Abstract
Systemic sclerosis (SSc) is characterized by vascular dysfunction that may lead to pulmonary artery hypertension (PAH). The N-terminal pro-B type natriuretic peptide (NT-proNBP), a marker of cardiac failure, is a diagnostic marker of early PAH in patients with SSc without heart failure. Our aim was to determine whether NT-proBNP levels may be a useful tool to evaluate the response to bosentan therapy in patients with PAH secondary to SSc. Ten patients with symptomatic, severe PAH secondary to SSc, received bosentan, 62.5 mg twice a day for 4 weeks followed by 125 mg twice a day for 7 months. Ten patients with SSc without PAH served as controls for basal level of NT-proBNP. Blood samples were obtained before the beginning of the therapy and after 3 and 7 months of treatment. SSc patients with PAH had significantly higher serum levels of NT-proBNP than those without PAH, at baseline. After 3 and 7 months of therapy, NT-proBNP concentration showed a progressive decrease, nearly approaching statistical difference at 7 months when compared to baseline levels (P = 0.953 and P = 0.600). Our results show that serum NT-proBNP levels may be a useful marker for the response to bosentan therapy in patients with PAH secondary to SSc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a connective tissue disease characterized by vascular damage, immunological abnormalities and fibrosis of the skin and internal organs [1]. Vascular involvement, with generalized disturbance of microcirculation, may lead to pulmonary artery hypertension (PAH) [2]. PAH is a major complication of both limited and diffuse SSc. Established, severe PAH is difficult to treat and has a poor prognosis [2]. The N-terminal pro-B type natriuretic peptide (NT-proBNP) is a potential marker of cardiac failure [3]. Also in patients with primary pulmonary hypertension (PPH), NT-proBNP levels are increased and correlate with functional class [4]. In patients with SSc and PAH without clinical heart failure, NT-proBNP is a diagnostic marker of early PAH [5] and its levels are directly related to the severity of PAH and are highly predictive of survival [6]. Bosentan is an oral endothelin receptor antagonist used in the therapy of primary and secondary PAH [7]. The aim of the present study was to determine whether the measurement of NT-proBNP may be a useful tool for the evaluation of the response to bosentan treatment in patients with PAH secondary to SSc.
Materials and methods
Patients
We enrolled 10 patients (9 females and 1 male, mean age 55 year, range 40–70) who had symptomatic, severe PAH associated with SSc (NYHA functional class III or IV) despite treatments including calcium channel blockers, anticoagulants, diuretics, cardiac glycosides or supplemental oxygen. Three patients were on stable oxygen supplementation started more than three months before the study. None of the patients was on sildenafil. These patients received 62.5 mg of bosentan tablets twice a day for 4 weeks, followed by 125 mg twice a day for 7 months. The inclusion criteria were a baseline 6 min walking test between 150 and 450 mt, a resting mean pulmonary artery pressure greater than 30 mmHg. As controls we enrolled 10 age, sex, disease duration and subtype matched SSc patients (9 females and 1 male, mean age 52 year, range 35–68) without PAH. All the participants to the study were from Northern Italy. Patients with heart failure and renal involvement were excluded from the study (patients characteristics are summarized in Table 1).
We collected blood samples before the beginning of the therapy with bosentan and after 3 and 7 months of continuous treatment. Blood was drown from all the subjects after obtaining written or oral informed consent.
Assay of proBNP and cardiac troponin T
Serum NT-proBNP concentration was determined on Elecsys 2010 instrument (Roche Diagnostics, Basel, Switzerland). A value lower than 14.8 pmol/L was considered normal.
In all patients blood sampling for cardiac troponin T (cTnT) was performed with highly sensitive third generation quantitative test (electrochemiluminescence method ECLIA Roche Diagnotics) to exclude patients with false positive value of NT-pro-BNP due to heart failure or acute coronary artery disease [8].
Statistics
Statistical analysis was performed using StartsDirect Statistical software (Starts Direct Inc, UK). Since NT-proBNP concentration presented a not-normal distribution, data were expressed as median values with minimum–maximum range and non-parametric tests were used for the analysis. More precisely the Mann–Whitney test was used to compare NT-proBNP concentrations at baseline between sclerodermic patients with and without PAH, whereas Wilcoxon signed ranks test was used to compare the concentrations after 3 and 7 months of therapy. P values < 0.05 were considered statistically significant.
Results
Sclerodermic patients with PAH had significantly higher levels of NT-proBNP than those without PAH (mean value: 23.4 pmol/L, min–max range 11.1–38 vs. 12.2, min–max range 2.5–23.7; P = 0.028 by Mann–Whitney test).
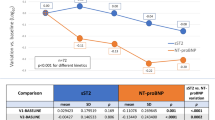
Considering the threshold values for normality (14,8 pmol/L) provided by the manufacturer of NT-proBNP assay kit, 9/10 sclerodermic patients with PAH had high concentrations of NT-proBNP, while 3/10 sclerodermic patients without PAH had high concentrations of NT-proBNP. In sclerodermic patients without PAH, the mean concentration of NT-proBNP remained stable during the study whereas in the patients with PAH the mean concentration was 26 pmol/L (range 4.45–144 pmol/L) after 3 months, and 15.7 pmol/L (range 6–79 pmol/L) after 7 months of therapy with bosentan. There was no significant difference in NT-proBNP concentration at 3 and 7 months of therapy compared with that at baseline (P = 0.953 and P = 0.600, respectively, by Wilcoxon signed ranks test), although a trend towards lower levels was observed after 7 months of treatment (Fig. 1). All patients had a cTnT value <0.01.
Of the ten patients with PAH enrolled in this study, three died after the follow-up period: one of scleroderma renal crisis, one of sepsis and one of cardiac arrhythmias. PAH remained stable during all the treatment period, the 6 min walking test was unchanged after 7 months of treatment compared to baseline and no adjunctive therapy was necessary. During bosentan therapy the leg ulcerations related to SSc did not improve. However, the three patients did not show the appearance of new ulcers.
Discussion
In our study, basal serum NT-proBNP concentration was significantly higher in patients with SSc and PAH than in SSc patients without PAH. These results are in accordance with those of other authors [2, 5, 6], who have reported that NT-proBNP is a useful biologic marker of early PAH in SSc patients without heart failure and that its levels are correlated with the severity of PAH. Moreover the test seems to have a negative predictive value, suggesting that patients with low NT-proBNP levels are highly unlikely to have PAH.
This is the first report on NT-proBNP serum levels variation during therapy with oral dual endothelin ET(A)/ET(B) receptor antagonist in patients with PAH secondary to SSc. We did not observe a statistically significant difference in NT-proBNP concentration after three and seven months of bosentan therapy and these findings may be related both to the low number of patients enrolled and to the duration of the study, since we observed a trends towards lower levels of NT-proBNP that nearly reached statistical difference compared to baseline levels, after seven months of therapy. Three patients without PAH had high NT-proBNP levels but no signs of heart cardiac failure: these patients are carefully monitored for the possibility that the high NT-proBNP levels may have a predictive value for the development of PAH.
Bosentan is clinically beneficial in patients with PAH secondary to SSc although pulmonary hemodynamics and pulmonary function tests seem to remain stable during the treatment [9]. These findings have been confirmed by other authors [10], who observed that in patients with connective tissue disease and PAH there is a progressive improvement in exercise capacity on bosentan therapy. However, pulmonary artery mean pressure remained unchanged. In a study conducted on 92 patients with SSc and PAH, the subjects receiving bosentan had a better survival rate compared to the group of patients not on bosentan therapy; in this last group pulmonary vascular resistance increased over a period of nine months while it remained stable in the group receiving bosentan [11]. Therefore it seems that patients with SSc and PAH experience an improvement in exercise capacity and walk distance during bosentan therapy although NT-proBNP levels and pulmonary vascular resistance remain stable. This behaviour seems different from that observed in patients with primary PAH who experience both a decrease in NT-proBNP concentration after 4 months of bosentan therapy [12] and an improvement in hemodynamic parameters when on bosentan therapy [13].
The identification of parameters able to evaluate the response to the treatment in subjects with PAH secondary to SSc is crucial and NT-proBNP was thought to be one such parameter. Indeed, our results confirm that NT-proBNP is a useful marker in discriminating SSc patients with PAH, and in monitoring the response to bosentan therapy after a long period of treatment. In our experience the 6 min walking test is not as useful as in patients with primary PAH or secondary to other connective tissue diseases since SSc patients may present with leg cutaneous ulcers and claudicatio that can invalidate the test. However, the 6 min walking test and the measurement of NT-proBNP at baseline and regularly after treatment initiation have been recently recommended, although these outcome measures lack some aspects of validation in PAH associated with connective tissue diseases [14].
Long-term follow-up in patients with PAH secondary to SSc is still necessary to understand the possible use of NT-proBNP as a prognostic marker for the response to therapy.
References
Siebold JR (2001) Scleroderma. In: Ruddy S, Harris ED, Sledge CB (eds) Kelley’s textbook of rheumatology, 6th edn. Saunders, Philadelphia, pp 1211–1240
Allanore Y, Borderie D, Meune C, Cabanes L, Weber S, Ekindjian OG, Kahan A (2003) N-terminal pro-brain natriuretic peptide as a diagnostic marker of early pulmonary artery hypertension in patients with systemic sclerosis and effects of calcium-channel blockers. Arthritis Rheum 48:3503–3508
Hobbs FD, Davis RC, Roalfe AK, Hare R, Davies MK, Kenkre JE (2002) Reliability of N-terminal pro-brain natriuretic peptide assay in diagnosis of heart failure: cohort study in representative and high risk community populations. Br Med J 324:1498–1500
Greig D, Castro P, Ferrada M, Lim J, López C, Braun S, Córdova S, Salazar M (2006) Brain natriuretic peptide in primary pulmonary hypertension. Rev Med Chil 134:299–304
Mukerjee D, Yap LB, Holmes AM, Nair D, Ayrton P, Black CM, Coghlan JG (2003) Significance of plasma N-terminal pro-brain natriuretic peptide in patients with systemic sclerosis-related pulmonary arterial hypertension. Respir Med 97:1230–1236
Williams MH, Handler CE, Akram R, Smith CJ, Das C, Smee J, Nair D, Denton CP, Black CM, Coghlan JG (2006) Role of N-terminal brain natriuretic peptide (NT-proBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J 27:1485–1494
Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G (2002) Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 346:896–903
Karthikeyan VJ, Lip GY (2007) N-terminal pro-brain natriuretic peptide and coronary artery disease. Eur J Clin Invest 37:18–25
Joglekar A, Tsai FS, McCloskey DA, Wilson JE, Seibold JR, Riley DJ (2006) Bosentan in pulmonary arterial hypertension secondary to sclerodermia. J Rheumatol 33:61–68
Cozzi F, Montisci R, Marotta H, Bobbo F, Durigon N, Ruscazio M, Sfriso P, Iliceto S, Todesco S (2006) Bosentan therapy of pulmonary arterial hypertension in connective tissue disease. Eur J Clin Invest 36:49–53
Williams MH, Das C, Handler CE, Akram MR, Davar J, Denton CP, Smith CJ, Black CM, Coghlan JG (2006) Systemic sclerosis associated pulmonary hypertension: improved survival in the current era. Heart 92:926–932
Souza R, Jardim C, Martins B, Cortopassi F, Yaksic M, Rabelo R, Bogossian H (2005) Effect of bosentan treatment on surrogate marker in pulmonary arterial hypertension. Curr Med Res Opin 21:907–911
Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ (2001) Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 358:1119–1123
Distler O, Pignone A (2006) Pulmonary arterial hypertension and rheumatic disease-from diagnosis to treatment. Rheumatology 45:22–25
Acknowledgment
We thank Dr. Nicola Martinelli for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simeoni, S., Lippi, G., Puccetti, A. et al. N-terminal pro-BNP in sclerodermic patients on bosentan therapy for PAH. Rheumatol Int 28, 657–660 (2008). https://doi.org/10.1007/s00296-007-0510-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-007-0510-7