Abstract
We examined the membrane expression of inducible Hsp70 and HSP receptors like TLR2, TLR4, CD14, CD36, CD40 and CD91 on fibroblast-like synovial cells (SC) derived from synovial tissue in 23 patients with rheumatoid arthritis (RA), who underwent synovectomy by using flow cytometric analysis. For comparison, autologous skin fibroblasts (SF) derived from the operation wound were tested. Significantly higher Hsp70 expression was found on synovial cells than on skin fibroblasts (median SC 21.4% × SF 5.0%, P < 0.001). Both synovial cells and skin fibroblasts expressed high levels of cell surface CD91 (median SC 80.2% × SF 79.2%), however, no or low levels of CD14, CD40, TLR2, TLR4 and CD36. Further, we observed high co-expression of CD91 and Hsp70 on RA synovial cells (median 18.6%), while skin fibroblasts showed only background Hsp70 expression (median 3.9%, P < 0.001). Since we demonstrated the high prevalence of inducible Hsp70 in RA synovial fluids, we speculate that Hsp70 might be captured onto the membrane of synovial cells from the extracellular space via the CD91 receptor. The significance of the Hsp70 interaction with synovial cells via CD91 remains undefined, but may mediate other non-immune purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current evidence suggests that the HSP70 family, heat shock proteins with a molecular weight of about 70 kDa, may play a role in the pathogenesis of various autoimmune diseases involving rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA).
By using enzyme-linked immunosorbent assay (ELISA), we could frequently detect antibodies against Hsp70, the major heat-inducible form of the HSP70 group, in sera of patients with JIA [1].
We also demonstrated that fibroblast-like synovial cells derived from synovial tissues of patients with a severe course of RA and JIA were strongly positive for membrane-expressed Hsp70 [2]. Similarly, Schett et al. reported an enhanced expression of Hsp70 in RA synovial tissue using Western blotting, immunohistochemistry and immunofluorescence [3].
Martin et al. detected dramatically increased level of Hsp70 in RA synovial fluid versus normal human sera, RA sera, osteoarthritis and gout synovial fluid. Moreover, the authors demonstrated high levels of Hsp70 on the surface of myeloid dendritic cells (DCs) in synovial fluids of patients with RA that occurred concurrently with CD91 and CD14 [4].
Members of the HSP70 family interact with a wide variety of antigenic peptides from pathogens, as well as self peptides discussed to be involved in autoimmunogenic processes [5]. It has been reported that HSP-chaperoned antigenic peptides can be presented via MHC classes I and II molecules and thus enhanced the activation of lymphocytes [5]. Extracellular Hsp70-peptide complexes can be detected in MHC class II-enriched compartments after receptor-mediated endocytosis [6]; complexes from the cytosol may reach the MHC class II presentation pathway via autophagic processes [7].
HSP70 molecules were found to bind peptide sequences comprising the shared epitope (SE) sequences 70QKRAA74, 70QRRAA74, 70RRRAA74, a highly conserved motif of similar amino acid sequences found in HLA-DRB1 molecules associated with an increased risk for rheumatoid arthritis [8]. It was reported that HSP70 molecules do not exclusively interact with the shared epitope sequences, but also with most other sequences found in the HV-3 region of HLA-DR molecules, with the exception of the amino acid sequence 70DERAA74 (SE), a sequence exclusively found in RA-protective HLA-DR molecules. It suggested a possible association of non-binding of Hsp70 to HLA-DR molecules or its 70–74 fragments and protection from RA [9].
It has been speculated that Hsp70 might be translocated to the cell surface from the cytosol in response to sustained stress and/or that Hsp70 might be captured onto the cell surface from the extracellular space via HSP receptors [2,4].
The goal of the current study was to estimate the expression of the most common HSP receptors such as toll-like receptor (TLR) 2 and 4, CD14 (a receptor for endotoxin—lipopolysaccharide), CD36 (collagen type I and thrombospondin receptor), CD40 (a receptor molecule on the cell surface of B cells, endothelial and epithelial cells) and CD91 (α2-macroglobulin/low-density lipoprotein receptor) on fibroblast-like synovial cells derived from synovial tissue and skin fibroblasts derived from the operation wound in patients with RA who underwent synovectomy.
Materials and methods
Patients
Local ethics committee approval and informed consent were obtained for all individuals involved in this study. The cohort consisted of 23 patients (22 females, 1 male) aged 29–79 years (mean 56.6, median 57 years) who fulfilled the American College of Rheumatology criteria for RA [10] with disease duration ranging from 3 to 32 years (median 16 years). A total of 22 patients suffered from rheumatoid factor (RF)-positive polyarthritis and one of them had RF-negative polyarthritis. Clinical disease activity was assessed using the physician’s global assessment of overall disease activity. All patients had active disease at the time of testing. The patients were treated depending on the stage of the disease with non-steroid anti-rheumatics (NSAIDs), corticosteroids (C) and/or disease-modifying antirheumatics (DMARDs).
Synovial cells were derived from RA affected synovial tissues (finger joint n = 1, metacarpophalangeal joint n = 3, elbow joint n = 1, shoulder joint n = 1; metatarsophalangeal joint n = 10, ankle joint n = 2, knee joint n = 1 and hip joint n = 4) and skin fibroblasts from RA non-affected operation wound.
ELISA
Using ELISA, we investigated the levels of inducible Hsp70 in synovial fluid and serum samples of patients with RA.
Sera of 24 age-matched healthy controls were included in the ELISA assays as a control.
Inducible Hsp70 was measured using a commercial quantitative sandwich ELISA (Stressgen, Canada) according to manufacturer’s instructions. Optical density was measured at 450 nm using ELISA plate reader (Dynex Technologies, MRX II, USA). Hsp70 concentrations from synovial fluid and serum samples were quantitated by interpolating absorbance readings from a standard curve and expressed as nanograms per milliliter.
Cell cultivation and preparation for fluorescent-activated cell sorting (or flow cytometry) analysis
Initially, the skin tissue was trimmed of epidermis, then both synovial and skin tissues were cut to 2 × 2 mm sections and cultured in AmnioMax medium (Gibco, Invitrogen Corporation, NY, USA) containing 1% antibiotics (Penicillin-Streptomycin, Sigma Biosciences, St Louis, MO, USA) and 1% Fungizone (Antimycotic, Gibco, Invitrogen Corporation, NY, USA) at 37°C with 5% C02 for 2 months on average. The medium was replaced twice a week. Upon reaching confluence, the cells were trypsinized using 0.05% trypsin/0.02% ethylenediamine tetraacetic acid (EDTA) in phosphate buffered saline (PBS) (PAN Biotech, GmbH, Germany) for 30 s, after which 1 ml of medium was added. The suspended cells were washed and resuspended again in 1 ml of PBS. The approximate cell number and viability were determined by Trypan Blue Exclusion method. All cells were viable after trypsinization. For the FACS analysis, the minimal amount used was 0.1 × 106 of the cells per tube.
Flow cytometry analysis
The adherent cells on primary cultures to third passages were used for FACS analysis. We identified the type of adherent synovial cells (type A: macrophage-like synovial cells or type B: fibroblast-like synovial cells) by their unique growth pattern, morphology, determination of most common leukocyte antigen (CD45) expression [11], detection of specific marker for macrophage-like synovial cells CD68 using FACS analysis [12], and staining using monoclonal mouse anti-human vimentin V9 IgG1 antibody (Immunotech, France) for cells of mesenchymal origin [2].
The intracellular staining of CD68 (IgG2b, Becton Dickinson) was done on fixed and permeabilized cells, as described previously [13]. Most common leukocyte antigens (CD45)-negative, non-vascular cells in synovium were regarded as synovial fibroblasts [11].
Flow cytometry was performed as previously described by Farkas et al. using a standard direct immunofluorescence technique and mouse anti-human monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC) and/or phycoerythrin (PE) on a FACStrak flow cytometer (Becton Dickinson, San Jose, USA) [14]. After washing in PBS containing 10% fetal calf serum (FCS, Sigma Biosciences), single-cell suspension of 0.1 × 106 cells per tube was dual-stained with anti-Hsp70 (cmHsp70.1-FITC, IgG1, Multimmune, Regensburg, Germany) and PE-conjugated monoclonal antibodies against CD91 (IgG1), CD14 (IgG2a), CD40 (IgG1), CD36 (IgM) (all these antibodies were purchased from Becton Dickinson, San Jose, USA), TLR2 and TLR4 (IgG2a, eBioscience, San Diego, CA, USA) for 30 min at 4°C in the dark. After one washing step, 7-amino-actinomycin D (7-AAD, Becton Dickinson)-negative, viable cells with intact cell membranes were analyzed. The percentage of specifically stained cells was defined as the number of positively stained cells minus the number of cells stained by the isotype-matched control antibody. Anti-major histocompatibility complex (MHC) class I (IgG1, Becton Dickinson) conjugated with FITC as well as PE was used as a positive control. Mouse IgG1-FITC, IgG1-PE, IgG2a-PE and IgM-PE (Becton Dickinson) were used as isotype-matched control antibodies for the detection of Hsp70 and Hsp70 receptors.
Jurkat cells, a human T-ALL cell line (American Type Culture Collection, Manassas, VA, USA, a kind gift from Professor Multhoff, University Hospital Regensburg, Germany) and CCRF-CEM cells, a human T-ALL cell line (ECACC, Porton Down, UK, a kind gift from Professor Dickinson, University of Newcastle upon Tyne, UK) were used as positive controls for screening of Hsp70 membrane expression. Cell lines were cultured in RPMI-1640 medium (Cambrex Bio Sciences Verviers, Verviers, Belgium) supplemented with heat-inactivated 10% FCS (Sigma Biosciences), sodium pyruvate (Sigma Biosciences), l-glutamine (Gibco, Invitrogen Corporation), Fungizone (Gibco, Invitrogen Corporation) and antibiotics (Sigma Biosciences), and the cells were dual stained with anti-Hsp70 (cmHsp70.1-FITC, IgG1, Multimmune) and anti-CD45 or anti- MHC I (PE, IgG1, Becton Dickinson).
A cut-off value for Hsp70 of 10% was chosen based on the results from previous screening of normal cells and tissues (peripheral blood lymphocytes and skin fibroblasts) by flow cytometry [14].
Statistical analysis
Two-tailed Student’s t-test was used for the statistical analysis. P < 0.05 were regarded as significant.
Results
A high prevalence of soluble Hsp70 detected in RA synovial fluids
Hsp70 positivity was detected in 100% RA synovial fluids (range 474.5–1078.9, mean 713.0, median 550.1 ng/ml) in contrast to control sera (range 8.0–53.4, mean 18.2, median 15.8 ng/ml; P < 0.001) and RA sera (range 12.0–44.6, mean 24.5, median 27.7 ng/ml; P < 0.001). The samples were considered to be positive if the OD values exceeded the mean plus 2 SD (standard deviation) of healthy control sera.
Expression of Hsp70 and HSP receptors on RA synovial cells and autologous skin fibroblasts
In our study, the adherent synovial cells were defined as fibroblast-like synovial cells (type B). The most common leukocyte antigen CD45 was not detected on the cells (CD45: mean 0.8%). No type A synovial cells, macrophage-like synovial cells of haematopoietic origin expressing CD68 (mean 0.1%), were identified in cell cultures using FACS analysis.
We examined the expression of inducible Hsp70 and HSP receptors like TLR2, TLR4, CD14, CD36, CD40 and CD91 on fibroblast-like synovial cells derived from synovial tissue and skin fibroblasts derived from the operation wound in patients with RA who underwent synovectomy.
Human leukemia cell lines, which were used as positive controls for the detection of membrane-bound Hsp70, expressed inducible Hsp70 on the cell surface continuously from the beginning till the end of the short-term culture (Jurkat cells d + 3–d + 11, range 74.8–97.7%, mean 87.4%, median: 87.1%; CCRF–CEM cells d + 5–d + 47, range 70.6–90.7%, mean 81.5%, median 81.8%).
Similar to our previous study, significantly higher Hsp70 membrane expression was found on fibroblast-like synovial cells (SC) than on autologous skin fibroblasts (SF) (SC: mean 26.8%, median 21.4% × SF: mean 5.5%, median 5.0%; P < 0.001).
Both synovial cells and skin fibroblasts expressed relatively high levels of cell surface CD91 (SC: mean 77.1%, median 80.2% × SF: mean 68.6%, median 79.2%). No or low expression of CD14 (SC: mean 0.9%, median 0.5% × SF: mean 0.5%, median 0.2%), CD40 (SC: mean 2.0%, median 0.6% × SF: mean 0.5%, median 0.2%), TLR2 (SC: mean 4.3%, median 3.2% × SF: mean 1.4%, median 1.0%), TLR4 (SC: mean 4.9%, median 1.7% × SF: mean 1.2%, median 0.6%) and CD36 (SC: mean 5.0%, median 3.6% × SF: mean 7.9%, median 5.3%) was detected on these cells of mesenchymal origin derived from arthritis affected synovial tissue and non-affected skin. The expression of MHC class I molecules exceeded 90% in most cases.
Similarly, we observed high co-expression of CD91 and Hsp70 on RA synovial cells (mean 19.4%, median 18.6%), while skin fibroblasts showed only background Hsp70 expression (mean 4.8%, median 3.9%; P < 0.001).
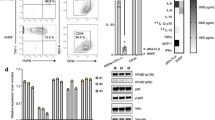
Figure 1 shows a representative flow cytometry analysis of RA fibroblast-like synovial cells and autologous skin fibroblasts. Hsp70 membrane expression was found on fibroblast-like synovial cells derived from arthritis-affected joints when compared with autologous skin fibroblasts either when single staining (Hsp70) or dual staining with a particular HSP receptor (CD91/Hsp70) was used.
Representative flow cytometry analysis on fibroblast-like synovial cells and skin fibroblasts derived from a 54-year-old female with RF-seropositive RA lasting for 20 years; therapy: C/DMARD. Synovial cells derived from synovial tissue of hip joint were cultivated for 40 days and skin fibroblasts derived from the operation wound for 49 days upon reaching the confluence. The adherent cells were trypsinized, resuspended in cell culture medium and dual stained with anti-Hsp70-FITC and CD91-PE conjugated antibodies. Only viable cells negative for 7-AAD were gated and analyzed. Both synovial cells and skin fibroblasts expressed high levels of CD91 on the cell surface (synovial cells: 88%; skin fibroblasts: 73.4%). About 23% of CD91-positive synovial cells carried also Hsp70, while only 2.9% of CD91-positive skin fibroblasts were simultaneously positive for Hsp70
Figure 2 shows cell surface expression of inducible Hsp70 and HSP receptors on fibroblast-like synovial cells derived from RA-affected joints (2a) and skin fibroblasts derived from the operation wounds (2b).
The surface expression of inducible Hsp70 and HSP receptors on fibroblast-like synovial cells derived from RA-affected joints (a) and skin fibroblasts derived from the operation wounds (b). The first bar, marked Hsp70, represents the percentage of Hsp70 expressing cells of all R1 events. The first of each pair of staples (the light bar) represents a mean or median value of the percentage of Hsp70-bound cells among fibroblasts expressing so-called HSP receptors. The second of each pair of staples (the dark bar) indicates the percentage of fibroblasts expressing the different receptors. Both synovial cells and skin fibroblasts expressed relatively high levels of cell surface CD91 (SC: mean 77.1%, median 80.2% × SF: mean 68.6%, median 79.2%). No or low expression of CD14 (SC: mean 0.9%, median 0.5% × SF: mean 0.5%, median 0.2%), CD40 (SC: mean 2.0%, median 0.6% × SF: mean 0.5%, median 0.2%), TLR2 (SC: mean 4.3%, median 3.2% × SF: mean 1.4%, median 1.0%), TLR4 (SC: mean 4.9%, median 1.7% × SF: mean 1.2%, median 0.6%) and CD36 (SC: mean 5.0%, median 3.6% × SF: mean 7.9%, median 5.3%) was detected on these cells of mesenchymal origin derived from arthritis-affected synovial tissue and non-affected skin. The expression of MHC class I molecules exceeds 90% in most cases. Similarly, we observed high co-expression of CD91 and Hsp70 on RA synovial cells (mean 19.4%, median 18.6%), while skin fibroblasts showed only background Hsp70 expression (mean 4.8%, median 3.9%; P < 0.001)
Discussion
RA is a disorder that has its origin in unfettered growth and activation of type B (fibroblast-like) synovial cells [15,16]. It is this cell type that initiates and propagates inflammation in RA-affected joints [16,17]. Fibroblast-like synovial cells are defined mainly by their high level of hyaluronan production. Further, they are responsible for the production of fibronectin, type IV collagen, laminin, chondroitin-6-sulfate-bearing proteoglycans, lubricin, phospholipids, etc. [11]. Not only are fibroblast-like synovial cells able to activate a series of cytokines such as tumor necrosis factor (TNF) α, interleukin (IL) IL-1β, IL-1α, IL-6, and IL-8, they are also able to activate molecules such as tissue factor, PAI-1, MCP-1 as well as a number of matrix metalloproteinases that are involved in tissue degradation. Furthermore, fibroblast-like synovial cells have at their disposal also the ability to activate a series of adhesion molecules such as VCAM and ICAM: genes that have been implicated in cell migration and cell activation [18,19]. Also of importance is the notion that fibroblast-like synovial cells are in command of a surprisingly extensive array of resources to respond to activation.
Patients with rheumatoid arthritis (RA) are confronted with a multitude of stressful events during the course of their disease such as mechanical stress, heat stress, cytokine stress and oxidative stress [20]. The function of HSPs is to protect the folding of nascent proteins, the refolding of denatured proteins and the solubilization of protein aggregates especially under stressful conditions [21].
In our study, we were interested in the expression of inducible Hsp70 purely on the cell surface of RA-derived synovial cells, which may be performed entirely using cells growing from synovial tissue and most simply using FACS analysis. Schett et al. detected enhanced Hsp70 expression in RA synovial tissue using Western blotting; further by immunohistochemistry performed on cryosections of RA synovial membranes, they revealed strong Hsp70 staining throughout the synovial tissue [3]. Independent of when Hsp70 expression was studied, both in vivo synovial tissues [3] and individual synovial cells derived from those synovial tissues, after a certain time of culturing [2], showed significant Hsp70 positivity.
In addition, using real-time PCR, we examined mRNA expression of inducible Hsp70 in RA-derived synovial tissues stored immediately after collection in RNA later. We found that relative mRNA expression was 2.04-fold higher on average, compared with healthy controls (the data in preparation for publication).
Hsp70 overexpression has been shown to be protective against apoptotic death; synovial cells expressing elevated Hsp70 levels might therefore develop a certain resistance to apoptosis [22,23]. This would be in line with the observation of the low frequency of apoptosis in rheumatoid synovium despite the abundance of apoptosis-inducing factors [24,25].
Since we observed a high membrane expression of Hsp70 on RA synovial cells and a high prevalence of soluble Hsp70 in RA synovial fluids, we examined whether extracellular Hsp70 might be bound to the surface of RA synovial cells via any HSP receptors.
Extracellular stress proteins including HSPs and glucose-regulated proteins (Grp) are emerging as important mediators of intercellular signaling and transport. Release of such proteins from cells is triggered by stress and during cell death by necrosis. After release into the extracellular fluid, HSP or Grp may then bind to the surfaces of adjacent cells and initiate signal transduction cascades as well as the transport of cargo molecules such as antigenic peptides [26]. Many of the effects of extracellular stress proteins are mediated through cell surface receptors.
Previous studies suggest that extracellular Hsp70 can initiate a potent, innate and adaptive immune response [27–29]. HSPs interacts with antigen-presenting cells (APCs) through surface receptors such as scavenger receptors LOX-1 [30,31]; CD94 [32] and SR-A [33]; the LDL-receptor-related protein/α2-macroglobulin CD91 receptor [34,35]; the toll-like receptor (TLR) 2 and 4 [29,35]; CD14 [27]; CD36 [35] and CD40 [36,37]. Formation of Hsp70-HSP receptor complex is associated with the induction of the pro-inflammatory response including a cytokine production (IL-1β, TNF-α, IL-6, etc.), expression of MHC class II [38] and nitric oxide (NO) release [39].
In addition to APCs, Hsp70 can avidly bind to non-APC cell lines, especially those from epithelial or endothelial background [40].
We report here the high membrane expression of CD91 on the cells of mesenchymal origin derived from RA affected and non-affected tissues. Further, we observed high simultaneous membrane co-expression of CD91 and Hsp70 in cell cultures derived from RA synovial tissues, while autologous skin fibroblasts showed only background Hsp70 expression.
Skin fibroblasts, which were used as a control for study of Hsp70 membrane expression [2,14], are the major cell type in the dermis for synthesis and reorganization of extracellular matrix components including several types of collagens (predominantly type I), elastin, fibronectin, laminin and proteoglycans (perlecan, epican, cell associated syndecans, etc). They are also capable of secreting factors that regulate the growth and differentiation of other cells, e.g. keratinocytes, and vice versa [41].
It was proposed that the oxidized LDL-binding protein CD91/LRP found on antigen-presenting cells and other cell types could be the common receptor for all immunogenic HSPs, including Hsp60, 70, Gp96 and calreticulin [34]. The role of CD91 as a direct high/medium affinity HSP binder is still not clear. Theriault et al. examined the ability of Hsp70 in free solution to bind cells with, or without CD91 expression and observed minimal differences [40]. However, Basu et al. have shown that complexes of peptides with heat shock proteins Hsp90, calreticulin, and Hsp70 are taken up by macrophages and dendritic cells (DCs) and re-presented by MHC class I molecules by using the CD91 receptor [34]. The studies of Delneste et al. indicated that the CD91 ligand macroglobulin competes with Hsp70 binding to macrophages and DCs in vitro, which is indirect evidence for a role for CD91 in Hsp70 binding [30].
Several studies have shown that receptor-mediated mechanisms are involved in antigen cross-presentation by HSP. Postulated HSP receptors involve two functional sub-types: internalizing receptors and signaling receptors. Interaction of Hsp70-peptide complexes with CD91 receptor leads to receptor-mediated endocytosis, processing of the antigenic peptide by MHC class I and perhaps MHC class II molecules and representation on the cell surface [42,43]. Unfortunately, at this point of the study, a kinetic analysis of endocytosis [44] was not performed due to insufficient amount of the synovial tissue achieved for experimental work.
Since we simultaneously demonstrated the high prevalence of inducible Hsp70 in RA synovial fluids, de-novo synthesis of inducible Hsp70 in RA-derived synovial tissues and a high membrane co-expression of Hsp70-CD91 on RA synovial cells, we speculate that Hsp70 released from inflamed synovial tissue might be captured onto the cell surface of synovial cells from the extracellular space via CD91 receptor and/or translocated to the cell surface from the cytosol. Interestingly, skin fibroblasts highly expressing CD91 cultured by the same manner were found to be negative for membrane-bound Hsp70.
The significance of the Hsp70 interaction with synovial cells via CD91 remains undefined, but may mediate other non-immune purposes like development of a higher resistance to stress-induced apoptosis as was described, e.g., in adjacent neuronal cells taking up extracellular Hsp70 released from glial cells in normal conditions or during stress [45].
References
Zlacka D, Vavrincova P, Hien Nguyen TT, Hromadnikova I (2006) Frequency of anti-hsp60, -65 and -70 antibodies in sera of patients with juvenile idiopathic arthritis. J Autoimmun 27:81–88
Nguyen TTH, Gehrmann M, Zlacka D, Sosna A, Vavrincova P, Hromadnikova I (2006) Heat shock protein 70 membrane expression on fibroblast-like synovial cells derived from synovial tissue of patients with rheumatoid and juvenile idiopathic arthritis. Scand J Rheumatol 35:447–453
Schett G, Redlich K, Xu Q, Bizan P, Groger M, Tohidast-Akrad M, Kiener H, Smolen J, Steiner G (1998) Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J Clin Invest 102:302–311
Martin CA, Carsons SE, Kowalewski R, Bernstein D, Valentino M, Santiago-Schwarz F (2003) Aberrant extracellular and dendritic cell (DC) surface expression of heat shock protein (hsp) 70 in the rheumatoid joint: possible mechanisms of hsp/DC-mediated cross-priming. J Immunol 171:5736–5742
Haug M, Schepp CP, Kalbacher H, Dannecker GE, Holzer U (2007) 70-kDa heat shock proteins: specific interactions with HLA-DR molecules and their peptide fragments. Eur J Immunol 37:1053–1063
Lipsker D, Ziylan U, Spehner D, Proamer F, Bausinger H, Jeannin P, Salamero J, Bohbot A, Cazenave JP, Drillien R, Delneste Y, Hanau D, de la Salle H (2002) Heat shock proteins 70 and 60 share common receptors which are expressed on human monocyte-derived but not epidermal dendritic cells. Eur J Immunol 32:322–332
Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, Brock R, Driessen C, Rammensee HG, Stevanovic S (2005) Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA 102:7922–7927
Auger I, Escola JM, Gorvel JP, Roudier J (1996) HLA-DR4 and HLA-DR10 motifs that carry susceptibility to rheumatoid arthritis bind 70-kD heat shock proteins. Nat Med 2:306–310
Maier JT, Haug M, Foll JL, Beck H, Kalbacher H, Rammensee HG, Dannecker GE (2002) Possible association of non-binding of HSP70 to HLA-DRB1 peptide sequences and protection from rheumatoid arthritis. Immunogenetics 54:67–73
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Edwars JC (2000) Fibroblast biology. Development and differentiation of synovial fibroblasts in arthritis. Arthritis Res 2:344–347
Seidel MF, Koch FW, Vetter H (2006) Macrophage-like synoviocytes display phenotypic polymorphisms in a serum-free tissue-culture medium. Rheumatol Int 26:244–251
Kunisch E, Fuhrmann R, Roth A, Winter R, Lungershausen W, Kinne RW (2004) Macrophage specificity of three anti-CD68 monoclonal antibodies (KP1, EBM11, and PGM1) widely used for immunohistochemistry and flow cytometry. Ann Rheum Dis 63:774–784
Farkas B, Hantschel M, Magyarlaki M, Becker B, Scherer K, Landthaler M, Pfister K, Gehrmann M, Gross C, Mackensen A, Multhoff G (2003) Heat shock protein 70 membrane expression and melanoma-associated marker phenotype in primary and metastatic melanoma. Melanoma Res 13:147–152
Tanaka A, O’Sullivan FX, Koopman WJ, Gay S (1988) Etiopathogenesis of rheumatoid arthritis-like disease in MRL/1 mice: II. Ultrastructural basis of joint destruction. J Rheumatol 15:10–16
Geiler T, Kriegsmann J, Keyszer GM, Gay RE, Gay S (1994) A new model for rheumatoid arthritis generated by engraftment of rheumatoid synovial tissue and normal human cartilage into SCID mice. Arthritis Rheum 37:1664–1671
Markovic M, Stuhlmeier KM (2006) Short-term hyperthermia prevents activation of proinflammatory genes in fibroblast-like synoviocytes by blocking the activation of the transcription factor NF-kappaB. J Mol Med 84:821–832
Kriegsmann J, Keyszer GM, Geiler T, Brauer R, Gay RE, Gay S (1995) Expression of vascular cell adhesion molecule-1 mRNA and protein in rheumatoid synovium demonstrated by in situ hybridization and immunohistochemistry. Lab Invest 72:209–214
Seemayer CA, Kuchen S, Kuenzler P, Rihoskova V, Rethage J, Aicher WK, Michel BA, Gay RE, Kyburz D, Neidhart M, Gay S (2003) Cartilage destruction mediated by synovial fibroblasts does not depend on proliferation in rheumatoid arthritis. Am J Pathol 162:1549–1557
Schett G, Tohidast-Akrad M, Steiner G, Smolen J (2001) The stressed synovium. Arthritis Res 3:80–86
Morimoto RI (1990) The stress response, function of the proteins, and perspectives. Stress proteins in biology and medicine. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B (1997) Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol 17:5317–5327
Schett G, Steiner CW, Groger M, Winkler S, Graninger W, Smolen J, Xu Q, Steiner G (1999) Activation of Fas inhibits heat-induced activation of HSF1 and up-regulation of hsp70. FASEB J 13:833–842
Sugiyama M, Tsukazaki T, Yonekura A, Matsuzaki S, Yamashita S, Iwasaki K (1996) Localisation of apoptosis and expression of apoptosis related proteins in the synovium of patients with rheumatoid arthritis. Ann Rheum Dis 55:442–449
Asahara H, Hasunuma T, Kobata T, Inoue H, Muller-Ladner U, Gay S, Sumida T, Nishioka K (1997) In situ expression of protooncogenes and Fas/Fas ligand in rheumatoid arthritis synovium. J Rheumatol 24:430–435
Calderwood SK, Mambula SS, Gray PJ Jr, Theriault JR (2007) Extracellular heat shock proteins in cell signaling. FEBS Lett 19:3689–3694
Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435–442
Asea A, Kabingu E, Stevenson MA, Calderwood SK (2000) HSP70 peptide bearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones 5:425–431
Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277:15028–15034
Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K (2002) Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity 17:353–362
Binder RJ, Vatner R, Srivastava P (2004) The heat-shock protein receptors: some answers and more questions. Tissue Antigens 64:442–451
Gross C, Hansch D, Gastpar R, Multhoff G (2003) Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem 384:267–279
Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV (2003) Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J 22:6127–6136
Basu S, Binder RJ, Ramalingam T, Srivastava PK (2001) CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14:303–313
Binder RJ, Srivastava PK (2004) Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci USA 101:6128–6133
Wang Y, Kelly CG, Karttunen JT, Whittall T, Lehner PJ, Duncan L, MacAry P, Younson JS, Singh M, Oehlmann W, Cheng G, Bergmeier L, Lehner T (2001) CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity 15:971–983
Becker T, Hartl FU, Wieland F (2002) CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol 158:1277–1285
Tobian AA, Canaday DH, Harding CV (2004) Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4+ T cells. J Immunol 173:5130–5137
Panjwani NN, Popova L, Srivastava PK (2002) Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol 168:2997–3003
Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK (2005) Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett 579:1951–1960
Tuan TL, Keller LC, Sun D, Nimni ME, Cheung D (1994) Dermal fibroblasts activate keratinocyte outgrowth on collagen gels. J Cell Sci 107:2285–2289
Calderwood SK, Theriault J, Gray PJ, Gong J (2007) Cell surface receptors for molecular chaperones. Methods 43:199–206
Li Z, Menoret A, Srivastava P (2002) Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol 14:45–51
Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G (2000) The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem 275:17187–17194
Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B (2001) In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res 914:66–73
Acknowledgments
This study was supported by TRANSEUROPE, No. QLRT-2001- 01936; Trans-net, No. MRTN-CT-2004-512253 and VZ FNM MZO 00064203.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hromadnikova, I., Nguyen, T.T.H., Zlacka, D. et al. Expression of heat shock protein receptors on fibroblast-like synovial cells derived from rheumatoid arthritis-affected joints. Rheumatol Int 28, 837–844 (2008). https://doi.org/10.1007/s00296-008-0532-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-008-0532-9