Abstract
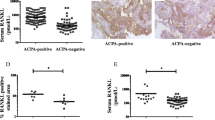
Osteoprotegerin (OPG) and soluble receptor activator of NF-kappa B ligand (sRANKL) together regulate the bone metabolism among other cytokines, whereby cathepsin K has a potent collagen-degrading activity. An imbalance of this system may be partly responsible for the skeletal complications of RA. Expanding on a previous study, we investigated the relationship between OPG, sRANKL and cathepsin K levels in the serum of patients with longstanding RA. We measured serum levels of OPG, sRANKL and cathepsin K of 100 patients with active, longstanding RA. We detected elevated serum levels of cathepsin K (median 54.8 pmol/l) and OPG (median 4.8 pmol/l), but normal sRANKL levels (median 0.2 pmol/l). Cathepsin K did not show a correlation with the overexpressed OPG (P = 0.64) and sRANKL (P = 0.81). The radiological destruction correlates significantly with cathepsin K (P = 0.004) and OPG (P = 0.007). We speculate that the increased levels of OPG are effective in compensating the action of sRANKL, but do not directly prevent bone degradation, as reflected by the elevated serum levels of cathepsin K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The major pathology in rheumatoid arthritis (RA) is the destruction of bone and cartilage, whereby cytokines of the TNF alpha family play an important role. Alteration of bone and cartilage structures is a critical step in the development of a rheumatoid joint.
Osteoprotegerin (OPG) is a soluble decoy receptor, produced by cells of the osteoblastic line and cells of the synovium in RA [1]. The receptor activator of NF-kappa B ligand (RANKL) exists as a membrane bound and free, soluble molecule, and is expressed on T-lymphocytes and pre-osteoblasts. The expression of soluble RANKL (sRANKL) leads to osteoclast activation and maturation, and inhibits osteoclast apoptosis [2, 3]. OPG is known to inhibit the function of RANKL, to prevent osteoclast maturation and, therefore, influences the expression of cathepsin K as well. Cathepsin K, a cystein protease, produced by osteoclasts and synovial fibroblasts, is secreted into the extracellular department [4], where it cleaves collagen type I and type II, leading to degradation of organic matrix between the osteoclasts and bone surface. Hence, normal cathepsin K expression is important for bone resorption and for bone remodelling. Proinflammatory cytokines like interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-alpha) induce an over expression of cathepsin K, which is responsible for increased bone resorption and skeletal complications in RA.
Objective
In continuation of a previously published study [5], we investigated the relationship between OPG, sRANKL and cathepsin K levels in the serum of patients with longstanding RA.
Patients and methods
One hundred patients (mean age 63.0 years, range from 18 to 75 years) meeting the American College of Rheumatology (ACR) criteria for rheumatoid arthritis [6] were enrolled in this study. All patients had a history of a treatment with at least one disease modifying antirheumatic drug (DMARD). Most frequently used was methotrexate, followed by leflunomide, sulfasalzopyrine, gold, azathioprine and chloroquine; however, no biological therapy was prescribed. The number of tender joints was determined by the Ritchie index, the number of swollen joints by the 44-swollen joint count (44 SJC) [7, 8]. Further clinical and laboratory variables assessed were the disease activity score (DAS), the radiological progression (Larsen Score) [9], cathepsin K, OPG, sRANKL, the erythrocyte sedimentation rate (ESR), and the C-reactive protein. Serum was obtained in the morning from the routinely taken blood samples and centrifuged immediately. The samples were kept at −80°C prior to determination of cathepsin K, sRANKL, OPG (Biomedica group, Vienna, Austria) and CRP (CRP, Rheumajet CRP® by Biokit). Cathepsin K, sRANKL and OPG were only determined in serum samples taken for routine blood examinations on the day of hospitalisation, thus the patients had no separate blood collection for quantification of the three parameters. Clinical data from a database, developed for the long-term observation of patients with RA in our institutions, were used.
The cathepsin K test kit is an enzyme-linked immunoassay that was developed for determination of cathepsin K directly in biological fluids (serum, plasma, cell culture supernatants) with mean serum levels of a healthy cohort from 12.7 pmol/l [5]. This ELISA uses antibodies specific for AA 1–20 and AA 196–210 of the mature enzyme and were produced by immunisation of sheep with peptides of these amino acid sequences coupled to keyhole limpet hämocyanine (KLH, primary immunisation 0.5 mg, boost 0.25 mg). Antisera were purified using the biotinylated peptides coupled to streptavidine sepharose (Amersham-Pharmacia Biotech Ltd, UK.) and synthetic cathepsin K (Pichem GmbH, Austria) was used as calibrator. Signal generation was accomplished by labelling with horse radish peroxidase (HRP). Briefly, the assay procedure consists of incubating 50 μl of sample with 200 μl HRP-labelled detection antibody on capture antibody pre-coated plates over night at room temperature. After a washing step to remove unbound detection antibody, tetramethylbenzidine (TMB) was added as substrate. After 30 min, the reaction was stopped by adding 50 μl of 0.9% H2SO4. The yellow colour, which is directly proportional to the amount of cathepsin K present in the sample, was measured on a standard microplate reader at 450 nm with 620 nm as reference. The detection limit of the assay was calculated as 1.1 pmol/l (0 standard plus 5× SD).
The sandwich type ELISA for the measurement of OPG developed by the Biomedica group Austria is based on two OPG-specific antibody preparations [10]. The capture antibody is coated to a microtiter plate and a biotinylated detection in combination with streptavidin–peroxidase and TMB is used for signal generation. The assay measures the free and the bound fraction of OPG. By this method, the mean value of a healthy control group consisting of 170 blood donors is 2.2 pmol/l (2 pmol/l for males, 2.35 pmol /l for females). The reference serum concentration is 0.25–7.25 pmol/l (5th–95th percentile). The presence of rheumatoid factor does not interfere with the measurement.
Soluble RANKL was measured by a sandwich type ELISA developed by Biomedica Group, Austria [11]. The sample and the biotinylated anti-sRANKL detection antibody were pipetted into the wells. Human sRANKL binds to the precoated recombinant osteoprotegerin (OPG) and forms a sandwich with the detection antibody.
After a washing step, the conjugate was added to the wells and tetramethylbenzidine (TMB) was added to the wells as substrate. The amount of colour developed is directly proportional to the amount of sRANKL present in the sample. The assay detects only free, not OPG-bound sRANKL. In a healthy group, mean of serum sRANKL levels were calculated as 1.3 pmol/l (median 0.9 pmol/l). No interferences with human anti-mouse antibodies (HAMA) or rheumatoid factors were observed.
Statistical analysis
The results were analysed by Spearman correlation statistics and Wilcoxon two-sample test. A P-value below 5% was considered statistically significant.
Results
The baseline characteristics of the patients are given in Table 1. The measured serum levels of cathepsin K (median 54.8 pmol/l) as well as that of OPG (median 4.8 pmol/l) were elevated to the reference that is 12.7 pmol/l or 2.2 pmol/l, respectively. The sRANKL concentration in the serum of the patients (median 0.2 pmol/l) was reduced compared to serum concentrations of a healthy control group (non selected healthy blood donors, n = 50) (median 0.9 pmol/l). Cathepsin K did not show a significant correlation with OPG (P = 0.64) and sRANKL (P = 0.81), although OPG was increased in this RA group. OPG correlated negatively with sRANKL (P = 0.029). Cathepsin K (P = 0.59) and sRANKL (P = 0.60) were not age dependent, in contrast to OPG (P = 0.0001) [10, 12]. Although 20 patients were untreated, the larger groups were treated either with methotrexate or leflunomide, the most frequently used DMARDs. There was no significant difference of cathepsin K, OPG, and sRANKL levels between these three groups (untreated, MTX, leflunomide). sRANKL (P = 0.009) and cathepsin K (P = 0.038) but not OPG (P = 0.36) correlated with the DAS. The increased OPG and cathepsin K levels were significantly associated with more severe joint damage as measured by the Larsen score (Table 2). Furthermore, we calculated the cathepsin K/OPG ratio, which was also significantly correlated with the Larsen score (P = 0.05).
Discussion
RA synovium contains a typical inflammatory infiltrate composed of macrophages, dendritic cells, T-lymphocytes, plasma cells, fibroblasts, and other immunocompetent cells. OPG is produced by osteoblasts and in the inflamed synovium of RA by dentritic cells, B cells, and other immunocompetent cells as a decoy receptor, which inhibits the osteoclast formation by neutralising the RANKL [13, 14].
Activated synovial lymphocytes produce RANKL, which activates receptor activator of NF-κB (RANK), a cell bound receptor on dendritic cells, osteoclasts and its precursor cells, chondrocytes, mammary gland epithelial cells and trophoblast cells [15–17]. Both RANK and RANKL are also expressed in chondrocytes [18] and are suggested to play a role in cartilage growth and homeostasis. OPG could protect the cartilage by preventing the subchondral bone structure against inflammatory cell infiltrates. Elevated levels of OPG in RA normalize after anti-TNF-alpha treatment [19, 20].
RANKL, interleukin 1, the macrophage colony stimulating factor (M-CSF), and RANK are a part of osteoclast formation and activation. RANKL inhibits osteoclast apoptosis and OPG acts as an antagonist [21].
The increased OPG levels seem to suppress the action of RANKL. OPG appears to be a protective agent against the development of erosions in RA, but RANKL may be not be solely responsible for erosive joint destructions [22, 29].
OPG has also been shown to inhibit the expression of cathepsin K, a central protease involved in bone resorption [23].
The aggrecan-degrading activity of cathepsin K and the aggrecan cleavage products increase the collagenolytic effects against collage type I and type II [24]. Cathepsin K was found to be increased around lymphocytic infiltrates in synovial tissue, which seems to be responsible for the degradation of articular tissue in rheumatoid joints [25, 26]. Proinflammatory cytokines like interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-alpha), and also sRANKL induce an over expression of cathepsin K in RA.
To the best of our knowledge, no study, which investigated the interactions between OPG, sRANKL, and cathepsin K on their regulation in the serum of patients with RA, has been performed thus far.
Cathepsin K has been demonstrated to be increased in the serum of patients with rheumatoid arthritis [5]. The increased levels of OPG, which we found in our cohort, seem to be an ineffective protective mechanism in RA. The up-regulation of cathepsin K and the correlation with the Larsen score as a parameter for radiological changes mirrors the destruction of bone structures in inflammatory diseases like RA. The increased production of OPG inhibits RANKL indeed, but the over expression of cathepsin K may lead to an imbalance in this system.
Conclusion
This is the first study that shows statistical correlations, possibly indicating functional relations between cathepsin K, a proteolytic enzyme, with OPG and sRANKL in the serum of patients with RA. The role of OPG and RANKL has been clarified in several in vitro studies, but the interaction of these cytokines and their importance in the serum of patients with RA remains under investigation. Our results suggest a possible imbalance or breakdown of the physiological balance of bone metabolism in this disease. We speculate that the increased levels of OPG are effective in compensating the action of sRANKL, but do not directly prevent bone degradation as reflected by the elevated serum levels of cathepsin K. Therefore, the inhibition of cathepsin K may provide an effective tool to prevent irreversible bone destructions in RA.
Further investigations are necessary to prove OPG and cathepsin K as valuable serum markers for diagnosis and monitoring of therapeutic interventions or disease progression. Either the therapeutic use of OPG or the development of cathepsin K inhibitors may provide an effective tool to prevent irreversible bone damages in RA [27, 28].
Abbreviations
- RA:
-
Rheumatoid arthritis
- OPG:
-
Osteoprotegerin
- (s)RANKL:
-
(soluble) Receptor activator of NF-kappa B ligand
- DMARD:
-
Disease modifying antirheumatic drug
- 44 SJC:
-
44 Swollen joint count
- DAS:
-
Disease activity score
- GH:
-
General health score
- RF:
-
Rheumatoid factor (U/l)
- ESR:
-
Erythrocyte sedimentation rate (mm/h)
- CRP:
-
C-reactive protein (mg/l)
- Leuco:
-
Leukocytes (G/l)
- TNF-alpha:
-
Tumor necrosis factor alpha
- TMB:
-
Tetramethylbenzidine
References
Haynes DR, Barg E, Crotti TN, Holding C, Weedon H et al (2003) Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondylarthropathies and osteoarthritis and normal controls. Rheumatology 42:123–34
Crotti TN, Smith MD, Weedon H, Ahern MJ, Findlay DM, Kraan M et al (2002) Receptor activator NF-kappa B ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondylarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis 61:1047–1054
Hou WS, Li Z, Keyszer G, Weber E, Levy R, Klein MJ, Gravallese EM, Goldring SR, Bromme D (2002) Comparison of cathepsin K and S expression within the rheumatoid and osteoarthritic synovium. Arthritis Rheum 46(3):663–674
Kaneko M, Tomita T, Nakase T, Ohsawa Y, Seki H, Takeuchi E, Takano H, Shi K, Takahi K, Kominami E, Uchiyama Y, Yoshikawa H, Ochi T (2001) Expression of proteinases and inflammatory cytokines in subchondral bone regions in the destructive joint of rheumatoid arthritis. Rheumatology (Oxford) 40(3):247–255
Skoumal M, Kolarz G, Woloszczuk W, Hawa G, Klingler A (2005) Serum cathepsin K levels of patients with destructive rheumatoid arthritis: correlation with radiological destruction. Arthritis Res Ther 7:R65–R70
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Ritchie DM, Boyle JA, McInnes JM, Jasani MK, Dalakos TG, Grieveson P (1968) Clinical studies with an articular index for the assessments of joint tenderness in patients with rheumatoid arthritis. Q J Med 147:393–406
Smolen JS, Breedveld FC, Eberl G (1995) Validity and reliability of the twenty-eight-joint count for the assessment of rheumatoid arthritis. Arthritis Rheum 38:38–43
Larsen A, Dale K, Eek M (1976) Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn 18:481–491
Kudlacek S, Schneider P, Pietschmann P, Woloszczuk W, Willvonseder R (2003) Serum levels of osteoprotegerin increase with age in a healthy adult population. Bone 32:681–686
Hawa G, Brinskelle-Schmal N, Glatz K, Maitzen S, WoloszczukW (2003) Immunoassay for soluble RANKL (receptor activator of NF-κB ligand) in serum. Clin Lab 49(9–10):461–463
Skoumal M, Kolarz G, Woloszczuk W, Hawa G, Klingler A (2004) Serum osteoprotegerin but not receptor activator of NF-kappa B ligand correlates with the Larsen score in rheumatoid arthritis. Ann Rheum Dis 63(2):216–217
Schett G, Redlich K, Smolen JS (2003) The role of osteoprotegerin in arthritis. Arthritis Res Ther 5:239–245
Collin-Osdoby P, Rothe L, Anderson F, Nelson M, Maloney W, Osdoby P (2001) Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem 276:20659–20672
Jones DH, Kong YY, Penninger JM (2002) Role of RANKL and RANK in bone loss and arthritis. Ann Rheum Dis 61(Suppl II):ii32–ii39
Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S (1997) RANK is the intrinsinc hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA 97(4):1566–1571
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Komuro H, Olee T, Kuhn K, Quach J, Brinson DC, Shikhman A, Valbracht J, Creighton-Achermann L, Lotz M (2001) The osteoprotegerin/receptor activator of nuclear factor kappa B/receptor activator of nuclear factor kappa B ligand system in cartilage. Arthritis Rheum 44:2768–2776
Ziolkowska M, Kurowska M, Radzikowska A, Luszczykiewicz G, Wieland P, Dziewczopolski W, Filipowicz-Sosnowska A, Pazdur J, Szechinski J, Kowalczewski J, Rell-Bakalarska M, Maslinski W (2002) High levels of osteoprotegerin and soluble receptor activator of nuclear factor kappa B ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor alpha treatment. Arthritis Rheum 46(7):1744–1753
Marotte H, Maslinski W, Miossec P (2005) Circulating tumor necrosis factor-alpha bioactivity in rheumatoid arthritis patients treated with Infliximab: link to clinical response. Arthritis Res Ther 7:R149–R155
Haynes DR, Crotti TN, Loric M, Bain GI, Atkins GJ, Findlay DM (2001) Osteoprotegerin and receptor activator of nuclear factor-(B ligand (RANKL) regulate osteoclast formation by cells in the human rheumatoid arthritic joint. Rheumatology 40:623–630
Hofbauer LC, Heufelder AC (2001) Role of receptor activator of nuclear factor-(B ligand and osteoprotegerin in bone cell biology. J Mol Med 79:243–253
Wittrant Y, Couillaud S, Theoleyre S, Dunstan C, Heymann D, Redini F (2002) Osteoprotegerin differentially regulates protease expression in osteoclast cultures. Biochem Biophys Res Commun 293(1):38–44
Troen BR (2004) The role of cathepsin K in normal bone resorption. Drug News Perspect 17(1):19–28
Hou WS, Li Z, Buttner FH, Bartnik E, Bromme D (2003) Cleavage site specifity of cathepsin K toward cartilage proteoglycans and protease complex formation. Biol Chem 384(6):691–697
Hummel KM, Petrow PK, Franz JK, Maller-Ladner U, Aicher WK, Gay RE, Brämme D, Gay S (1998) Cysteine protease cathepsin K mRNA is expressed in synovium of patients with rheumatoid arthritis and is detected at sites of synovial bone destruction. J Rheumatol 25(10):1887–1894
Votta BJ, Levy MA, Badger A, Bradbeer J, Dodds RA, James IE, Thompson S, Bossaard MJ, Carr T, Connor JR, Tomaszek TA, Szewczuk L, Drake FH, Veber DF, Gowen M (1997) Peptide aldehyde inhibitor of cathepsin K inhibit bone resorption both in vitro and in vivo. J Bone Miner Res 12(9):1396–1406
Tavares FX, Boncek V, Deaton DN, Hassell AM, Long ST, Miller AB, Payne AA, Miller LR, Shewchuk LM, Wells-Knecht K, Willard DH, Wright LL, Zhou HQ (2004) Design of potent, selective and orally bioavailable inhibitors of cysteine protease cathepsin K. J Med Chem 47(3):588–599
Skoumal M, Kolarz G, Haberhauer G, Woloszczuk W, Hawa G, Klingler A (2005) Osteoprotegerin and the receptor activator of NF-kappa B ligand in the serum and synovial fluid. A comparison of patients with longstanding rheumatoid arthritis and osteoarthritis. Rheumatol Int 26(1):63–69
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skoumal, M., Haberhauer, G., Kolarz, G. et al. The imbalance between osteoprotegerin and cathepsin K in the serum of patients with longstanding rheumatoid arthritis. Rheumatol Int 28, 637–641 (2008). https://doi.org/10.1007/s00296-007-0506-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-007-0506-3