Abstract
This study aims to review the cumulative clinical and laboratory data of 1,790 Chinese patients with systemic lupus erythematosus. Data were compared separately between male and female patients for each disease onset age groups and among three disease onset age groups in male and female patients. The ratio of female to male was 9.2:1, with differences among three age groups. There was no difference in mean age at onset between females and males. But diagnosis delay in male patients is shorter than in females. When compared with females, in adult-onset patients, males presented more frequently with serositis, pleuritis and discoid rash, but less frequently with malar rash, alopecia, oral ulcers, elevated erythrocyte sedimentation rate, anti-nuclear, anti-SSA and anti-SSB antibodies. In younger-onset group, males have less discoid rash. In older-onset group, males have less anti-SSA antibodies. In male patients, only anti-SSB antibodies were different in three age groups and negatively correlate to age. Among female patients, age had negative correlations with malar rash, discoid rash, photosensitivity, anti-dsDNA, anti-Sm, anti-SSB and anti-rRNP antibodies, but positive correlation with leucopenia. We conclude that women of childbearing age possess a distinct clinical and laboratory profile. In addition, differences in disease manifestations seem to be correlated with female sex hormones rather than age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disease in which a broad spectrum of clinical and immunological manifestations exists. The pathogenesis and etiology remains unclear, but multiple genetic and environmental factors are likely to play a role in its pathogenesis. It is well recognized that SLE predominantly affects women, especially in reproductive age. An increase in frequency of SLE among females is believed to be related to the effect of endogenous sex hormones [1]. In fact, supporting evidence is provided by the literature showing that the ratio of female to male is much lower in prepuberal children and after menopause, although a female predominance remains [2–4]. In addition, the importance of sex hormones in the pathogenesis of SLE is also illustrated in the lupus-prone mouse models; females do worse than their male counterparts and have early autoimmune disease and mortality. An administration of exogenous estrogens exacerbates SLE, while androgens have a protective role [5–7].
Clinical and laboratory characteristics are variable and are influenced by age of onset and gender. Onset of SLE under the age of 15 years or beyond the age of 50 years is unusual, and many previous studies have suggested that these groups of patients have a clinical presentation different from that seen in adult patients; the differences were also observed between male and female lupus patients. However, the conclusions about the distinguishing disease features in different groups were not consistent. These discrepancies may be influenced by genetic differences and the small sample size in several studies [2, 3, 8–19]. In addition, most previous studies compared the prevalence of various disease manifestations between male and female SLE patients or among different age groups, but only a few of studies had excluded the potential confounding factors, such as age that may affect the results when the analysis is influenced by sex.
Therefore, in the present study, we performed a retrospective analysis from a large series of consecutive selected Chinese patients with SLE to evaluate whether different disease manifestations exists between male and female in different age groups at disease onset. In addition, the differences among younger-onset, adult-onset and older-onset groups in male or female patients were also analyzed.
Materials and methods
Patients
Consecutive patients (n = 1,790) with SLE were recruited from the Departments of Rheumatology at Anhui Provincial Hospital and at the First Affiliated Hospital to Anhui Medical University. SLE was diagnosed by the presence of four or more ACR diagnostic criteria (1982), revised in 1997 [20, 21]. All patients were divided into three groups according to the age at disease onset: younger-onset group (<15 years), adult-onset group (15≥ and <50 years) and older-onset group (≥50 years).
Collection of clinical and laboratory data
Demographic data, clinical data and laboratory data were collected from hospital records or by questionnaire, and reviewed by experienced physicians.
Clinical manifestations were recorded and analyzed separately. Renal involvement was identified by the presence of at least one of the following four criteria: (1) persistently elevated urinary pH value (≥6.0); (2) persistently elevated serum creatinine level (>1.5 mg/dl) and/or impaired creatinine clearance (<50 ml/min); (3) persistent proteinuria (≥500 mg/day) for more than 3 months; (4) a pathologic urine sediment (consisting of >10 red blood cells per high-power field or red blood cell casts), which was confirmed by kidney biopsy, according to standard criteria. The occurrence of central nervous system (CNS) involvement was defined according to the ACR nomenclature and case definitions for neuropsychiatric lupus syndromes [22]. Serositis was defined as pleurisy documented by clinical examination and pleural effusion on chest radiography or pericarditis documented by pericardial effusion on echocardiography. Myositis was documented by the presence of proximal muscle weakness associated with increased aldolase or creatine phosphokinase levels and compatible findings on electromyography and muscle biopsy. Diagnosis of vasculitis was established with duplex sonography. Other common clinical features of SLE patients such as malar rash, discoid rash, oral ulcer, arthritis, alopecie and photosensitivity were recorded.
Laboratory abnormalities were also recorded, mainly including leukopenia (white blood cell count <4,000/mm3), thrombocytopenia (platelet count <100,000/mm3), the occurrence of proteinuria, elevated erythrocyte sedimentation rate (ESR) (male >15 mm/h; female >20 mm/h), complement reduction (by immunoturbidimetry; ITM). The present antibodies to nuclear, Sm, SSB, SSA and ribosomal RNP (rRNP) were evaluated by indirect immunofluorescence technique and anti-dsDNA antibodies (by enzyme-linked immunosorbent assay; ELISA).
Statistical analysis
All data were analyzed using SPSS 10.01 software (SPSS Inc, 2000). Continuous variables were described as mean with standard deviation (SD). Categorical variables were described as percentages. The clinical and laboratory manifestations of SLE were compared between male and female lupus patients in different disease onset age groups, and disease manifestations also compared among three disease onset age groups in male and female patients. Comparisons between continuous variables were made using the Student’s t test when appropriate. Chi-square or Fisher’s exact test was used for categorical variables. Probability level less than 0.05 in two-tailed test were used as a criterion of significance.
Results
Demographic data
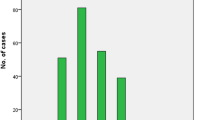
The demographic characteristics of the patients are presented in Table 1. The cohort consisted of 1,790 Chinese patients with SLE. 108 (6.0%) patients age under 15 years, 131 (7.3%) patients at or over the age of 50 years, and a majority of the patients, age 15–50 years, accounted for 86.6%. There were 1,614 (90.2%) were females and 176 (9.8%) were males; the ratio of female to male was 9.2:1 and was different in three age groups, in adult onset patients was 10.5:1, in younger onset group (4.7:1) and older onset group (5.0:1; P < 0.001). In 106 out of 176 male patients (60.1%), the onset of the disease was in the range of 15–25 years of age, with the maximum peak between 15 and 28 years of age. On the other hand, the female patients had their maximum peak between 21 and 35 years of age (Fig. 1).
The mean age at onset was 31.0 ± 12.0 years. Diagnosis delay was 32.0 ± 59.0 weeks. There was no significant difference in mean age at onset between female and male patients (30.9 ± 11.5 vs. 31.5 ± 15.9 years, P > 0.05). But the diagnosis delay in male patients is shorter than in females (24.0 ± 44.1 vs. 32.8 ± 60.4 weeks, P < 0.05).
Cumulative clinical and laboratory manifestations with gender
In adult onset patients, when compared with females, male gender was significantly associated with serositis (24.4 vs. 16.0, P < 0.05), pleuritis (15.6 vs. 10.0, P < 0.05) and discoid rash (11.9 vs. 5.0, P < 0.01). But the frequency of malar rash (42.2%), alopecia (13.4%) and oral ulcers (6.7%) were less than that in female patients (51.7, 23.3 and 14.4%, respectively, P < 0.05). There was no significant difference between the two groups in other clinical features (including fever, renal involvement, photosensitivity, pericarditis, vasculitis, CNS involvement, myositis, thrombocytopenia, leukopenia, proteinuria, hematuria, low C3, anti-dsDNA, anti-Sm and anti-rRNP antibodies).
In younger-onset patients, there was only difference in discoid rash between male and female patients (0 vs. 14.6%, P < 0.05). No significant difference in clinical features was observed between two sexes in older onset patients (Table 1).
In adult-onset SLE patients, elevated ESR was observed in 75.0% of male patients, which was less than that in females (83.7%, P < 0.05). ANA was positive in 74.6% of male patients and 83.6% of female patients (P < 0.01). Anti-SSA antibodies were in 23.3 versus 36.6% (P < 0.05), and Anti-SSB antibodies in 7.5 versus 16.5% (P < 0.05). No significant differences were found in other laboratory parameters (including thrombocytopenia, leucopenia, proteinuria, hematuria, low C3, anti-dsDNA, anti-Sm and anti-rRNP antibodies) between males and females.
In younger-onset patients, no difference was found in laboratory results between male and female patients. In older-onset groups, there were also no significant differences in most laboratory results between two groups, except anti-SSA antibodies more frequently presented in females than in males (25.3 vs. 4.5.%, P < 0.05) (Table 2).
Cumulative clinical and laboratory manifestations with age
In male patients, only the prevalence of anti-SSB antibodies were different among three onset patient groups (P < 0.05), and then, further test by Spearman rank correlation analysis, there was adverse correlation between anti-SSB antibodies and age group (correlation coefficient [r s ] = −0.163, P < 0.05). No difference in other clinical or laboratory features among the three groups were found (P > 0.05) (Table 3).
In female patients, the frequencies of malar rash, discoid rash, photosensitivity, leukopenia and some autoantibodies, including anti- dsDNA, anti-Sm, anti-SSB and anti-rRNP antibodies were different among three age groups (P < 0.05). Spearman rank correlate analysis showed adverse correlations of malar rash, discoid rash, photosensitivity, anti-dsDNA, anti-Sm, anti-SSB and anti-rRNP antibodies with age groups (P < 0.01). A positive correlation was found between leucopenia and age groups (r s = −0.163, P < 0.05) (Table 4).
Discussion
In the present study, we analyzed whether gender and age have influence on the manifestations of SLE. Although previous studies have compared the prevalence of clinical and laboratory features between different age groups or sex groups, the potential confounding factors were not controlled in most studies, such as age that may affect the results when the analysis is influenced by sex. In order to eliminate the effect of confounding factors, in our study, all patients were divided into three groups according to the age at disease onset, and data were compared separately between male and female patients for each disease onset age groups and among three disease-onset age groups in male and female patients.
There was no significant difference in the mean age at onset between male and female patients, which was similar to the most previous studies. The peak incidence of males was occurred in age 15–28 years and 22–35 years in female patients. In our study, the general ratio of female to male was 9.2:1; this ratio in adult onset patients was 10.5:1, and decreased in younger onset group (4.7:1) and older onset group (5.0:1), which is in agreement with previous studies [3, 4]. In our series the diagnosis delay was significantly lower in men, similar to Garcia et al. [23] reported in 1,214 Latin-American patients with SLE. The possible reason for this is the severity of the manifestation and the fast progression of symptoms until they fulfilled the ACR classification criteria [24].
Many studies have published conflicting results concerning gender or age differences in the clinical and laboratory manifestations of SLE (Table 5). In the current study, we analyzed the association of sex with clinical and laboratory manifestation, stratified by age at onset. Except for discoid rash more likely presented in younger-onset female patients and anti-SSA antibodies more frequently presented in older-onset female patients, there was no significant difference in other common clinical and laboratory features between two sexes in younger- or older-onset patients. However, when compared with females in adult-onset group, males were significantly associated with higher presence of serositis, pleuritis and discoid rash, but less in malar rash, alopecia, oral ulcers and some laboratory abnormal, including ESR, ANA, anti-SSA and anti-SSB antibodies. In accordance with our results, Koh et al. [11] reported that anti-SSA and anti-SSB antibodies was less common in 61 Oriental (43 Chinese) male patients. Mok et al. [13] also found a lower prevalence of alopecia and anti-SSA antibodies in 51 Chinese male patients with SLE. Voulgari et al. [12] revealed that malar rash and increased ESR are more frequently present in women. No significant difference in laboratory features between males and females in our younger-onset patients is similar to Alsaeid et al. [4] revealed from 35 Kuwaiti children. Major organ involvements such as nephritis and CNS involvements, however, were not significantly different between the two sexes in our series. This is consistent with Mok et al. [13] who also failed to show a difference in major organ disease of SLE between males and females. Significant differences in discoid rash between males and females have been reported by previous studies [14]. Interestingly, in this study, a higher frequency of discoid rash was found in adult onset patients but less in younger onset patients among males.
We also examined the association of age at disease onset with clinical and laboratory manifestation in male and female patients groups, respectively. The results showed that only anti-SSB antibodies were different in three age groups in male patients and negatively correlated to age. Among female patients, there were adverse correlations of malar rash, discoid rash, photosensitivity, anti-dsDNA, anti-Sm, anti-SSB and anti-rRNP antibodies with age groups, but leucopenia was positively correlated to age. Our results are in agreement with those from Voulgari et al. [14] who also mentioned that anti-SSB antibodies were less common in older women with SLE. Ho et al. [3] reported lower prevalence of malar rash in 25 older onset patients. By multivariate regression analysis, after adjusting the confounding factors (including gender), Ward et al. [8] found that malar rash and anti-dsDNA antibodies were less frequent with increasing age.
Diversity in genetic, environmental and sociodemographic factors might partly explain the differences among previous studies. In addition, the lack of standardization of patients’ selection, difference in study design as mentioned previous and the low sample size in some studies were also partly accounted for the discrepancy.
There are several limitations in the present study. First, data were collected retrospectively; the potential diagnostic bias was unavoidable. Second, the patients in our research were composed of those who were referred to the tertiary hospitals, which may misrepresent the patients who were admitted to hospitals of different degrees.
Taken together, this study demonstrates that women of childbearing age possess a distinct clinical and laboratory profile. In addition, differences in disease manifestations seem to be correlated with female sex rather than age.
References
Hochberg MC (1990) Systemic lupus erythematosus. Rheum Dis Clin North Am 16:617–639
Tucker LB, Menon S, Schaller JG et al (1995) Adult-and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Br J Rheumatol 34:866–872. doi:10.1093/rheumatology/34.9.866
Ho CT, Mok CC, Lau CS et al (1998) Late onset systemic lupus erythematosus in southern Chinese. Ann Rheum Dis 57:437–440
Alsaeid K, Kamal H, Haider MZ (2004) Systemic lupus erythematosus in Kuwaiti children: organ system involvement and serological findings. Lupus 13:613–617. doi:10.1191/0961203304lu1075xx
Carlsten H, Tarkowski A (1993) Histocompatibility complex gene products and exposure to oestrogen: two independent disease accelerating factors in murine lupus. Scand J Immunol 38:341–347. doi:10.1111/j.1365-3083.1993.tb01736.x
Roubinian JR, Papoian R, Talal N (1977) Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. J Clin Invest 59:1066–1070. doi:10.1172/JCI108729
Roubinian JR, Talal N, Greenspan JS et al (1979) Delayed androgen treatment prolongs survival in murine lupus. J Clin Invest 63:902–911. doi:10.1172/JCI109390
Ward MM, Studenski S (1990) Systemic lupus erythematosus in men: a multivariate analysis of gender differences in clinical manifestations. J Rheumatol 17:220–224
Aydintug AO, Domenech I, Cervera R et al (1992) Systemic lupus erythematosus in males: analysis of clinical and laboratory features. Lupus 1:295–298. doi:10.1177/096120339200100504
Costallat LT, Coimbra AM (1993) Systemic lupus erythematosus in 18 Brazilian males: clinical and laboratory analysis. Clin Rheumatol 12:22–25. doi:10.1007/BF02231783
Koh WH, Fong KY, Boey ML (1994) Systemic lupus erythematosus in 61 Oriental males. A study of clinical and laboratory manifestations. Br J Rheumatol 33:307–308. doi:10.1093/rheumatology/33.4.339
Molina JF, Drenkard C, Molina J et al (1996) Systemic lupus erythematosus in males. A study of 107 Latin American patients. Medicine 75:124–130. doi:10.1097/00005792-199605000-00002
Mok CC, Lau CS, Chan TM et al (1999) Clinical characteristics and outcome of southern Chinese males with systemic lupus erythematosus. Lupus 8:188–196. doi:10.1191/096120399678847605
Voulgari PV, Katsimbri P, Alamanos Y et al (2002) Gender and age differences in systemic lupus erythematosus. A study of 489 Greek patients with a review of the literature. Lupus 11:722–729. doi:10.1191/0961203302lu253oa
Othmani S, Louzir B, Group d’etude du lupus (2002) Systemic lupus erythematosus in 24 tunisian males: clinico-biological analysis and clinical course. Rev Med Interne 23:983–990. doi:10.1016/S0248-8663(02)00684-7
Pande I, Sekharan NG, Kailash S et al (1993) Analysis of clinical and laboratory profile in Indian childhood systemic lupus erythematosus and its comparison with SLE in adults. Lupus 2:83–87. doi:10.1177/096120339300200204
Costallat LT, Coimbra AM (1994) Systemic lupus erythematosus: clinical and laboratory aspects related to age at disease onset. Clin Exp Rheumatol 12:603–607
Font J, Cervera R, Espinosa G et al (1998) Systemic lupus erythematosus (SLE) in childhood: analysis of clinical and immunological findings in 34 patients and comparison with SLE characteristics in adults. Ann Rheum Dis 57:456–459
Font J, Pallarés L, Cervera R et al (1991) Systemic lupus erythematosus in the elderly: clinical and immunological characteristics. Ann Rheum Dis 50:702–705. doi:10.1136/ard.50.10.702
Tan EM, Cohen AS, Fries JF et al (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277. doi:10.1002/art.1780251101
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725. doi:10.1002/art.1780400928
ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature (1999) The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 42:599–608. doi:10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F
Garcia MA, Marcos JC, Marcos AI et al (2005) Male systemic lupus erythematosus in a Latin-American inception cohort of 1214 patients. Lupus 14:938–946. doi:10.1191/0961203305lu2245oa
Arbuckle MR, James JA, Dennis GJ et al (2003) Rapid clinical progression to diagnosis among African-American men with systemic lupus erythematosus. Lupus 12:99–106. doi:10.1191/0961203303lu334oa
Acknowledgments
This work was partly supported by grants from the National Natural Science Foundation of China (30571608, 30771848) and the key program of National Natural Science Foundation of China (30830089). We are indebted to Dr. Bo-Ke Zhang, the First Affiliated Hospital of Anhui Medical University, and Dr. Guo-Sheng Wang, Anhui Provincial Hospital, for assistance in case identification. We also thank Hang Hong, Xiao-Yue Chu, Bing Dai and Guo-Ping Chen, students of Anhui Medical University, for assistance in data collection. Their cooperation was invaluable to this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
J.-B. Feng and J.-D. Ni contributed equally to this work and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Feng, JB., Ni, JD., Yao, X. et al. Gender and age influence on clinical and laboratory features in Chinese patients with systemic lupus erythematosus: 1,790 cases. Rheumatol Int 30, 1017–1023 (2010). https://doi.org/10.1007/s00296-009-1087-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-009-1087-0