Abstract
In rheumatoid arthritis (RA) patients treated with infliximab (IFX), QuantiFERON-TB Gold (QFT-G), an interferon γ assay for diagnosing tuberculosis infection, was performed to compare its effectiveness to conventional diagnostic procedures (tuberculin skin test, imaging and medical history) in diagnosing latent tuberculosis infection (LTBI). QFT-G was measured bimonthly in 14 rheumatoid arthritis patients during IFX treatment. Seven of 14 patients were confirmed as LTBI positive by at least one method. Of these, four were positive on QFT-G during the study period, and two were positive before the start of IFX administration. For two of the four QFT-G-positive patients, LTBI was diagnosed only by QFT-G. The rate of agreement between QFT-G and conventional procedures was 64.3%. A total of 5% of QFT-G tests were impossible to judge due to decreased reactions in the positive control. These results suggest that QFT-G is able to detect LTBI in RA patients overlooked by conventional methods. Conventional procedures and QFT-G should be employed in parallel, and LTBI should be assumed when one technique gives a positive result.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a therapeutic drug for rheumatoid arthritis (RA), anti-TNF inhibitors show prompt, specific anti-inflammatory action and joint destruction depression effects, and have thus become important in intractable RA treatment [1]. On the other hand, the frequent occurrence of opportunistic infections is of concern due to the artificial inhibition of TNF. As anti-TNF inhibitors have become more widely used in RA treatment, increases in the incidence of tuberculosis infection have been reported [2, 3]. Because these cases represent reactivation of endogenous infection, onset may be prevented by prophylactic administration of isoniazid (INH) if latent tuberculosis infection (LTBI) can be adequately detected before initiating anti-TNF therapy [4]. However, conventional techniques are insufficient to identify LTBI, i.e., in-depth history (history of treatment, contact with active TB patients), imaging (chest X-ray and CT) and tuberculin skin test (TST). Furthermore, it is difficult to diagnose LTBI based on the results of TST alone, as the Bacillus Calmette-Guerin (BCG) vaccine is widely used in various countries, including Japan [5].
Therefore, an interferon γ assay for ESAT-6 and CFP-10, both of which are specific proteins for Mycobacterium tuberculosis and are not present in BCG, has been put to practical use in recent years. The advantages of interferon γ assay in the diagnosis of LTBI are reported to be high in specificity and sensitivity [6–8]. However, there have been no reports regarding the utility of interferon γ assay in anti-TNF inhibitor treatment for RA. We performed an observational study focusing on the utility of LTBI monitoring by QuantiFERONTM-TB Gold test (QFT-G, Cellestis, Australia), an interferon γ assay, in RA patients scheduled for treatment or undergoing treatment with infliximab (IFX), an anti-TNF inhibitors, and the results were compared with conventional diagnostic approaches. In addition, we examined the serial changes in measurement of QFT-G during IFX administration.
Materials and methods
Study population
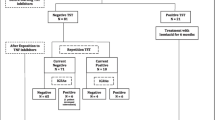
We performed an observational study of 14 patients with RA in whom the presence of active tuberculosis infection was excluded prior to initiation of IFX administration at Sapporo Medical University Hospital. For this, QFT-G measurement was started before IFX administration in three patients. QFT-G measurement was started before the fifth administration of IFX in two patients and before the seventh administration of IFX in nine patients. Chronologic measurement of QFT was performed in alternate months. The study was approved as a clinical audit by the hospital research committee and all participants provided written informed consent. We estimated patients as being LTBI according to the following conditions and INH prophylaxis was performed [9]; history of tuberculosis treatment or close contact with an infected person, abnormal findings on chest X-ray suggestive of old tuberculosis (calcification shadows more than 5 mm, pleural thickening, linear opacities), TST with redness at least 20 mm in diameter or the presence of induration, or positive result on QFT-G (more than 0.35 IU/ml). Treatment with INH was initiated at 300 mg/day and continued with pyridoxine supplementation.
Patient characteristics (Table 1)
The mean age was 48.6 years (range 18–64 years), and the male/female ratio was 3:11. Methotrexate (MTX) was used in all patients and prednisolone was administered at an average of 5.8 mg/day in 12 of 14 patients. There was no change about medications just before start of IFX therapy. The average number of treatments with IFX was 15 (range 1–24). IFX treatment was canceled in case 2 due to a history of tuberculous lymphadenitis and positive QFT-G after initiation of IFX treatment. INH prophylaxis was performed in cases 1, 2, 3, 6 and 7 by the condition mentioned above. Of these, INH administration was started based on medical history in case 3, but liver dysfunction developed, and INH was terminated in four weeks. However, INH was not used in spite of positive result of TST in case 8 because IFX treatment had been initiated before the release of Japanese guidelines for prescribing anti-TNF agents. INH was not used in case 4 due to the transient positivity of QFT-G.
QuantiFERON-TB gold
Testing was performed at the First Department of Pathology according to the manufacturer’s recommendations. Briefly, the test consisted of a negative control (nil well, i.e., whole blood without antigens or mitogen), a positive control (mitogen well, i.e., whole blood stimulated with the mitogen phytohemagglutinin [PHA]) and two sample wells, i.e., whole blood stimulated with either of the M. tuberculosis-specific antigens, Early Secretory Antigen Target 6 (ESAT-6) or Culture Filtrate Protein 10 (CFP-10). Whole blood specimens were incubated for 16–20 h (overnight) at 37°C in a humidified atmosphere. IFN γ levels in the nil well were considered background and were subtracted from the results of the mitogen well and the antigen-stimulated wells. The results were considered positive if the concentration of IFN γ in the sample well after stimulation with ESAT-6 and/or CFP-10 was greater than or equal to 0.35 IU/ml (after subtracting the value of the nil well), regardless of the results of the positive control (mitogen well). The results were considered negative if the response to the specific antigens (after subtracting the value of the nil well) was less than 0.35 IU/ml and if the IFN γ levels of the positive control (after subtracting the value of the nil well) were greater than or equal to 0.5 IU/ml. The results were considered indeterminate if both antigen-stimulated sample wells were negative (i.e., <0.35 IU/ml after subtracting the value of the nil well) and if the value of the positive control well was less than 0.5 IU/ml after subtracting the value of the nil well.
Results
QFT-G measurement results (Table 2)
Tuberculosis infection including extrapulmonary involvement was not seen during the study period. The mean observation period after initiation of IFX treatment was 28.3 months (range 13–44 months). The average number of QFT-G measurements was 4.3 (range 2–8). In cases 1–3, measurement of QFT-G was performed of before the start of IFX administration. Positive QFT-G was observed in four patients (cases 1, 2, 4 and 7) irrespective of IFX administration, and two patients were positive on QFT-G before IFX administration. Indeterminate results were observed in three of 60 tests (5%) due to inadequate production of IFN γ by PHA stimulation. Of these, case 8 recorded indeterminate results twice, and this patient was treated with cyclosporine A together with MTX.
QFT-G in LTBI detection and comparison with conventional diagnostic procedures (Table 3)
When it was defined as LTBI by positive result of either QFT-G or conventional diagnostic procedures, seven patients were taken as LTBI. QFT-G was positive in four of 14 patients. As for two of four patients, existence of LTBI was proved only by QFT-G. The number of cases diagnosed as having LTBI by TST, imaging (chest X-ray, CT), and medical history was 4/14, 1/14 and 4/14, respectively. Medical histories supporting diagnosis of LTBI included treatment history of pulmonary tuberculosis (two cases), tuberculous lymphadenitis (one case) and family history with childhood tuberculosis in patient’s son (one case). In QFT-G and traditional approaches (TST, chest X-ray, medical history), both were negative in seven cases and both were positive in two cases. Accordingly, the rate of agreement was 64.3%.
Chronologic changes in QFT-G during administration of anti-TNF inhibitor (Fig. 1)
Measurement of QFT-G was repeated more than four times in nine patients. The chronologic changes are shown in Fig. 1 according to each antigen (ESAT-6 and CFP-10). INH prophylaxis was performed in cases 1, 2, 3, 6 and 7. QFT-G levels (ESAT-6 in particular) tended to decrease after initiating INH in case 2 and case 7. On the other hand decreased levels on QFT-G were not observed in case 1, in which QFT-G was positive before administration of IFX. Drastic changes were absent during the observational period in cases giving negative results at the beginning of IFX administration.
Discussion
Due to the drawbacks of LTBI diagnosis by conventional approaches (medical history, imaging and TST), QFT-G, an interferon γ assay for M. tuberculosis-specific ESAT-6 and CFP-10, was developed. QFT-G is reported to have high specificity and sensitivity for diagnosis of tuberculosis infection, as it is not affected by BCG vaccination [8]. According to recent reports, interferon γ assay exceeded TST in both sensitivity and specificity in the diagnosis of tuberculosis infection in immunocompetent individuals [6, 7]. To our knowledge, however, there have been no reports regarding the utility of interferon γ assay in LTBI detection with anti-TNF inhibitor use, and even the CDC guidelines do not mention the utility of QFT-G [10, 11]. In order to compare QFT-G with conventional approaches for the diagnosis of LTBI during IFX administration, we thus performed this observational study.
Although it was impossible to calculate the sensitivity and specificity due to the lack of a gold standard for LTBI, the present study showed that seven patients were judged as having LTBI according to conditions described above. Accordingly, the rate of agreement between conventional methods and QFT-G was 64.3%. On the other hand, excellent agreement between TST and QFT-G was reported (more than 90%) in contacts in a tuberculosis outbreak [8, 12, 13]. This difference may be explained by the following problems related to TST; false positivity due to BCG vaccination and atypical mycobacterial infection, and false negativity caused by immunosuppression (aging, immunosuppressive drugs such as corticosteroid, diabetes mellitus and renal failure). In particular, RA patients treated with MTX and IFX tend to be under immunosuppressive conditions and the utility of TST decreases.
In particular the concern of our study was that only QFT-G was able to confirm LTBI in two patients. Although caution is required when QFT-G is positive and conventional approaches are negative, it is reasonable to consider QFT-G positivity as being LTBI, as several reports have demonstrated the high sensitivity and specificity of QFT-G [6–8]. QFT-G positivity should be judged as Th1 type T cells responding M. tuberculosis, and thus INH prophylaxis is imperative during IFX treatment in RA patients [14]. In some cases, however, QFT-G did not present positive results where LTBI was diagnosed by conventional procedures. QFT-G was negative in three of five patients judged as having LTBI conventionally. These results are consistent with reports that TST has higher sensitivity than interferon γ assay for diagnosis of LTBI in old tuberculosis infection cases [15, 16]. In addition, since longer incubation periods are necessary for detection of LTBI, assay conditions may need to be adjusted in the case of RA patients treated with IFX [17].
Further examination is necessary regarding cases 2, 3, 6 and 8, as INH prophylaxis was necessary according to conventional procedures, while QFT-G was negative. In fact, QFT-G negativity during anti-TNF inhibitor administration does not necessarily support the absence of tuberculosis infection according to CDC recommendations [10]. Therefore, it is impossible to confirm the presence of LTBI based on the results of QFT-G alone. The results of conventional diagnostic methods (TST, imaging and medical history) and QFT-G should be used in parallel, and INH prophylaxis should be initiated if any method gives a positive result.
It has been reported that the rate of indeterminate test results is higher for QFT-G and is associated with immunosuppression [6]. One study in children reported a 17% rate of indeterminate QFT-G results [16]. However, only 5% cases could not be evaluated in the present study, despite the use of IFX associated with MTX and corticosteroids. Thus, there do not seem to be any serious problems with the clinical application of QFT-G to RA patients treated with IFX and MTX.
The influence of INH on QFT-G responsiveness remains undetermined, although it has been reported that sensitivity of interferon γ assay is low in comparison with TST after treatment for active tuberculosis infection [13]. In this study, INH prophylaxis was performed in four spatients (cases 1, 2, 6 and 7). Of these patients, a decrease in QFT-G value under INH prophylaxis was observed in cases 2 and 7. These findings support the notion that administration of INH causes a decrease in QFT-G value. At present, it is uncertain whether QFT-G negativity is sufficient grounds to stop INH administration. Cases presenting positive QFT-G should thus be followed for future investigations.
Several potential limitations in this study should be considered when interpreting the results. Firstly, the study population was small and we could not perform QFT-G before the start of IFX administration in all cases. Secondly, we could not establish a study group in which no INH prophylaxis was performed despite a positive QFT-G. To confirm the utility of QFT-G in LTBI diagnosis for the purposes of preventing tuberculosis onset associated with anti-TNF inhibitor treatment, it is necessary to compare the incidence rate of tuberculosis onset among four groups classified with respect to positive or negative QFT-G before IFX and INH use or non-use. However, it is unrealistic to perform the above-mentioned comparison because of the present condition that IFX is used for RA treatment, with due consideration given to tuberculosis prophylaxis. Thus, we performed an observational study and compared QFT-G with conventional approaches for diagnosis of LTBI. Further investigations into the performance of QFT-G in those with immunosuppression are required in order to understand the better performance of QFT-G in detecting LTBI.
Conclusion
Although the advantages of QFT-G in the diagnosis of LTBI are reported to be high in specificity and sensitivity, there have been no reports regarding the utility of QFT-G in anti-TNF inhibitor treatment for RA. We performed the study focusing on the utility of LTBI monitoring by QFT-G in RA patients with IFX treatment, and the results were compared with conventional approaches (tuberculin skin test, imaging and medical history). Seven of fourteen patients were confirmed as LTBI positive by at least one method. Of these, four were positive on QFT-G during the study period, and two were positive before the start of IFX administration. The rate of agreement between QFT-G and conventional procedures was 64.3%. A total of 5% of QFT-G tests were impossible to judge due to decreased reactions in the positive control. These results suggest that QFT-G is able to detect LTBI in RA patients overlooked by conventional methods during IFX administration. Conventional procedures and QFT-G should thus be employed in parallel, and LTBI should be assumed when one technique gives a positive result, after which prophylactic INH should be administered.
References
Hochberg MC, Lebwohl MG, Pelvy SE (2005) sThe benefit/risk profile of TNF-blocking agents: findings of a concensus panel. Semin Arthritis Rheum 34:819–836
Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM (2001) Tuberculosis associated with infliximab, a tumor necrosis factor a-neutralizing agent. N Engl J Med 345:1098–1104
Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD (2003) Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk. Arthritis Rheum 48:2122–2127
Jasmer RM, Nahid P, Hopewell PC (2002) Latent tuberculosis infection. N Engl J Med 347:1860–1866
Tissot F, Zanetti G, Francioli P, Zellweger JP, Zysset F (2005) Influence of Bacilli Calmette-Guerin vaccination on size of tuberculin skin test reaction: to what size? Clin Infect Dis 40:211–217
Richeldi L (2006) An update on the diagnosis of tuberculosis infection. Am J Respir Crit Care Med : [Epub ahead of print]
Ferrara G, Losi M, D’Amico R, Roversi P, Piro R, Meacci M, Meccugni B, Dori IM, Andreani A, Bergamini BM, Mussini C, Rumpianesi F, Fabbri LM, Richeldi L (2006) Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 367:1328–1334
Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, Shigeto E, Harada N, Mitarai S, Okada M, Suzuki K, Inoue Y, Tsuyuguchi K, Sakaki Y, Mazurek GH, Tsuyuguchi I (2004) Specific detection of tuberculosis infection. An interferon-γ-based assay using new antigens. Am J Respir Crit Care Med 170:59–64
Miyasaka N, Takeuchi T, Eguchi K (2006) Guidelines for the proper use of eternercept in Japan. Mod Rheumatol 16:63–67
Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A (2005) Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 54:49–55
CDC (2005) Guidelines for the investigation of contacts of persons with infectious tuberculosis. MMWR Recomm Rep 54:1–37
Brock I, Weldingh K, Lillebaek T, Follmann F, Anderson P (2004) Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med 170:65–69
Pai M, Riley LW, Colford MC (2004) Interferon-γ assay in the immunodiagnosis of tuberculosis: a systemic review. Lancet Infect Dis 4:761–776
Gardam MA, Keystone EC, Menzies R, Manners S, Skamene E, Long R, Vinh DC (2003) Anti-tumor necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect Dis 3:148–155
Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, Ravn P (2006) Latent tuberculosis in HIV positive, diagnosed by the M. Tuberculosis specific interferon gamma test. Respir Res 7:56
Connell TG, Curtis N, Ranganathan SC, Buttery JP (2006) Performance of a whole blood interferon gamma assay in detecting latent infection with Mycobacterium tuberculosis in children. Thorax 61:616–620
Dheda K, Udwadia ZF, Huggett JF, Johnson MA, Rook GAW (2005) Utility of the antigen-specific interferon-g assay for the management of tuberculosis. Curr Opin Pulm Med 11:195–202
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, H., Shigehara, K., Yamamoto, M. et al. Interferon γ assay for detecting latent tuberculosis infection in rheumatoid arthritis patients during infliximab administration. Rheumatol Int 27, 1143–1148 (2007). https://doi.org/10.1007/s00296-007-0361-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-007-0361-2