Abstract
In Japan, the recommended dosage regimens of infliximab (IFX) for treatment of rheumatoid arthritis (RA) and Crohn’s disease (CD) are different. However, the differences have not been analyzed theoretically. In a previous study, we constructed a pharmacokinetic–pharmacodynamic model to investigate the effects of IFX for CD and found it useful to establish a rational dosage regimen of IFX for individual patients with CD. In the present study, we investigated whether the theory-based model could be used for cases of RA and also used it to evaluate the validity of the dosage regimen. The results obtained with our model were in good agreement with observed tender joint count (TJC) ratio data, which was considered to show the validity of our analysis. Thus, we concluded that the model could be used for patients with RA. Furthermore, a second administration of IFX given 2 weeks after the first infusion was important to achieve remission in the early stage of RA. We also compared the estimated pharmacodynamic parameters of RA with those of CD. The elimination rate constant of inflammation in RA was greater than that in CD, suggesting that the recovery from inflammation in RA is faster than that in CD, and indicating a reason for the difference in dosage between RA and CD. In conclusion, use of our model in light of the individual quantitative factor of tumor necrosis factor (TNF)-α allows establishment of IFX dosage regimens for individual patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation and progressive joint destruction [1]. In affected individuals, the destruction of cartilage and bone occurs by erosion mainly in the junction of cartilage, bone, and synovium, a region known as the pannus [2]. The peak age at onset ranges from 30 to 60 years, with a preponderance in females ranging from 3:1 to 4:1 [3].
Tumor necrosis factor (TNF)-α is a mediator of immunity and inflammation, with proinflammatory cytokines, such as IL-1, IL-6, and GM-CSF, linked in a network with TNF-α at its apex [2]. TNF-α induces a number of proinflammatory changes in endothelial cells and has been detected in the synovial fluid of patients with RA [4]. Thus, TNF-α plays a major role in RA and is a therapeutic target.
It has been reported that measurement of stool TNF-α concentrations may provide a valuable means of monitoring intestinal disease activity in patients with inflammatory bowel disease [5]. Crohn’s disease (CD) is characterized by chronic transmural inflammation in any part of the gastrointestinal tract [6], with major symptoms including diarrhea, abdominal pain, fistulas, and weight loss, and a peak age at onset ranging from the late teens to 20 years of age [3, 6].
Infliximab (IFX), a chimeric murine-human monoclonal IgG1 antibody, targets TNF-α and is used as a therapeutic agent for RA and CD, as it binds with a high affinity to TNF-α and neutralizes its effects [2, 7–9]. In Japan, the recommended dosage regimens of IFX for treatment of RA and CD are 3 and 5 mg/kg of body weight, respectively, at weeks 2 and 6 after the initial dose, and then every 8 weeks thereafter [10]. Although these dosage regimens have been established through clinical trial experience, they have not been analyzed theoretically.
In a previous study, we constructed a pharmacokinetic–pharmacodynamic model to determine the effects of IFX for CD and found it useful to establish a rational dosage regimen of IFX for individual patients [11]. In the present study, we investigated whether the model could be applied to cases of RA and also used it to evaluate the validity of the dosage regimen.
Methods
Pharmacokinetic and pharmacodynamic data for analysis
We collected the data about the time course of serum concentrations of IFX after administration of a single infusion of IFX in patients with RA at doses of 1, 3, or 5 mg/kg, [12] as well as in patients with CD at doses of 5, 10, or 20 mg/kg [13]. Clinical responsiveness of RA was evaluated by tender joint count (TJC), which has been used in clinical studies to determine the severity of RA activity. Values representing sequential changes in TJC after repeated intravenous infusions of 1, 3, or 5 mg/kg of IFX at 0, 2, and 6 weeks, were obtained at 0, 3, 7, 14, 42, and 70 days after starting administration [10]. In addition, the clinical responsiveness of CD was evaluated using Crohn’s disease activity index (CDAI), which is commonly utilized in clinical studies to determine the severity of CD activity. In another previous study [11], values for sequential changes in CDAI with repeated IFX treatments were used to investigate 2 groups of patients who responded to a single infusion of IFX within 2 weeks, according to the following protocol [14]. Following assessment of response after week 2, all patients were randomly grouped with those who received repeated infusions of 5 mg/kg of IFX at weeks 2 and 6, and then every 8 weeks thereafter until week 46 (group 1), or those who received 5 mg/kg of IFX at weeks 2 and 6, followed by a single administration of 10 mg/kg (group 2).
Analysis of time courses of serum concentration of IFX
Previous findings showed that with dosage amounts ranging from 1 to 20 mg/kg, the clearance of IFX is unchanged by increasing the dose, whereas linear relationships can be observed among the dose administered, maximum serum concentration (C max), and area under the curve (AUC) [15]. Therefore, in the present study, the time courses of serum concentration after a single infusion of IFX were analyzed using the following one-compartment pharmacokinetic model.
where C IFX (μM), \( C_{{_{\text{IFX}} }}^{0} \) (μM), k e (day−1), and D (mg/kg) represented the serum concentration of IFX, serum concentration of IFX immediately after administration of 5 mg/kg of IFX (t = 0), elimination rate constant, and dose, respectively. To estimate the pharmacokinetic parameters of \( C_{{_{\text{IFX}} }}^{0} \) and k e in patients with RA, the serum concentration of IFX after a single infusion of IFX at a dose of 1, 3, or 5 mg/kg was simultaneously fitted to Eq. 1 using the nonlinear least squares methods. On the other hand, those of patients with CD were derived from previous study [11]. We used the nonlinear least squares program MLAB (Civilized Software) for analysis.
Pharmacodynamic model of IFX administered for patients with RA and CD
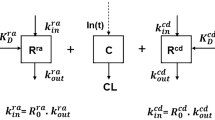
The pharmacodynamic model derived from the pharmacokinetic profile of IFX and turnover rate of TNF-α are shown in Fig. 1. With this model, we assumed that the degree of inflammation was changed by the binding of IFX to TNF-α, which served to neutralize its biological activity.
Pharmacokinetic and pharmacodynamic model for anti-inflammatory effects of infliximab (IFX). IFX was shown to be eliminated at the rate constant of k e (day−1), while tumor necrosis factor (TNF)-α was generated at the rate constant of k s (μM day−1) and eliminated at the rate constant of k T (day−1). TNF-α forms a complex with IFX at the binding rate constant of k on (μM−1 day−1) and is dissociated from the TNF-IFX complex at a dissociation rate constant of k off (day−1). Inflammation occurs at the rate constants of K (day−1) and K′ (day−1) and is remitted in vivo at the rate constant of k r (day−1)
TNF-α is generated at a rate constant of k s (μM day−1) and eliminated at a rate constant of k T (day−1). It forms a complex with IFX at a binding rate constant of k on (μM−1 day−1) and is dissociated from the TNF-IFX complex at a dissociation rate constant of k off (day−1). Thus, the concentration of TNF-α (C TNF) and TNF-IFX complex (C TNF-IFX) was represented as follows:
where k I (μM−1) is the dissociation constant. In the absence of IFX, C TNF was expressed as C TNF = k s/k T.
Assuming that the inflammation caused by TNF-α at the rate constant of K (day−1) was according to the proportion of the unbound TNF-α, K was determined according to Eq. 5, as follows:
where K max (day−1) is K in the absence of IFX. We considered that inflammation caused by factors other than TNF-α at the rate constant of K′ (day−1), as well as inflammation caused by K and K′, would be remitted in vivo at the rate constant of kr (day−1). Therefore, the value of the number of tender joints at t (weeks) (Et) in the presence of IFX was represented as follows:
Furthermore, the value for TJC in the absence of IFX (E 0) was expressed by the following equation:
In addition, the values for sequential changes of TJC were represented by the ratio (TJC/ER) of TJC at t (weeks) (E t) to E 0 were expressed by:
Analysis of clinical response of IFX administered for patients with RA and CD
Since k on and k T represented binding rate constants of IFX with TNF-α and the elimination rate constant of TNF-α, respectively, we concluded that these were common values between CD and RA. Then, to estimate k on, k T, k s, K max, K′, and k r values, we simultaneously fitted ER data from patients administered repeated infusions of IFX at the tested doses to the aforementioned Eqs. 2–8. A previously reported k I value (0.046 nM) [8] was adopted for this study. We used the nonlinear least squares program MLAB (Civilized Software) for analysis.
Simulation of values for sequential changes of TJC following administrations of IFX at various intervals
Changes in ER following repeated infusions of IFX at a dose of 3 mg/kg at various intervals (Fig. 2) were simulated using the estimated pharmacokinetic and pharmacodynamic parameters and assessed to determine a rational dosage regimen. For the present study, the threshold of positive clinical remission was defined as a decrease in TJC of 50%.
Results
Time course analysis of serum concentration of IFX
The time courses of serum concentrations of IFX following a single infusion at a dose of 1, 3, or 10 mg/kg for patients with RA, along with fitted curves based on the nonlinear least squares methods, are shown in Fig. 3. The fitted curves were well matched to the observed data. The estimated pharmacokinetic parameters of IFX administered for RA and pharmacokinetic parameters of IFX administered for CD, which were reported previously, [11] are shown in Table 1. The pharmacokinetic parameters of \( C_{{_{\text{IFX}} }}^{0} \) and k e were not significantly different between RA and CD.
Analysis of sequential changes of TJC and CDAI
The sequential changes in the TJC and CDAI ratios following repeated infusions of IFX and the fitted curves based on the simultaneous nonlinear least squares methods are shown in Fig. 4. The estimated pharmacodynamic parameters are shown in Table 2. The relationships between the time course effects of IFX derived from the pharmacodynamic model in the present study were matched to data reported for TJC and CDAI, while the estimated pharmacodynamic parameters of CD were matched to reported values [11]. As a result, we found that K′ and k r for patients with RA were greater than those for patients with CD.
Sequential changes in a CDAI ratio and b TJC ratio after repeated infusions. Symbols were derived from (a) data reported in literature [14] or an interview form from patients receiving REMICADE® (b). The lines shown are fitted curves
Simulation of values for sequential changes of TJC following administrations of IFX at two intervals
The time courses of TJC ratios following repeated infusions of IFX at a dose of 3 mg/kg at the tested intervals were simulated from the estimated pharmacokinetic parameters (Fig. 5). With Schedule 2 a greater therapeutic effect was required to reach clinical remission than with Schedule 1. Therefore, our results confirmed the importance of administration at 2 weeks after the first infusion to maintain the therapeutic effect.
Discussion
In the present study, we analyzed whether the present pharmacokinetic–pharmacodynamic model of the effect of IFX on CD was in good agreement with observed TJC ratio data, as well as the validity of the IFX dosage regimen for patients with RA. We found that results obtained with the model were in good agreement with observed TJC ratio data, which was considered to show the validity of our analysis. Thereafter, the model was shown useful for patients with RA.
We also compared estimated pharmacodynamic parameters of RA with those of CD. The estimated K′ value of RA was about 3 × 105 times greater than that of CD, suggesting that inflammation associated with RA is more affected by factors other than TNF-α than that associated with CD. On the other hand, the estimated K max value of RA was about 380 times greater than the K′ value, indicating that TNF-α is the major factor of inflammation in RA. This result is considered reasonable, as it has been reported that the disease state of RA is correlated with the serum concentration of TNF-α [16]. The estimated k r value of RA was also about 40 times greater than that of CD, suggesting that the recovery from inflammation in RA is faster than that in CD. Together, these findings indicate a reason for the differences in dosage between RA and CD.
The estimated half-life value of TNF-α, calculated from k T, was 16.5 days, which was greater than the half-life of serum TNF-α, reported to be several minutes [17, 18]. Therefore, it is considered that IFX does interact not only with soluble TNF-α but also with transmembrane TNF-α, which is produced by monocytes, macrophages, and T cells, and released from cells as soluble TNF-α by metalloproteinase [12]. Although soluble TNF-α is rapidly inactivated and eliminated, transmembrane TNF-α may exhibit the same behavior with these cells, whose lifetime has been reported to be several months [19]. Thus, we considered that the estimated value for k T obtained in the present study consisted of the elimination rate constants for both transmembrane and soluble TNF-α.
When IFX is given as repeated infusions for RA, administration at 2 weeks after the initial infusion is important. If not given at that 2-week time point, the therapeutic effect is not maintained from weeks 2 to 6. Our results confirmed the importance of administration at 2 weeks after the first infusion to maintain an adequate therapeutic effect.
In conclusion, the present results indicated that our theory-based model can be used for RA and is also useful to establish a rational dosage regimen of IFX for patients with RA and CD. Furthermore, we found that the amount of TNF-α is correlated with inflammation associated with RA and CD, as well as the therapeutic effect of IFX. It is considered that a dosage regimen of IFX for individual patients can be established with this model in view of the individual quantitative factor of TNF-α.
References
Kaneko M, Tomita T, Nakase T, Ohsawa Y, Seki H, Takeuchi E, Takano H, Shi K, Takahi K, Kominami E et al (2001) Expression of proteinases and inflammatory cytokines in subchondral bone regions in the destructive joint of rheumatoid arthritis. Rheumatology (Oxford) 40:247–255
Feldmann M, Brennan FM, Maini RN (1996) Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 14:397–440. doi:10.1146/annurev.immunol.14.1.397
Imura Y (2008) Wakariyasui Naikagaku. bunkoudou, Tokyo
Eigler A, Sinha B, Hartmann G, Endres S (1997) Taming TNF: strategies to restrain this proinflammatory cytokine. Immunol Today 18:487–492
Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT (1992) Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 339:89–91
Garnett WR, Yunker N (2001) Treatment of Crohn’s disease with infliximab. Am J Health Syst Pharm 58:307–316 quiz 317–309
Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, Wagner C (2002) Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 301:418–426
Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J (1995) Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions. Cytokine 7:251–259. doi:10.1006/cyto.1995.0029
Siegel SA, Shealy DJ, Nakada MT, Le J, Woulfe DS, Probert L, Kollias G, Ghrayeb J, Vilcek J, Daddona PE (1995) The mouse/human chimeric monoclonal antibody cA2 neutralizes TNF in vitro and protects transgenic mice from cachexia and TNF lethality in vivo. Cytokine 7:15–25. doi:10.1006/cyto.1995.1003
REMICADE for I.V. infusion100 (2007) Product Information
Furuya Y, Ozeki T, Takayanagi R, Yokoyama H, Okuyama K, Yamada Y (2007) Theory based analysis of anti-inflammatory effect of infliximab on Crohn’s disease. Drug Metab Pharmacokinet 22:20–25
Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S et al (1997) A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385:729–733. doi:10.1038/385729a0
Cornillie F, Shealy D, D’Haens G, Geboes K, Van Assche G, Ceuppens J, Wagner C, Schaible T, Plevy SE, Targan SR et al (2001) Infliximab induces potent anti-inflammatory and local immunomodulatory activity but no systemic immune suppression in patients with Crohn’s disease. Aliment Pharmacol Ther 15:463–473
Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W et al (2002) Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 359:1541–1549. doi:10.1016/S0140-6736(02)08512-4
Couriel DR, Hicks K, Giralt S, Champlin RE (2000) Role of tumor necrosis factor-alpha inhibition with inflixiMAB in cancer therapy and hematopoietic stem cell transplantation. Curr Opin Oncol 12:582–587
Robak T, Gladalska A, Stepien H (1998) The tumour necrosis factor family of receptors/ligands in the serum of patients with rheumatoid arthritis. Eur Cytokine Netw 9:145–154
Blick M, Sherwin SA, Rosenblum M, Gutterman J (1987) Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res 47:2986–2989
Bocci V (1991) Interleukins. Clinical pharmacokinetics and practical implications. Clin Pharmacokinet 21:274–284
Furusawa SIJ (1998) Laboratory hematology, 3rd edn. Igaku-Shoin, Tokyo
Acknowledgments
We are grateful to Dr. Takeshi Ozeki for advising this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimura, K., Takayanagi, R., Yokoyama, H. et al. Theory-based analysis of anti-inflammatory effect of infliximab on Crohn’s disease and rheumatoid arthritis. Rheumatol Int 32, 145–150 (2012). https://doi.org/10.1007/s00296-010-1553-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-010-1553-8