Abstract
To determine the efficacy of oral vitamin D [25(OH)D] in patients with active rheumatoid arthritis (RA) who are in methotrexate (MTX) therapy, patients receiving stable doses of MTX were randomized to one of two dose groups and received 12 weeks of double-blind vitamin D[25(OH)D] (50,000 IU per week) or matching placebo. The moderate and major efficacy measure was the proportion of patients with >0.6 and >1.2 improvement in RA based on the Disease Activity Score 28(DAS 28) at 12 weeks. Safety measures included adverse events and laboratory assessments. On a background of MTX, the percentage of patients with a moderate/major DAS 28 response at week 12 in the vitamin D groups (76/44%) was not significantly different from placebo (64.6/33.4%). Adverse events were typically mild and included small hepatic enzyme elevation; we did not have any undesirable events resulting in discontinuation of study drug. In patients with active RA receiving stable doses of MTX, vitamin D showed non-significant improvement in efficacy outcomes compared to placebo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most studies indicate that blood levels of vitamin D linked to immune system activity, and vitamin D has an immunomodulatory effect on this system. However, some studies failed to prove this relationship. In one study, more vitamin D consumption was associated with reduced risk of rheumatoid arthritis and low vitamin level in northern European people when compared with southern Europe, was associated with high RA prevalence. In this study, 25(OH)D levels in Estonian were acceptably higher. In addition, there was a significant negative association between the level of 25(OH)D and DAS28 in summer months in Italian patients. Considerably, lower levels of 25(OH)D were seen in RA patients and a seasonal rhythm of summer and winter were observed. Furthermore, there was a significant negative association between 25(OH)D levels and RA disease activity (DAS28) both in the northern and southern Europe [1].
The immunoregulatory effect of Vitamin D in chronic diseases such as inflammatory bowel disease, multiple sclerosis and diabetes type 1 is clear. An interaction exists between vitamin D metabolites and inflammatory cytokines such as TNF-alpha, which is involved in osteoclasts-mediated bone resorption and bone loss. Some preliminary studies showed the association between seasonal variations in vitamin D serum levels, disease activity (DAS28), and latitude in rheumatoid arthritis. Osteoporosis and Metabolic Bone Diseases Study Group of SIR believe that there are many reasons to measure vitamin D levels in patients with rheumatoid arthritis [2]. Patients with rheumatoid arthritis have vitamin D metabolic disturbance; this might play a role in osteoporosis associated with RA [3].
The optimal amount of vitamin D that can affect the immune system is not known yet, but is likely to be at least that required for healthy bones. The evidence showed that vitamin D has a role in self-tolerance [4]. In several studies on patients with rheumatoid arthritis, an association of bone resorption with high disease activity has been found [5]. Recently, more intake of vitamin D was associated with a lower risk of rheumatoid arthritis [6]. In a study of 190 cases of the SLE, and 722 cases of RA, increasing levels of vitamin D intake had no association to the relative risk of developing either SLE or rheumatoid arthritis [7]. In another prospective cohort study of 29,368 women between 55–69 years without a history of RA, through 11 years of following up additional intake of vitamin D was inversely associated with risk of RA [8]. Study of Craig SM and colleagues conducted to examine the prevalence of vitamin D insufficiency, and the associations of vitamin D concentration with disease status in African Americans with rheumatoid arthritis. In unadjusted analyses, vitamin D concentrations were inversely associated with baseline pain, swollen joints, and Disease Activity Score (DAS28), but not with measures at 3 years’ disease duration. There were no multivariate associations of 25(OH)-D with any disease measures at baseline or at 3 years. There were not strong associations of 25(OH)-D concentration with symptoms or disease severity in African Americans with RA [9]. We conducted this study to answer this question: “Can oral vitamin D reduce the disease activity index (DAS28) in rheumatoid patients under the MTX therapy?”
Patients and methods
Patients
This study included 117 patients from rheumatology clinic of Alzahra Hospital in Isfahan. Inclusion criteria were: ≥18 years of age, active rheumatoid arthritis (RA) by the American College of Rheumatology (ACR) 1987 criteria; initiated MTX ≥24 weeks prior to the study, with stable dosing (7.5–20 mg/week) for the last ≥8 weeks; DAS28 of >3.2; morning stiffness ≥45 min.
Patients with overlap of rheumatoid arthritis and other autoimmune diseases, RF negativity, abnormal serum calcium, phosphorus, and alkaline phosphatase were excluded from the study. Serum calcium, phosphorus, and alkaline phosphatase measured by the photometric method, and abnormal ranges excluded from the study [10].
Patients were randomized to vitamin D [25(OH)D] regimens (50,000 IU weekly) or placebo. Patients were stratified for using hydroxychloroquine (HCQ) or chloroquine (CQ). Permitted medications included oral corticosteroids (≤10 mg/day prednisone), HCQ (≤400 mg/day) and CQ (150 mg/day) if stable for ≥8 weeks prior to baseline. For control of confounders, serum 25-dihydroxy vitamin D, calcium and alkaline phosphatase measured before and after intervention.
Clinical assessments
The primary efficacy end point was the proportion of patients with a 0.6 improvement in Disease Activity Score in 28 joints (DAS28) score, at week 12. Secondary end point included 1.2 improvements from baseline in the analyses of DAS28. Safety assessments included hematology, blood chemistry, fasting lipids, and urinalysis.
Statistical analysis
Data analysis carried out by paired T test and independent T test. We used SPSS 15 software. We used general linear model for the control of confounder’s effect. P value less than 0.05 was significant. In data analysis, efficacy analyses were based on the intent-to-treat (ITT) population, including all randomized patients who had ≥1 dose of double-blind study medication. All participants in the study signed informed consent.
Primary efficacy was analyzed using the Cochran–Mantel–Haenszel (CMH) test, with the use of HCQ/CQ as stratification factors. Patients with missing DAS 28 values more than 0.6 at week 12 were classified as non-responders. For secondary efficacy, categorical parameters were analyzed as described for the primary efficacy.
Results
Patient characteristics
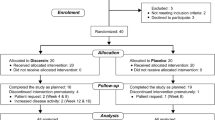
Demographics and baseline disease activity was similar across treatment groups. At baseline, most of the patients had a positive rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) test; 85% reported having morning stiffness of ≥45 min; and 68% of patients had ESR values that were ≥28 mm/h. At baseline, the median MTX dose was 10 mg; 94% of patients were taking either HCQ or CQ and 96% were using corticosteroids. 84% of patients completed 12 weeks of treatment. Premature discontinuations were attributed to: Two cases due to travel and one case death due to heart disease was excluded from the study. We determined five cases at the end of the study that refused to eat drugs, and two cases did not follow study. In the control group, one case due to dizziness, two cases because of their husband disagreement, and six cases due to refusal medication were excluded from the study. At the end of the study, 50 cases remained in vitamin D group and 48 cases in the control group. (N = 98)). Mean age of patients in the intervention group (49.9 ± 13) and placebo (50 ± 12.7). There was no statistically significant difference between two groups (P > 0.05). Nine of 98 patients (9.2%) were men and 89 patients (90.8%) were women. Sex distribution of the two groups was the same (P > 0.05). Table 1 shows clinical and biochemical characteristics of patients in both groups before intervention.
Comparison of DAS28 index in two patient groups at the end of study, showed that there is not any significant difference in baseline disease severity in intervention and placebo groups (DAS28 = 5.5 ± 1.2 and 4.8 ± 1.2, respectively) (P > 0.05). After three-month periods, we analyzed clinical and biochemical factors in both groups. Before and after intervention serum calcium, phosphor, and alkaline phosphatase in two groups did not have significant differences (P > 0.05) (Table 1, 2). After the intervention, serum 25(OH)D level was significantly higher in the vitamin D group than the placebo group (P < 0.05), that it was predictable. Data analysis after the intervention depicted in Table 2.
Clinical efficacy
The percentage of patients with a DAS 28 response at week 12 was similar or slightly higher in the vitamin D groups (76% moderate response and 44% major response) compared with placebo (64.6% moderate response and 33.4% major response); the differences were not statistically significant. With definition of 12 week improvements in DAS28 score (major response), the following results were found out:
Of 10 patients who took chloroquine (CQ) regardless of VitD, 100% had a positive response to treatment (pure effect of CQ). Of 31 patients who took hydroxychloroquine (HCQ) 200 mg plus 25(OH)D, 14 (45.2%) reached DAS28 <2.6 as defined in the study. Of 33 patients, using HCQ without 25(OH)D, 9 (27.3%) had a positive response to treatment. Of 18 patients who received 400 mg HCQ, none of the patients had the positive response, (response rate was zero). After stratification for adjutant therapy with HCQ and CQ, we had carried out Cochran–Mantel–Haenszel (CMH) analysis. The result was as follows:
Adding 25(OH)D to treatment protocol changes Odd ratio from 2.22 (CI 95% .9–5.3) to 2.1 (CI 95% .7–6.2). (P > 0.05 for both estimations).
Mean DAS28 scores decreased over time and were the same by week 12 in two groups. Changes in the DAS28 component criteria were similar in the vitamin D and placebo groups. Modest, but not significant, improvement observed in the vitamin D group compared to placebo for DAS28 parameters [tender joint count, swollen joint count, visual analog scale (VAS) and ESR]. The percentage of patients reporting reduced duration of morning stiffness indicated similar improvement across the treatment groups.
In this study, there were a weak but significant negative correlation between 25(OH)D levels and DAS28. (R = 0.14; P < 0.05).
Safety
The percentage of patients experiencing at least one adverse event was 6% in the placebo group and 12% in the vitamin D group. Mild elevations in liver enzymes (<2 times the upper limit of normal) occurred in three placebo-treated patients and six 25(OH)D-treated patients. We did not see hypercalcemia in both groups. There were no deaths or serious adverse event because of drug or placebo.
Discussion
Most studies indicate that the blood level of vitamin D linked to immune system activity, and high consumption of vitamin D associated with reduced risk of rheumatoid arthritis. Vitamin D commonly prescribed by rheumatologists for treatment of osteoporosis. High disease activity of rheumatoid arthritis changes vitamin D metabolism and increased bone resorption. Low Level of 1, 25-dihydroxy vitamin D3 in patients causes a negative calcium balance and inhibits bone production [3.] Most studies, heretofore, were cross-sectional [2, 5, 11] or only survey correlation between disease activity and levels of vitamin D [5, 11]. Of course, most of these studies found an inverse correlation between the disease activity and serum vitamin D levels. However, our study was a placebo-controlled clinical trial that measured influence of oral 25(OH)D on disease activity, and in contrast to previous studies could find a weak inverse correlation between 25(OH)D levels and disease activity index (DAS28). We could not find any association between serum 25(OH)D levels and ESR, VAS, swollen joints count and tender joints count. Of course, oral 25(OH)D improved above indices. However, it was not statistically significant. Study of Costenbader KH and colleagues conducted in 190 cases of SLE and 722 cases of RA, and followed from 1980 to 2002. Association between vitamin D consumption and incidence of RA and SLE studied in this prospective cohort. This study elucidated that increased consumption of vitamin D do not decrease incident risk of RA or SLE [7]. In contrast, the study of Merlino LA et al. [8] conducted on 29,368 women between ages from 55 to 69 years old without history of RA. In 11 years of follow-up, more consumption of vitamin D (as nutritional or supplement) associated with lower risk of RA (RR = 0.67). In our study, we did not evaluate the effects of vitamin D on the incidence of rheumatoid arthritis, but assayed the effect of this substance, as an adjuvant treatment in these patients. Of course, we found that vitamin D consumption had a little effect on disease activity. Andjelkovic Z, et al. reviewed the immunoregulatory effects of high dose 1 alpha (OH) D3 in RA patients. It was a three-month open label study on 19 RA patients under the treatment of standard DMARDs. Patients divided in two groups, highly active and moderate active. They received two micrograms daily of alpha calcidiol. Results evaluated by ESR, CRP, morning stiffness, Richie and Lee index. After 3 months, a positive effect on disease activity was 89% complete remission and 44% significant remission. Only two patients did not improve. This study concluded that: “alpha calcidiol is an immune regulator substance and can use as adjuvant treatment in RA patients” [12]. Although this study was a clinical trial that evaluated the influence of vitamin D on rheumatoid disease activity, but was the open-label, not placebo-controlled and not blinded. We designed the present study because of controversies about vitamin D effect on disease control. In this context, there was not any randomized placebo control trial to evaluate the immunomodulatory effect of vitamin D on rheumatoid patients. In our study, two groups at the beginning of the study did not have significant differences in severity of disease. After 3 months, all clinical and laboratory parameters of disease activity decreased significantly in both groups. Of course, this decrement in the vitamin D group was more than the placebo group, but was not statistically significant. We could find a negative correlation between serum 25(OH)D levels and disease activity index, but it was not statistically significant. We also could not find a significant correlation between 25(OH)D levels and ESR, VAS, swollen joints count, and tender joints count. By Cochran–Mantel–Haenszel (CMH) test, with the use of HCQ/CQ as stratification factors, we found that adding Vitamin D to treatment protocol could not change odd ratio significantly. Generally, we concluded that oral 25(OH)D can use in the regimen of patients with rheumatoid arthritis as an adjuvant with other DMARDs. Our Limitations were: 1 Small sample size and 2 Low dose of 25(OH)D, because we were afraid of hypercalcemia. This study demonstrated that if we add 25(OH)D to drug regimen of patients with inadequate clinical responses to MTX, it has minimal efficacy that was not different from placebo. We found mild liver transaminase elevations in a small percentage of patients.
References
Cutolo M, Otsa K, Laas K, Yprus M, Lehtme R, Secchi ME et al (2006) Circannual vitamin D serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin Exp Rheumatol 24(6):702–704
Frediani B, Rossini M, Adami S, Bianchi G, Di Munno O, Sinigaglia L et al (2006) Study of vitamin D status of rheumatoid arthritis patients. Rationale and design of a cross-sectional study by the osteoporosis and metabolic bone diseases study group of the Italian Society of Rheumatology (SIR). Reumatismo 58(4):314–318
Kröger H, Penttilä IM, Alhava EM (1993) Low serum vitamin D metabolites in women with rheumatoid arthritis. Scand J Rheumatol 22(4):172–177
Cantorna MT, Mahon BD (2004) Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence, Exp Biol Med (Maywood) 229(11):1136–1142; 67(4):530–535. Epub 2007 Jul 31
Oelzner P, Müller A, Deschner F, Hüller M, Abendroth K, Hein G et al (1998) Relationship between disease activity and serum levels of vitamin D metabolites and PTH in rheumatoid arthritis. Calcif Tissue Int 62(3):193–198
Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B (2007) Vitamin D in rheumatoid arthritis. Autoimmun Rev 7(1):59–64 Epub 2007 Aug 14
Costenbader KH, Feskanich D, Holmes M, Karlson EW, Benito-Garcia E (2008) vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis
Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG (2004) Iowa Women’s Health Study. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum 50(1):72–77
Craig SM, Yu F, Curtis JR, Alarcón GS, Conn DL, Jonas B et al (2010) Vitamin D status and its associations with disease activity and severity in African Americans with recent-onset rheumatoid arthritis. J Rheumatol 37(2):275–281 Epub 2009 Dec 23
(2007) Cecil Text book of Medicine[book online]. 23rd edn Elsevier, Saunders
Arnson Y, Amital H, Shoenfeld Y (2007) Vitamin D and autoimmunity: new aetiological and therapeutic considerations; Ann Rheum Dis 66(9):1137–1142 Epub 2007 Jun 8
Andjelkovic Z, Vojinovic J, Pejnovic N, Popovic M, Dujic A, Mitrovic D et al (1999) Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin Exp Rheumatol 17(4):453–456
Conflict of interests
None.
Ethical approval
Obtained from the ethics committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salesi, M., Farajzadegan, Z. Efficacy of Vitamin D in patients with active rheumatoid arthritis receiving methotrexate therapy. Rheumatol Int 32, 2129–2133 (2012). https://doi.org/10.1007/s00296-011-1944-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-011-1944-5