Abstract
To observe the effect of resveratol on cartilage,chondrocyte apoptosis, and nitric oxide in experimental osteoarthritis (OA) of rabbit and to study the mechanism of resveratol in the treatment of osteoarthritis. Thirty New Zealand rabbits were randomly divided into 5 groups: group A (normal control group), group B (model control group), group C (resveratrol intervention high-dosage group), group D (resveratrol intervention middle dosage group), and group E (resveratrol intervention low-dosage group). The model of OA of the knee was established using Hulth technique in groups B, C, D, and E. After 4 weeks, group A and group B rabbits were administered daily a knees injection of dimethylsulfoxide (DMSO), whereas groups C, D, and E were administered daily a knees injection of resveratrol in DMSO in different dosages for 2 weeks. Daily dosage for rabbits of groups C, D, and E was fixed at 50, 20, and 10 μmol/kg, respectively. Then, the rabbits were killed, and the lateral cartilage sections of right femoral medial condyle were evaluated using a histological scoring system (H&E and safranin-O staining) and analyzed by TUNEL for apoptosis. Nitric oxide (NO) in synovial fluid was measured by nitrate reduction method. Histological evaluation of cartilage tissue revealed a significantly reduced cartilage destruction; the evaluation also revealed the loss of matrix proteoglycan content in cartilage in resveratrol intervention groups compared to the model control. Resveratrol reduced the apoptosis rate of chondrocyte and level of NO in the synovial fluid. In the above experiments of OA rabbits, the protective effects of resveratrol were enhanced with increased resveratrol dosage. Resveratrol controls the progression of experimental OA. One of the mechanism(s) responsible for this effect would include lowering of the apoptosis rate of chondrocyte and reducing the production of NO in experimental OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a chronic disease characterized by the progressive degeneration of cartilage, causing pain, and loss of articular function. The primary pathogenic events in OA include loss and abnormal remodeling of cartilage extracellular matrix (ECM). Chondrocytes are the only type of cells constituting the articular cartilage. Their functions include maintaining tissue homeostasis, responding to injury, and executing the cartilage remodeling process that characterizes OA. The earlier concepts on OA pathogenesis merely focused on the role of synthesis and degradation of the ECM in the articular cartilage. More recent findings indicate that chondrocyte death and survival are closely linked with cartilage matrix integrity [1]. Chondrocytes are differentiated from mesenchymal cells during embryonic development. Differentiated chondrocytes synthesize sufficient amounts of cartilage-specific ECM—including type II collagen and proteoglycan—to maintain matrix integrity [1, 2]. It has been found that this homeostasis is destroyed by chondrocyte apoptosis in the progressive stage of OA. So, apoptosis inhibition appears feasible and should be considered and explored as a therapeutic target of OA. Researches showed that there appears to be at least two independent pathways for the induction of chondrocyte apoptosis—the Fas and the NO pathways [3]. Pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), which are thought to play an important role in the pathogenesis of arthritis, differentially regulate the apoptotic pathway in human chondrocytes by NO pathway [4]. In addition, NO mediates the inflammatory response involved in the matrix metalloproteinases degradation, thereby inhibiting the synthesis of collagen and proteoglycans [5].
Resveratrol (trans-3, 4-trihydroxystilbene) is a natural phytoalexin (polyphenolic compound) found in the skin of red grapes, cranberries, peanuts, and root extracts of the weed Polygonum cuspidatum. It has been reported that resveratrol has antitumor activity, immunomodulatory, antioxidative, anti-inflammatory functions, apart from numerous biological activities [6].
In this study, we intend to reveal the effect of resveratol on cartilage, chondrocyte apoptosis, and nitric oxide in experimental osteoarthritis (OA) of rabbit.
Materials and methods
Animals and surgical model
In this study, all experiments were performed in accordance with the guidelines for animal research from the National Institutes of Health and were approved by the Committee on Animal Research at the Second Xiangya Hospital.
Resveratrol was purchased from Sigma (500 mg) and prepared according to the manufacturer’s protocol. In brief, the outline of the procedure is as follows: 100 μmol resveratrol was dissolved in dimethylsulfoxide (DMSO) and prepared stock resveratrol solution. It was diluted in serum physiologic as 30 μMol in 100 μL.
Thirty adult New Zealand white rabbits (equal number of male and female rabbits, weight 1.5–2.5 kg) were divided randomly into 5 groups (each group consists of 6 rabbits): group A (normal control group), group B (model control group), group C (resveratrol intervention high-dosage group), group D (resveratrol intervention middle dosage group), and group E (resveratrol intervention low-dosage group).
The model of OA was established in groups B, C, D, and E using the Hulth-Telhag modeling method. The surgical procedure for the modeling groups was performed under sterile conditions in an animal surgical suite under general endotracheal anesthesia (Halotane 2%). Intramuscular antibiotic (Enrofloxacin 5 mg/kg) was administered. A midline skin incision was then made over the right knee: a medial parapatellar incision was made through the retinaculum. The medial collateral ligament was sharply divided.
A medial parapatellar arthrotomy was then performed, and the patella was dislocated laterally. Care was taken to protect and retract the vascular intraarticular fat pad. With the knee flexed, the anterior cruciate ligament and the posterior cruciate ligament were transected. The knee joint was then dislocated to excise the medial meniscus.
The joint was irrigated with sterile saline solution. The capsule and the synovium were then closed together using a 4.0 interrupted Vicryl and the skin was closed. A sham procedure was performed on the left hind limb serving as a control within the same animal. An arthrotomy was performed, as described above. After dislocating the patella laterally, the knee was irrigated; but the ligaments and menisci were left intact. Postoperatively, the rabbits were kept in single-subject cages for 4 days and analgesics (Buprenex) were administered as per requirement. The rabbits were allowed to bear weight as per their individual tolerance.
After 4 days, the rabbits were transferred to three large free-roaming communal cages (10 × 15 feet) where they had unlimited activity. Four weeks later, rabbits of group A and group B were administered daily knees injection containing DMSO, while rabbits of groups C, D, and E were administered daily knees injection containing resveratrol in DMSO at different dosages for 2 weeks. Daily dosage of groups C, D, and E were 50, 20, and 10 μmol/kg, respectively. Then, the rabbits were killed, and the lateral cartilage sections of right femoral medial condyle and synovial fluid were analyzed.
Histology
For histologic examination, the cartilage specimens of knees were fixed in 10% neutral-buffered formalin; then they were decalcified with 10% Na2 ethylenediaminetetraacetate (EDTA) buffered at pH 7.4 and embedded in paraffin. Several sections of 4 mm thickness were cut from the lateral articular cartilage of right femoral medial condyle on a rotary microtome.
For histological evaluation, sagittal sections derived from chondral tissues were stained with hematoxylin and eosin (H&E) and safranin O. A semiquantitave scoring on a grading scale for cartilage erosions was used by an independent observer for each knee. A characteristic parameter in arthritis is the progressive loss of articular cartilage. The depth of cartilage erosion determined by H&E staining was graded according to Mankin scoring criteria (total score: 14 scores). In addition, cartilage proteoglycan depletion was determined using safranin-O staining. The loss of proteoglycans was scored from 0 to 3, ranging from fully stained cartilage to destained cartilage or complete loss of articular cartilage.
TUNEL method
The DNA cleavage was assessed by the terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL); therefore, the “In situ cell death detection, fluorescein” kit was used. Cells were gently washed with phosphate-buffered saline (PBS) and fixed with 250 μl of 4% (w/v) paraformaldehyde prepared freshly in PBS (pH = 7.4) for 30 min at room temperature.
After washing twice in PBS, cells were incubated in 200 μl of permeabilization solution (0.1% [w/v] Triton X-100 in 0.1% [w/v]sodium citrate) for 2 min on ice. Cells were again washed in PBS before incubation with 55 μl of TUNEL reagents (terminal deoxynucleotide transferase and fluorescein-labeled nucleotide mixture) or 55 μl of LABEL (without terminal transferase) used as negative control for 2 h at 37°C in a humidified chamber.
As a negative control, staining was performed without terminal deoxynucleotidyl transferase. The apoptosis ratio (%) was measured: it is defined as ratio between the total number of positive nuclei and the total number of the present cells in the six randomly allocated cellular fields. Positive nuclei stained brown and negative ones stained blue.
Nitric oxide assays
NO was measured in terms of NO metabolite (nitrates and nitrites) release using a Nitric Oxide Detection Kit (Shenzhen Jingmei Bioengineering Institute, China), according to the manufacturer’s instructions. NO is chemically active and rapidly converts to NO3− and NO2− in vivo. This method uses nitrate reductase to specifically reduce NO3− to NO2−, and the content of NO2− is determined colorimetrically. This kit is sensitive, stable, and simple to use, having a major advantage of measuring the total amount of NO3− and NO2− through nitrate reductase.
Statistical analysis of data
The data obtained in this study were introduced into a database specifically designed for analysis with SPSS 11 .0 statistical package. Data were expressed as the mean ± SD for each group. One-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was used to compare differences in nitric oxide production and apoptosis rates among the groups. In all cases, P < 0.05 was considered as significant.
Results
All rabbits in each experimental group completed the study. No signs of resveratrol or DMSO toxicity were noted. The levels of daily activity were similar in all rabbits.
Histological analyses
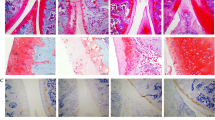
In groups B and A, significantly increased cartilage destruction was determined by H&E staining (1.1 ± 0.32 vs 9.2 ± 0.57 and P < 0.05). Cartilage destruction in groups C, D, and E (4.2 ± 0.78, 6.9 ± 0.36, 7.8 ± 0.41) was less compared to that in group B. According to Mankin scoring criteria, significant differences of the scores were found among groups C, D, and E (P < 0.05) (Table 1; Fig. 1).
Representative histologic staining (hematoxylin and eosin, H&E) of lateral cartilage sections in all groups. H&E staining showed resveratrol at different dosage prevented cartilage destruction of OA, including cartilage thinning, hypocellularity, and fissures of the cartilage surface to different extents
In addition, loss of matrix proteoglycan content in the cartilage was much lower in group A compared to group B (0.4 ± 0.12 vs 2.6 ± 0.34 and P < 0.05), as determined by safranin-O staining. Specimens of cartilage from the group B exhibited morphologic changes characteristic of arthritis. These included fibrillation and fissures of the cartilage surface and loss of safranin-O staining. Groups C, D, and E (1.3 ± 0.23, 1.9 ± 0.12, 2.1 ± 0.27) reduced the loss of matrix proteoglycan content compared to group B (P < 0.05) (Table 1; Fig. 2).
Apoptosis by tunnel
The apoptosis rates of chondrocyte in groups B, C, D, and E were significantly higher than that of group A (31.64 ± 4.05%, 18.15 ± 2.21%, 22.92 ± 3.57%, 27.42 ± 2.71%, 3.28 ± 1.02%, P < 0.01). The apoptosis rates of chondrocyte in groups C, D, and E were decreased compared with that of group B (P < 0.01, P < 0.01, and P < 0.05, respectively). Significant differences of apoptosis rate were found among groups C, D, and E (P < 0.05) (Fig. 3).
Nitric oxide assays
The nitric oxide content of synovial fluid in groups B, C, D, and E (138.71 ± 4.96; 87.13 ± 2.87, 93.36 ± 2.63, 105.42 ± 3.85) were significantly higher than that of group A (46.39 ± 5.98 and P < 0.05). The nitric oxide content of synovial fluid in groups C, D, and E were decreased compared with that of group B (P < 0.05, respectively). Significant differences of nitric oxide content were found among groups C, D, and E (P < 0.05).
Discussion
Resveratrol (3,5,4′-trihydroxystilbene) is a phytoalexin present in a wide variety of plant species, including mulberries, peanut sand grapes, and thus is a constituent of the human diet. This compound, like other members of stilbene family, is produced in response to pathogen attack, UV-irradiation, and exposure to ozone. Researches showed that resveratrol have anti-inflammatory, anticarcinogen, antiaging, and antioxidant properties [6]. Resveratrol attenuated oxidative stress and inhibited iNOS expression, reducing the production of NO in many kinds of cell in vivo and in vitro [7].
Apart from the observations, resveratrol has been shown to be a potent inhibitor of both NF-kB activation and NF-kB-dependent gene expression [8, 9]. Resveratrol inhibits COX-2 and iNOS expression by blocking NF-kB activation [10]; it also blocks TNF-α and IL-1β-induced activation of the NF-kB [11]. NF-kB can regulate chondrocyte apoptosis induced by NO, IL-1β, IL-17 [12–16].
In vitro study, Shakibaei and his colleagues found the antiapoptotic effects of resveratrol on IL-1β-stimulated chondrocytes [17]. Moreover, resveratrol was defensive to the IL-1β-induced catabolic effects of chondrocytes by elevating proteoglycan synthesis, suppressing COX–2 expression and enzyme activity, and decreasing production of MMPs 1, 3, and 13 [18]. A vivo study demonstrated that intraarticular injections of resveratrol reduced the severity of cartilage lesions by histologic examination in the experimental OA model [19].
In this study, we also found that resveratrol exhibited cartilage protective effect in the experimental OA model of rabbits. For histologic examination, the degree of the cartilage lesions and loss of matrix proteoglycan content in the cartilage were significantly improved by treatment with resveratrol.
Apoptosis plays a pivotal role in OA pathogenesis. The apoptosis ratio is higher (18–51%) in the articular cartilage of OA than that of the normal [1, 2, 20]. Our result (31.64 ± 4.05%) is in accordance with these studies. The number of apoptotic cells was correlated with matrix degradation, the corruption of fibrillar architecture, and the OA grade [1, 2]. In the animal experimental study, intraarticular administration of the pan-caspase inhibitor Z-VAD-FMK, which can inhibit apoptosis significantly, reduced cartilage degradation in experimental OA [21]. This result provides direct support for a role of apoptosis in OA pathogenesis, suggesting that inhibition of chondrocyte apoptosis would be an effective therapeutic approach for OA. A study showed antiapoptotic effects of resveratrol on IL-1β-stimulated chondrocytes as an in vitro model of OA [18]. In the animal model of OA, our study presented resveratrol decrease apoptosis rates of chondrocyte. The results demonstrated resveratrol inhibit chondrocyte apoptosis of OA in vivo. It also implied inhibition of chondrocyte apoptosis may be one of the mechanisms resveratrol protecting cartilage destruction.
NO plays an important role in synthesis, degradation of ECM, and chondrocyte apoptosis. A study in an experimental model of OA revealed a selective inhibition of iNOS by L-NIL and the subsequent decreased production of NO resulting in decreased level of chondrocyte apoptosis and the progression of experimental OA [22, 23]. Our study shown that resveratrol reduce the production of NO in the synovial fluid. The results indicated that resveratrol reduced the production of NO and interfered in the NO pathway for the induction of chondrocyte apoptosis. The protective effects of resveratrol in experimental OA are attributed, in part, to its capacity to inhibit chondrocyte apoptosis and reduce the production of NO.
Previous data showed that resveratrol is effective at concentrations ranging from 5 to 100 μM in chondrocytes in vitro [17, 18]. Elmali N selected 10 μmol/kg/day resveratrol as therapeutic dosage in animal model exhibiting protective effect [19]. In our study, we chose 50, 20, 10 μmol/kg, respectively, as daily dosages of three groups to identify the protective effect of resveratrol at different dosages.
Significant differences of Mankin scores, chondrocyte apoptosis rates, and NO content were found among the three groups. The protective effects of resveratrol were enhanced with increased resveratrol dosage. The results suggested the protective effects of resveratrol were dose dependent in the dose range of 10–50 μmol/kg.
In conclusion, we have shown that 2 weeks intraarticular administration of resveratrol (10–50 μmol/kg/day) protect against cartilage destruction by inhibiting chondrocyte apoptosis and reducing the production of NO in the rabbit OA model. From the results of this study, we believe resveratrol might provide a novel and alternative approach as a disease-modifying OA drug. We also hope that the results of this study could bring more studies to confirm the therapeutic benefits and explore the mechanisms of resveratrol in the treatment of OA.
References
Hashimoto S, Ochs RL, Komiya S et al (1998) Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum 41(9):1632–1638
Chen MH, Wang JL, Wong CY et al (2005) Relationship of chondrocyte apoptosis to matrix degradation and swelling potential of osteoarthritic cartilage. J Formos Med Assoc 104(4):264–272
Hashimoto S, Setareh M, Ochs RL et al (1997) Fas/Fas ligand expression and induction of apoptosis in chondrocytes. Arthritis Rheum 40(10):1749–1755
López-Armada M, Caramés B, Lires-Deán M et al (2006) Cytokines, tumor necrosis factor-α and interleukin-1β, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthr Cartil 14(7):660–669
Abramson SB (2008) Osteoarthritis and nitric oxide. Osteoarthr Cartil 16(Suppl 2):S15–S20
de la Lastra CA, Villegas I (2005) Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol Nutr Food Res 49(5):405–430
Udenigwe CC, Ramprasath VR, Aluko RE et al (2008) Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev 66(8):445–454
Manna SK, Mukhopadhyay A, Aggarwal BB (2000) Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappaB, activator protein-1 and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol 164(12):6509–6519
Holmes-McNary M, Baldwin AS Jr (2000) Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res 60(13):3477–3483
Surh YJ, Chun KS, Cha HH et al (2001) Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res 1(480–481):243–268
Estrov Z, Shishodia S, Faderl S et al (2003) Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood 102(3):987–995
Kuhn K, Hashimoto S, Lotz M (2000) IL-1 beta protects human chondrocytes from CD95-induced apoptosis. J Immunol 164(4):2233–2239
Kim SJ, Chun JS (2003) Protein kinase C alpha and zeta regulate nitric oxide-induced NF-kappa B activation that mediates cyclooxygenase-2 expression and apoptosis but not dedifferentiation in articular chondrocytes. Biochem Biophys Res Commun 303(1):206–211
Mendes AF, Carvalho AP, Caramona MM et al (2002) Role of nitric oxide in the activation of NF-kappaB, AP-1 and NOS II expression in articular chondrocytes. Inflamm Res 51(7):369–375
Martel-Pelletier J, Mineau F, Jovanovic D et al (1999) Mitogen-activated protein kinase and nuclear factor kappaB together regulate interleukin-17-induced nitric oxide production in human osteoarthritic chondrocytes: possible role of transactivating factor mitogen-activated protein kinase-activated protein kinase (MAPKAPK). Arthritis Rheum 42(11):2399–2409
Shalom-Barak T, Quach J, Lotz M (1998) Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem 273(42):27467–27473
Shakibaei M, John T, Seifarth C et al (2007) Resveratrol inhibits IL-1beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann N Y Acad Sci 1095(1):554–563
Dave M, Attur M, Palmer G et al (2008) The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum 58(9):2786–2797
Elmali N, Esenkaya I, Harma A et al (2005) Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm Res 54(4):158–162
Blanco FJ, Guitian R, Vazquez-Martul E et al (1998) Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum 41(2):284–289
D’Lima D, Hermida J, Hashimoto S et al (2006) Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum 54(6):1814–1821
Pelletier JP, Jovanovic DV, Lascau Coman V et al (2000) Selective inhibitor of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase–3 level. Arthritis Rheum 43(6):1290–1299
Pelletier JP, Lascau-Coman V, Jovanovic D et al (1999) Selective inhibition of inducible nitric oxide synthase in experimental osteoarthritis is associated with reduction in tissue levels of catabolic factors. J Rheumatol 26(9):2002–2014
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Gao, JS., Chen, JW. et al. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol Int 32, 1541–1548 (2012). https://doi.org/10.1007/s00296-010-1720-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-010-1720-y