell contact-dependent and contact-independent mechanisms [5]. Antigen recognition and activation of the Tregs via the T-cell receptor is required to suppress immune response [6]. Tregs have been found to produce IL-10, IL-4, TGF-beta. However, there is a lack of evidence that these cytokines play a role in Treg-mediated suppression [5, 7, 8].
Forkhead box P3 (FoxP3), an intracellular marker for functional Tregs in both mice and humans, is critical for the development and function of Tregs [9–12]. In humans, Foxp3 expression is also induced in CD4+CD25− Teffs upon stimulation and leads to acquisition of a regulatory phenotype that is able to suppress in vitro proliferation of autologous CD4+CD25− T cells [13, 14]. Another marker for human Treg delineation is CD127, the interleukin 7 receptor-α (IL-7R-α) chain. It has been reported that the expression of CD127 inversely correlates with Foxp3 expression and Treg suppressor activity [15, 16].
In a study of antigen-induced arthritis, depletion of Tregs in immunized animals before arthritis induction was shown to exacerbate disease, whereas transfer of Tregs into immunized mice at the time of induction decreased the severity of disease [17]. It has been shown that early active, antirheumatic drug-naïve RA patients have a smaller proportion of Tregs in the peripheral blood than controls, whereas stable, well-controlled RA patients receiving therapy have Treg numbers similar to controls [18]. In addition, it has been reported that Tregs from RA patients are unable to suppress pro-inflammatory cytokine production, and treatment with anti-tumor necrosis factor (TNF)-α antibodies restores the capacity of Tregs to inhibit cytokine production [19]. TNF has been shown to inhibit suppressive activity and downregulate Foxp3 expression in Tregs [20]. These findings suggest that Treg suppressive function in patients with active RA is decreased significantly and can be restored by appropriate treatments. However, the direct effects of various disease-modifying anti-rheumatic drugs (DMARDs)—the mainstay in the treatment of RA—on Tregs have not yet been systematically addressed. An in vitro study showed that the suppressor function of umbilical cord blood-derived Tregs was modestly reduced by methotrexate (MTX), suggesting that such drugs may adversely affect the suppressive function of Tregs [21]. However, given that the function of Tregs in RA patients could be recovered after appropriate treatment (e.g., anti-TNF agents) and MTX is the first-line drug in the treatment of RA, it is unclear whether the function of Tregs are also reduced by MTX or other DMARDs, including sulfasalazine (SSZ) and leflunomide (LEF). The aim of this study was to evaluate the direct effects of these DMARDs (MTX, SSZ, and LEF) on the function of Tregs in peripheral blood.
Materials and methods
Isolation of CD4+CD25+ and CD4+CD25− T cells from peripheral blood mononuclear cells
Peripheral blood was obtained from five healthy volunteers after obtaining informed written consent. This study protocol was approved by the Asan Medical Center Institutional Review Board. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Ficoll/Plaque (Pharmacia Biotech, Uppsala, Sweden) at 400g for 30 min. CD4+ T cells were isolated from PBMCs by negative selection using an LD column (Miltenyi Biotech, Sunnyvale, CA, USA). Purified CD4+ T cells were incubated with anti-human CD25 magnetic beads (Miltenyi Biotech) and separated into CD4+CD25+ and CD4+CD25− fractions by positive selection using an LS column (Miltenyi Biotech). The selected CD4+CD25+ cell fractions were separated again over an LS column to achieve higher purities.
The isolated cells were activated by incubation with 100 U/ml recombinant human interleukin (IL)-2 (R&D Systems, Minneapolis, MN, USA), 1 μg/ml anti-CD3 antibody, and 1 μg/ml anti-CD28 antibody (Serotec Ltd., Oxford, UK) for 3 days. Purity was assessed using a fluorescence-activated cell sorter (FACS; Becton–Dickinson, Oxnard, NJ, USA). Isolated cells (2 × 105 cells in 90 μl of phosphate-buffered saline) were incubated with 10 μl RPE-conjugated mouse anti-human CD25 and FITC-conjugated mouse anti-human CD4 antibodies (Serotec Ltd., Kindlington, Oxford, UK) for 30 min.
Cell viability assay
The viability of isolated CD4+CD25+ T cells before and after culturing with each DMARD was assessed using an XTT-based cell proliferation kit (Roche, Karlsruhe, Germany). Briefly, activated CD4+CD25+ T cells (105 cells/well) were cultured with vehicle (control) or different doses of individual DMARDs (0.1, 1, 10, 100, and 1,000 nM MTX; 0.1, 1, 10, and 100 μg/ml SSZ; 1, 10, 30, and 100 μg/ml LEF; 1, 2.5, 10, and 100 μM INF) for 1 week. XTT labeling mixture (50 μl) and electron-coupling reagent (1 μl) were added to each well and plates were incubated for 18 h at 37 °C. Absorbance of wells was measured using a microtiter plate reader at 450 nm, with a reference wavelength of 650 nm. Cell viability assay results were used to determine a maximum dose of each DMARD at which cell viability was unaffected for use in subsequent experiments: MTX, 100 nM; SSZ, 100 μg/ml; LEF, 30 μM; INF, 10 μg/ml.
Proliferation and suppression assays
The suppressive function of isolated CD4+CD25+ Tregs on the proliferation of CD4+CD25− Teffs was assessed by coculturing-activated CD4+CD25+ Treg from healthy individuals with CD4+CD25− Teffs (105 cells/well) in 96-well plates at different ratios of Tregs and Teffs (1:1, 0.5:1, 0.25:1, 0:1). The cells were cultured with 10 μg/ml phytohemagglutinin (PHA; Sigma, St. Louis, MO, USA) for 7 days; 1 μCi [3H]thymidine (Amersham Pharmacia Biotech, Uppsala, Sweden) was added for the last 16 h. Cell proliferation was measured on the last day of culture using a β-counter (Perkin-Elmer LAS, Wellesley, MA, USA).
The effect of DMARDs on the suppressive function of CD4+CD25+ Tregs was determined by culturing activated CD4+CD25+ Treg (104 cells/well) with vehicle control or individual DMARDs (MTX, 100 nM; SSZ, 100 μg/ml; LEF, 30 μM; INF, 10 μg/ml) for 7 days. Cultures were performed in α-MEM (Gibco-BRL, Grand Island, NY) supplemented with 10 % FBS, 2 mM l-glutamine 1 %, 100 U/ml penicillin, and 100 μg/ml streptomycin with the medium replaced every 3 days. After removal of media to the extent possible (to minimize the possible effect of DMARDs on CD4+CD25− Teffs), CD4+CD25+ Tregs were cocultured with CD4+CD25− Teffs (104 cells/well) in 96-well plates. Cell proliferation assays were then performed as described above.
RNA extraction and reverse transcription-polymerase chain reaction for Foxp3 mRNA expression
Activated CD4+CD25+ Tregs (2 × 105 cells/well) were cultured with vehicle control or each DMARD (MTX, 100 nM; SSZ, 100 μg/ml; LEF, 30 μM; INF, 10 μg/ml) for 7 days and then lysed by addition of 1 ml TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was isolated, and first-strand cDNA was synthesized by incubating for 1 h at 37 °C with 1 μl M-MLV reverse transcriptase (200 U), 5 μl 5 × RT buffer (25 mM Tris–HCl, 375 mM KCl, 15 mM MgCl2, 50 mM DTT), 5 μl of a 2.5-mM dNTP mixture, and 0.5 μl RNase inhibitor, in accordance with the manufacturer’s instructions (Promega, Madison, WI, USA). Foxp3 mRNA was estimated by semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) using the primers 5′-ACA CCA CCC ACC ACC GCC ACT-3′ (sense) and 5′-TCG GAT GAT GCC ACA GAT GAA GC-3′ (antisense). The amplification protocol consisted of an initial denaturation at 95 °C for 2 min, followed by 30 cycles of denaturation at 95 °C for 20 s, annealing at 64 °C for 10 s, and extension at 72 °C for 50 s, followed by a final extension at 70 °C for 5 min. The PCR products were electrophoresed on 1.5 % agarose gels (Amersham Pharmacia Biotech, Uppsala, Sweden) and stained with ethidium bromide (0.5 μl/ml) for 30 min.
Statistical analysis
The Mann–Whitney test was used to compare the effects of each DMARD with that of vehicle control. Comparison between 3 or more experimental groups was performed by Kruskall–Wallis test or Jonckheere-Terpstra test where appropriate. p-Values <0.05 were considered statistically significant. All data were analyzed using SPSS v14.0 software (SPSS, Chicago, IL, USA).
Results
Isolation of CD4+CD25+ Tregs and CD4+CD25− Teffs from PBMCs
CD4+CD25+ and CD4+CD25− T cells were isolated from PBMCs by negative and repeated positive selection, and their purity was assessed by FACS. The purity of CD4+CD25+ Tregs and CD4+CD25− Teffs was 95.1 and 98.1 %, respectively (Fig. 1a). CD25+ T cells constituted about 3 % of the CD4+ T-cell population.
a Purification of CD4+CD25+ and CD4+CD25− T cells from a healthy individual. CD4+CD25+ and CD4+CD25− T cells were isolated from PBMCs by negative and positive selection, and purity was assessed by FACS. b Suppression of CD4+CD25− effector T cells (Teffs) proliferation by CD4+CD25+ regulatory T cells (Tregs). Activated Tregs isolated from healthy individuals were cocultured with Teffs (105 cells/well) in 96-well plates at different ratios of Tregs and Teffs (1:1, 0.5:1, 0.25:1, 0:1). Cells were cultured in wells containing PHA for 7 days; proliferation was determined by [3H]thymidine uptake. The suppressive effect of Tregs on Teffs proliferation was proportional to the ratio of Tregs to Teffs (p < 0.05). Data represent the mean ± SD of 3 independent experiments
Suppressive function of CD4+CD25+ Tregs on CD4+CD25− Teffs
Activated CD4+CD25+ Tregs from healthy individuals were cocultured with CD4+CD25− Teffs (105 cells/well) in 96-well plates at different ratios of Tregs and Teffs (1:1, 0.5:1, 0.25:1, 0:1) and incubated in the presence of PHA for 7 days. Cell proliferation assays using [3H]thymidine uptake showed that the suppressive effect of CD4+CD25+ Tregs on CD4+CD25− Teff proliferation was proportional to the ratio of CD4+CD25+ Tregs to CD4+CD25− Teffs (Fig. 1b).
Cell viability after culturing with different doses of various DMARDs
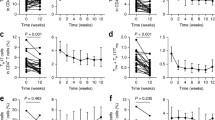
To assess the effects of DMARDs on the survival of CD4+CD25+ Treg, we cultured activated CD4+CD25+ Tregs with different doses of each DMARD (MTX, SSZ, LEF, INF) for 1 week, and then performed XTT-based cell proliferation assays. We found that CD4+CD25+ Treg proliferation was sustained in the presence of all tested doses of each DMARD (Fig. 2).
Viability of CD4+CD25+ regulatory T cells (Tregs) after culturing with different doses of each DMARD. Activated Tregs from healthy individuals were cultured with various doses of methotrexate (MTX), sulfasalazine (SSZ), leflunomide (LEF), or infliximab (INF) for 7 days, and then cell viability was assessed using an XTT-based cell proliferation assay. Cell viability was not affected by any tested dose of individual DMARDs (p = not significant for each drug). Data represent the mean ± SD of 3 independent experiments
Effects of DMARDs on the suppressive function of CD4+CD25+ Tregs
To assess the effect of each DMARD on the anti-proliferative function of CD4+CD25+ Tregs, we cultured activated CD4+CD25+ Tregs with each DMARD for 7 days and then plated CD4+CD25− Teffs as a responder in 96-well plates at a 1:1 ratio (104 cells/well). Cell proliferation assays using [3H]thymidine uptake showed that the anti-proliferative function of CD4+CD25+ Tregs was not influenced by MTX or INF, but was significantly reduced by SSZ (p < 0.01) and LEF (p < 0.01) (Fig. 3).
Effect of various disease-modifying anti-rheumatic drugs (DMARDs) on the anti-proliferative function of CD4+CD25+ regulatory T cells (Tregs) from healthy individuals. CD4+CD25− effector T cells (Teffs) were used as responder cells. After activation and culture with various DMARDs for 7 days, Tregs (104 cells/well) were cocultured with Teffs (104 cells/well) in 96-well plates. Cells were cultured in wells containing PHA for 7 days; proliferation was determined by [3H]thymidine uptake. The anti-proliferative function of Tregs was significantly reduced by sulfasalazine (SSZ) and leflunomide (LEF), but was not influenced by methotrexate (MTX) or infliximab (INF). * p < 0.05. Data represent the mean ± SD of 3 independent experiments
The effects of DMARDs on Foxp3 mRNA expression in CD4+CD25+ Tregs
To assess the effect of each DMARD on Foxp3 expression in CD4+CD25+ Tregs, we cultured activated CD4+CD25+ Tregs with each DMARD for 7 days and then assayed Foxp3 mRNA expression by RT-PCR. Foxp3 mRNA expression in CD4+CD25+ Tregs was reduced by SSZ and LEF, but not by MTX or INF (Fig. 4).
Foxp3 mRNA expression in CD4+CD25+ regulatory T cells (Tregs) from a healthy individual. Activated Tregs were cultured with various DMARDs for 7 days. Foxp3 mRNA expression in Tregs was assayed by RT-PCR. Foxp3 mRNA expression in Tregs was reduced by sulfasalazine (SSZ) and leflunomide (LEF), but not by methotrexate (MTX) or infliximab (INF)
Discussion
CD4+CD25+ Tregs are known to play a crucial role in preventing the development of autoimmune disease. Given that RA, an autoimmune disease, develops in the presence of Tregs in peripheral blood and synovial fluid, it is possible that Teffs are less susceptible to Treg-mediated suppression [22], or that Tregs in RA lose their regulatory function.
There are some controversies on the role of Treg in pathogenesis of RA. Some studies reported that the proportion of CD4+CD25+ Tregs in the peripheral blood of RA patients is smaller than controls [23], whereas other studies reported different results [19, 22, 24]. Regarding the function of Tregs, some studies reported that the synovial fluid CD4+CD25+ Tregs show increased regulatory activity compared with peripheral blood Tregs [22], while Ehrenstein et al. [19] have demonstrated that Tregs derived from peripheral blood of patients with active RA are defective in their ability to suppress pro-inflammatory cytokine production, but not proliferation. This finding differs from other studies using cells from synovial fluid [22–24]. Some of this variability may be explained by differences in the populations of patients, especially in disease stage and therapy, the methods used to purify Treg, or how the suppression assays were performed.
A number of different DMARDs have been used to reduce inflammation and prevent joint destruction in RA. Various actions of each DMARD on RA have been documented, but the exact mechanisms are not fully understood. MTX, as a folate antagonist, inhibits the synthesis of purines and pyrimidines and exerts anti-inflammatory effects by inhibiting proliferation and inducing apoptosis of immune/inflammatory cells as well as inhibiting the production of both monocytic and lymphocytic proinflammatory cytokines involved in RA [25]. MTX, regarded as a cornerstone of RA treatment, is currently recommended as the first DMARD of choice and as the anchor drug to which other DMARDs can be combined and new drugs can be evaluated [26, 27]. LEF inhibits pyrimidine synthesis, resulting in blockade of T-cell proliferation [28]. It also alters the synthesis of cytokines by augmenting the immunosuppressive cytokine TGF-β1 and suppressing the immunostimulatory cytokine IL-2 [29]. SSZ has been shown in vitro to possess multiple anti-inflammatory properties. It inhibits T-cell proliferation, natural killer cell activity, and B-cell activation, resulting in a decrease in immunoglobulin synthesis. Cytokine profiles also are altered by SSZ, resulting in inhibition of the T-cell cytokines IL-2 and interferon-γ, and the monocyte/macrophage cytokines IL-1, TNF-α, and IL-6 [30–32]. The mechanism of these DMARDs in rheumatic disease is still not fully elucidated. Moreover, the effects of these DMARDs on Treg function are not yet known.
The therapeutic effects of anti-TNF agents in RA are thought to be mediated by blocking the TNF-α-mediated inflammatory cytokine cascade. Additionally, anti-TNF agents can induce apoptosis of membrane-bound TNF-α-expressing cells or reduce their numbers through antibody- or complement-dependent cytotoxicity [33–35]. Anti-TNF agents have also been reported to neutralize TNF-α, which binds to its receptor on Tregs and thereby downregulates Treg function [20].
Regarding other drugs being used to treat RA, glucocorticoids (GCs) therapy had been reported to increase the frequency of Treg in early clinical studies on patients with different autoimmune diseases [36, 37]. However, other larger studies on patients with autoimmune diseases showed the opposite result [38]. A recent study suggests that short-term GC therapy did not change the relative frequency of circulating Tregs in vivo, neither in immunocompetent human subjects nor in mice. GC treatment in vitro did not have any direct effect on the functional ability of the Treg cells [39]. Calcineurin inhibitors (CNIs), cyclosporin A and tacrolimus, has been shown to inhibit FOXP3 expression and possible suppressor function of Tregs in several in vitro and in vivo studies [40, 41] and to reduce the frequencies of circulating Tregs in renal or liver transplant recipients [42, 43].
In this study, we focused on the possible inhibitory (adverse) effect of DMARDs on the regulatory function of healthy Treg population which are unaffected by such an inflammatory condition. SSZ and LEF significantly attenuated Treg expression of Foxp3 mRNA and suppression of Teffs. In contrast, neither MTX nor INF had any effect on these Treg properties, indicating that these DMARDs do not reduce the suppressive capacity of Tregs. Theoretically, DMARD which do not disturb the suppressive function of Treg might be the ideal choice for RA. For example, in individuals who are in preclinical stage or remission state of RA without any evidence of inflammation, some DMARDs such as SSZ or LEF might disturb autoregulatory function of Treg.
There had been several reports that sulfasalazine induced exacerbation of intestinal and/or extraintestinal manifestations of ulcerative colitis [44, 45]. Although the relationship between the disease flare and Treg function was not known, it deserves much consideration. In a recent study, LEF increased the proportion of CD4+CD25+ Tregs and FoxP3 mRNA expression in spleen lymphocytes from collagen-induced arthritis rats both in vivo and in vitro [46]. In contrast, LEF decreases peripheral Treg in a mouse model of allogeneic bone marrow transplantation [47]. Because the environment where the Treg was studied has variable disease states in most studies, it is difficult to know whether the change in Treg function is caused by the drug itself or through environmental changes by the drug. As far as we know, there has been no in vitro human study on the effect of these drugs on Treg function in non-disease condition. Although the intracellular mechanism of SSZ and LEF as well as other DMARDs has not been known exactly, we thought that the negative effect of SSZ and LEF on Treg could be related to downregulation of Foxp3 activation. However, more research is needed to know which intracellular component is affected by these drugs and then makes an influence on Foxp3 activation.
Because this was an in vitro study with a small number of samples, there may be limits to which its results can be extrapolated to explain the effects of DMARDs on Tregs in vivo, where complex mechanisms and interactions among various inflammatory cells and cytokines are likely involved. Although Tregs suppress the activation and/or proliferation and cytokine formation of Teff even in the absence of antigen presenting cells (APCs) in vitro [8, 48], much of the effect of Treg on Teff has been known to depend on the effect on the function of APCs. Our experiment performed in the absence of APCs has a limitation in the evaluation of APCs-mediated effect of Treg. In addition, we defined Treg based on expression of CD4 and CD25 as many previous studies. Although the “classic” CD4+CD25+ Tregs are generally Foxp3+ and highly immunosuppressive, these population also includes effector T cells [49]. Thus, identification of Treg by low levels of CD127 expression in combination with CD4 and CD25 expression would be a better approach to evaluate the pure FoxP3+Treg function [15]. However, a notable finding in this study is that each DMARD may have a different effect on the regulatory function of Tregs. A better understanding of the mechanisms underlying the therapeutic action of each DMARD on Tregs may provide a direction for research into more effective combination regimens of conventional DMARDs or lead to the development of new treatment strategies for RA.
Conclusions
We have shown that each DMARD had a different effect on Treg function. SSZ and LEF inhibited the anti-proliferative function of Tregs on cocultured Teffs and reduced Treg expression of Foxp3 mRNA, whereas MTX and INF did not.
References
Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S (1999) Thymus and autoimmunity: production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol 162(9):5317–5326
Taams LS, Akbar AN (2005) Peripheral generation and function of CD4+ CD25+ regulatory T cells. Curr Top Microbiol Immunol 293:115–131
Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP (2005) Modulation of monocyte/macrophage function by human CD4+ CD25+ regulatory T cells. Hum Immunol 66(3):222–230. doi:10.1016/j.humimm.2004.12.006
Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV (2004) Cutting edge: human CD4+ CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol 172(8):4676–4680
Shevach EM (2009) Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30(5):636–645. doi:10.1016/j.immuni.2009.04.010
Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY (2004) Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity 21(2):267–277. doi:10.1016/j.immuni.2004.07.009
Sakaguchi S (2004) Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 22:531–562. doi:10.1146/annurev.immunol.21.120601.141122
Thornton AM, Shevach EM (1998) CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 188(2):287–296
Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD (2001) The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27(1):20–21. doi:10.1038/83713
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol 4(4):330–336. doi:10.1038/ni904
Khattri R, Cox T, Yasayko SA, Ramsdell F (2003) An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol 4(4):337–342. doi:10.1038/ni909
Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, Ziegler SF, Roncarolo MG, Levings MK (2005) The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest 115(11):3276–3284. doi:10.1172/JCI24685
Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF (2003) Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+ CD25- T cells. J Clin Invest 112(9):1437–1443. doi:10.1172/JCI19441
Pillai V, Ortega SB, Wang CK, Karandikar NJ (2007) Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol 123(1):18–29. doi:10.1016/j.clim.2006.10.014
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203(7):1701–1711. doi:10.1084/jem.20060772
Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203(7):1693–1700. doi:10.1084/jem.20060468
Frey O, Petrow PK, Gajda M, Siegmund K, Huehn J, Scheffold A, Hamann A, Radbruch A, Brauer R (2005) The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+ CD25+ T cells. Arthritis Res Ther 7(2):R291–R301. doi:10.1186/ar1484
Lawson CA, Brown AK, Bejarano V, Douglas SH, Burgoyne CH, Greenstein AS, Boylston AW, Emery P, Ponchel F, Isaacs JD (2006) Early rheumatoid arthritis is associated with a deficit in the CD4+ CD25 high regulatory T cell population in peripheral blood. Rheumatology (Oxford) 45(10):1210–1217. doi:10.1093/rheumatology/kel089
Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C (2004) Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med 200(3):277–285. doi:10.1084/jem.20040165
Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE (2006) TNF downmodulates the function of human CD4+ CD25hi T-regulatory cells. Blood 108(1):253–261. doi:10.1182/blood-2005-11-4567
Porter SB, Liu B, Rogosheske J, Levine BL, June CH, Kohl VK, Wagner JE, Miller JS, Blazar BR (2006) Suppressor function of umbilical cord blood-derived CD4+ CD25+ T-regulatory cells exposed to graft-versus-host disease drugs. Transplantation 82(1):23–29. doi:10.1097/01.tp.0000225824.48931.af
van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS (2004) CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthr Rheum 50(9):2775–2785. doi:10.1002/art.20499
Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V (2004) CD25 bright CD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthr Res Ther 6(4):R335–R346. doi:10.1186/ar1192
Liu MF, Wang CR, Fung LL, Lin LH, Tsai CN (2005) The presence of cytokine-suppressive CD4+ CD25+ T cells in the peripheral blood and synovial fluid of patients with rheumatoid arthritis. Scand J Immunol 62(3):312–317. doi:10.1111/j.1365-3083.2005.01656.x
Cutolo M, Sulli A, Pizzorni C, Seriolo B, Straub RH (2001) Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis 60(8):729–735
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376(9746):1094–1108. doi:10.1016/S0140-6736(10)60826-4
Pincus T, Yazici Y, Sokka T, Aletaha D, Smolen JS (2003) Methotrexate as the “anchor drug” for the treatment of early rheumatoid arthritis. Clin Exp Rheumatol 21(5 suppl 31):S179–S185
Fox RI (1998) Mechanism of action of leflunomide in rheumatoid arthritis. J Rheumatol Suppl 53:20–26
Cao WW, Kao PN, Aoki Y, Xu JC, Shorthouse RA, Morris RE (1996) A novel mechanism of action of the immunomodulatory drug, leflunomide: augmentation of the immunosuppressive cytokine, TGF-beta 1, and suppression of the immunostimulatory cytokine, IL-2. Transp Proc 28(6):3079–3080
Barrera P, Haagsma CJ, Boerbooms AM, Van Riel PL, Borm GF, Van de Putte LB, Van der Meer JW (1995) Effect of methotrexate alone or in combination with sulphasalazine on the production and circulating concentrations of cytokines and their antagonists. Longitudinal evaluation in patients with rheumatoid arthritis. Br J Rheumatol 34(8):747–755
Imai F, Suzuki T, Ishibashi T, Dohi Y (1991) Effect of sulfasalazine on B cells. Clin Exp Rheumatol 9(3):259–264
Fujiwara M, Mitsui K, Yamamoto I (1990) Inhibition of proliferative responses and interleukin 2 productions by salazosulfapyridine and its metabolites. Jpn J Pharmacol 54(2):121–131
Shen C, Van Assche G, Rutgeerts P, Ceuppens JL (2006) Caspase activation and apoptosis induction by adalimumab: demonstration in vitro and in vivo in a chimeric mouse model. Inflamm Bowel Dis 12(1):22–28. doi:10.1097/01.MIB.0000194185.69800.07
Shen C, Assche GV, Colpaert S, Maerten P, Geboes K, Rutgeerts P, Ceuppens JL (2005) Adalimumab induces apoptosis of human monocytes: a comparative study with infliximab and etanercept. Aliment Pharmacol Ther 21(3):251–258. doi:10.1111/j.1365-2036.2005.02309.x
Nesbitt A, Fossati G, Bergin M, Stephens P, Stephens S, Foulkes R, Brown D, Robinson M, Bourne T (2007) Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis 13(11):1323–1332. doi:10.1002/ibd.20225
Suarez A, Lopez P, Gomez J, Gutierrez C (2006) Enrichment of CD4+ CD25high T cell population in patients with systemic lupus erythematosus treated with glucocorticoids. Ann Rheum Dis 65(11):1512–1517. doi:10.1136/ard.2005.049924
Xu L, Xu Z, Xu M (2009) Glucocorticoid treatment restores the impaired suppressive function of regulatory T cells in patients with relapsing-remitting multiple sclerosis. Clin Exp Immunol 158(1):26–30. doi:10.1111/j.1365-2249.2009.03987.x
Banica L, Besliu A, Pistol G, Stavaru C, Ionescu R, Forsea AM, Tanaseanu C, Dumitrache S, Otelea D, Tamsulea I, Tanaseanu S, Chitonu C, Paraschiv S, Balteanu M, Stefanescu M, Matache C (2009) Quantification and molecular characterization of regulatory T cells in connective tissue diseases. Autoimmunity 42(1):41–49. doi:10.1080/08916930802282651
Sbiera S, Dexneit T, Reichardt SD, Michel KD, van den Brandt J, Schmull S, Kraus L, Beyer M, Mlynski R, Wortmann S, Allolio B, Reichardt HM, Fassnacht M (2011) Influence of short-term glucocorticoid therapy on regulatory T cells in vivo. PLoS ONE 6(9):e24345. doi:10.1371/journal.pone.0024345
Baan CC, van der Mast BJ, Klepper M, Mol WM, Peeters AM, Korevaar SS, Balk AH, Weimar W (2005) Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation 80(1):110–117. doi:10.1097/01.TP.0000164142.98167.4B
Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS (2006) Inhibition of CD4+ CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 108(1):390–399. doi:10.1182/blood-2006-01-0329
Segundo DS, Ruiz JC, Izquierdo M, Fernandez-Fresnedo G, Gomez-Alamillo C, Merino R, Benito MJ, Cacho E, Rodrigo E, Palomar R, Lopez-Hoyos M, Arias M (2006) Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+ CD25+ FOXP3+ regulatory T cells in renal transplant recipients. Transplantation 82(4):550–557. doi:10.1097/01.tp.0000229473.95202.50
Chu Z, Zhang J, Zhao Y, Ji Q, Zhong J, Zhang C, Zhang B (2010) Influence of immunosuppressive drugs on the development of CD4(+)CD25(high) Foxp3(+) T cells in liver transplant recipients. Transp Proc 42(7):2599–2601. doi:10.1016/j.transproceed.2010.04.026
Chakraborty TK, Bhatia D, Heading RC, Ford MJ (1987) Salicylate induced exacerbation of ulcerative colitis. Gut 28(5):613–615
Schwartz AG, Targan SR, Saxon A, Weinstein WM (1982) Sulfasalazine-induced exacerbation of ulcerative colitis. N Engl J Med 306(7):409–412. doi:10.1056/NEJM198202183060708
Wang TY, Li J, Li CY, Jin Y, Lu XW, Wang XH, Zhou Q (2010) Leflunomide induces immunosuppression in collagen-induced arthritis rats by upregulating CD4+ CD25+ regulatory T cells. Can J Physiol Pharmacol 88(1):45–53. doi:10.1139/Y09-094
Jin D, Duan K, Zhang L, Peng J, Zhao Y (2011) The effects of leflunomide on CD4(+)CD25 (+)Foxp3 (+) T regulatory cells in mice receiving allogeneic bone marrow transplantation. Inflamm Res Off J Euro Histamine Res Soc (et al.). doi:10.1007/s00011-011-0388-4
Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S (1998) Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol 10(12):1969–1980
Michel L, Berthelot L, Pettre S, Wiertlewski S, Lefrere F, Braudeau C, Brouard S, Soulillou JP, Laplaud DA (2008) Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J Clin Invest 118(10):3411–3419. doi:10.1172/JCI35365
Acknowledgments
This study was supported by a grant (08-330) from the Asan Institute for Life Science, Seoul, Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ji Seon Oh and Yong-Gil Kim contributed equally to this research.
Rights and permissions
About this article
Cite this article
Oh, J.S., Kim, YG., Lee, S.G. et al. The effect of various disease-modifying anti-rheumatic drugs on the suppressive function of CD4+CD25+ regulatory T cells. Rheumatol Int 33, 381–388 (2013). https://doi.org/10.1007/s00296-012-2365-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2365-9