Abstract
Our aim was to investigate the efficacy, toxicity, and drug discontinuation in patients with ankylosing spondylitis (AS) treated with infliximab. Thirty-five patients with AS, who were enrolled between June 2001 and December 2002 were treated with infliximab. All patients fulfilled the New York revised criteria for AS and had axial disease. Infliximab (5 mg/kg weight), was given intravenously at weeks 0, 2, 6, and every 8 weeks thereafter. If this failed to give an acceptable treatment response, the interval was shortened to 6 or 4 weeks. The patients were followed-up at predefined times according to a standardized protocol. Data concerning infliximab efficacy, tolerability, adverse events, interval, and drug discontinuation were all recorded. Clinical improvement according to the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) 50% and the Ankylosing Spondylitis Assessment Study group (ASAS) 40%, and ASAS 5/6 response criteria were recorded. Infliximab treatment resulted in a rapid improvement in the BASDAI and ASAS scores in the first year of the treatment, which sustained throughout the fourth year. More specifically, after the third year of treatment 17/35 (48.6%) of patients achieved BASDAI 50% response criteria, 19/35 (54.3%) attained the ASAS 40% and 15/35 (42.9%) reached the ASAS 5/6. After the fourth year of treatment BASDAI 50% was reached by 17/35 (48.6%) of patients, ASAS 40% by 17/35 (48.6%), while ASAS 5/6 was attained by 15/35 (42.9%). The clinical improvement was associated with the reduction of acute phase reactants as measured by C-reactive protein levels. After the first year of treatment, the “survival rate” of infliximab was 94.3%, after the second year was 91.4%, after the third year was 85.7% and even after 4 years of treatment still maintained high 77.9%. Six (17.1%) patients were withdrawn during the observational period. Three because of lack of efficacy, two because of allergic reactions and one lost from follow-up. Infliximab was effective, safe, and well tolerated in patients with AS. The clinical response was maintained for a period of 4 years and over, with infliximab survival of 77.9%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic systemic inflammatory disease more common in young men, with a prevalence of 0.15–0.8% [1, 2]. It is associated with characteristic symmetric sacroiliitis and production of syndesmophytes, leading to ankylosis, kyphosis and disability, but also to extraspinal lesions [3].
Therapies for AS have been unsatisfactory until the last few years. Non-steroidal anti-inflammatory medications may control symptoms, but they have no effect on joint damage progression. Traditional disease modifying anti-rheumatic drugs (DMARDs) commonly used in RA have been used in AS. The response rates have been small, and there is no evidence that any of these drugs actually affect axial disease progression [4]. Recently, reports of efficacy of biological agents for AS in 2000 opened new horizons for patients [5–9]. These compounds allow for a specific intervention in the immune cascade underlying the disease pathogenesis. Their use is considered to be a “real revolution”, as they appear to fill the space of efficacy of previously used DMARDs [10, 11]. In fact, trials with infliximab and other anti-tumour necrosis factor alpha (TNF-α) agents have showed remarkable improvement in AS patients [10, 11].
Anti-TNF-α therapy should be considered for patients with severe AS and elevated serological markers of inflammatory activity and have responded inadequately to conventional therapy [12]. The efficacy and safety of infliximab have been established in a number of randomised and open label, controlled trials in patients with AS [7, 8, 9, 13]. Although these trials have shown that active AS responds dramatically to infliximab, there are no many long-term studies of their use [14–17]. Thus, questions concerning their efficacy and safety remain still unanswered in long-term use.
In compliance to our previous studies [13, 14], we investigated infliximab efficacy, toxicity, and reasons for drug discontinuation during long-term disease course in an observational study of patients with AS.
Materials and methods
Thirty-five patients with AS, who were enrolled between June 2001 and December 2002 were treated with infliximab. All patients fulfilled the New York revised criteria for AS [18]. All patients had axial disease. Patients were fully informed about the treatment regimen and entered the study after having read and signed an informed consent form. Infliximab (5 mg/kg weight), was given intravenously at weeks 0, 2, 6, and every 8 weeks thereafter. If this regimen failed to give an acceptable treatment response the interval was shortened to 6 or to 4 weeks. All patients were followed up at predefined times according to a standardised protocol. The protocol has been approved by the Institutional Scientific Committee of the University Hospital of Ioannina. Data concerning infliximab efficacy, tolerability, adverse events, interval, and drug discontinuation were all recorded. Clinical improvement according to the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) 50% and the Ankylosing Spondylitis Assessment Study Group (ASAS) 20 and 40% and ASAS 5/6 response criteria were also recorded [19, 20]. All patients had their last follow up by July 2006.
Definitions
Active disease was defined if patients had BASDAI ≥ 40/100 [20] and C-reactive protein (CRP) ≥ 10 mg/l (normal value < 6 mg/l). Refractory disease was defined by the failure of at least two non-steroidal anti-inflammatory drugs during a single 3-month period, failure of intra-articular steroids if indicated, and failure of sulfasalazine in patients with peripheral arthritis. A response to treatment according to ASAS criteria requires improvement of at least 20% and absolute improvement of at least 10 units on a scale of 0–100 in three of the following four domains: (1) patient’s global assessment of the disease activity; (2) pain; (3) function [in this study the Bath Ankylosing Spondylitis Functional Index (BASFI) score] [21]; and (4) inflammation [in this study the mean duration of morning stiffness related to the BASDAI, and 100 mm visual analogue scale (VAS) scores] and the absence of deterioration by 20% and by 10% of units in the four domains [22]. Lack of efficacy was defined as patients not fulfilling the BASDAI 20%, as well as the ASAS 20% response criteria. Failure of drug treatment was defined as patients who stopped receiving the drug for more than 2 months because of lack of efficacy. Adverse drug reactions were defined as patients who had reactions that required permanent discontinuation of infliximab owing to life threatening conditions, or because of intolerability. Discontinuation was decided when drug treatment failed or patients had adverse drug reactions.
Monitoring
A complete blood count with differential and platelet count, as well as serum values of liver enzymes, bilirubin, albumin, glucose, creatinine, and urine analysis were obtained before treatment and at each patient’s visit. Finally, 2 ml of blood serum of patients (at each visit) was taken and stored at −20°C for the measurement of an antibody profile.
Statistical analysis
Standard methods of survival analysis (Kaplan–Meier) were used, in which infliximab termination due to side effects and/or lack of efficacy, and/or failure of drug treatment was taken as the end points.
Results
During the observation period, 41 patients with AS were investigated. Of these, one refused treatment and five were excluded from the study. More specifically, three patients had a positive tuberculin skin test, one had congestive heart failure, and another presented with restrictive lung disease. Thus, 35 patients were studied who had negative tuberculin skin test and normal chest radiographs.
All patients had axial disease, 2 patients presented with peripheral arthritis and 11 had a history of anterior uveitis. The demographic, clinical and laboratory data at entry have been reported previously [14]. There were 34 men and 1 woman with a mean age of 43.6 ± 12.1 years and disease duration 15.7 ± 7.8 years. Two (5.7%) patients were on methotrexate (MTX), two (5.7%) were treated with sulfasalazine and two (5.7%) patients were also taking prednisone 5 mg/day. All patients had active disease as evaluated by the high BASDAI score and high levels of CRP (Table 1).
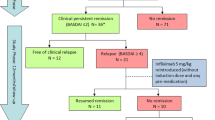
Infliximab treatment resulted in a rapid improvement in the BASDAI and ASAS scores in the first year of the treatment, which sustained throughout the fourth year (Fig. 1). More specifically, after three years’ treatment 17/35 (48.6%) of patients achieved BASDAI 50% response criteria, 19/35 (54.3%) attained the ASAS 40% and 15/35 (42.9%) reached the ASAS 5/6. After the fourth year of treatment BASDAI 50% was reached by 17/35 (48.6%) of patients, ASAS 40% by 17/35 (48.6%), while ASAS 5/6 was attained by 15/35 (42.9%) (Fig. 2). The clinical improvement was associated with the reduction of acute phase reactants as measured by CRP levels (Fig. 3).
Trial profile and response to treatment in an intention to treat analysis. Infliximab treatment resulted in a rapid improvement of Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Ankylosing Spondylitis Assessment Study group (ASAS) scores in the first year, which sustained throughout the fourth year of treatment. The number of patients in the middle arrow represents the patients in transition between the third and fourth year
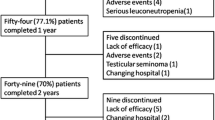
After the first year of treatment, the “survival rate” of infliximab was 94.3%, after the second year was 91.4%, after the third year was 85.7% and even after 4 years of treatment still maintained high 77.9% (Fig. 4).
Six patients (17.1%) were withdrawn during the observational period. The main reason for drug discontinuation was lack of efficacy (3 patients; 8.6%), followed by immediate hypersensitivity reactions (2 patients; 5.7%). One patient was lost from follow up. Finally, 13 (37.1%) patients developed antinuclear antibodies and two had low titer of double stranded DNA. None of the patients developed symptoms or signs of systemic lupus erythematosus.
The interval between infusions was shortened from 8 to 6 weeks in nine patients (for one patient after the first year, for four patients after the second year, and for four patients after the third year). These patients initially responded well to infliximab treatment, but 10 days before the next infusion they experienced neck pain and stiffness. After this treatment regimen the patients responded well and continued therapy.
Discussion
Infliximab is a monoclonal antibody that binds to TNF-α and blocks its biological activity. It is approved for use in patients with rheumatoid arthritis (RA), Crohn’s disease, AS and psoriatic arthritis [23]. Its administration has been shown to be highly efficacious in patients with active AS. Several placebo-controlled and open trials have shown that active AS responds dramatically to infliximab [7, 8, 9]. As in RA, treatment of AS must be continued because disease activity returns a few weeks after infliximab is stopped [24].
In this we report the results of an observational study from a single University center monitoring the biological agent infliximab in clinical practice. First, we wanted to evaluate whether infliximab is effective and for how long, and second, which are the most frequently and serious adverse events. One main finding of the present study is that the improvement of signs and symptoms observed in the majority of the patients during the first year was sustained throughout the fourth year of the study, as measured by the BASDAI 50%, the ASAS 20 and 40%, and ASAS 5/6 response criteria. Median CRP levels remained low. Our results are in line with those previously published by other investigators [15, 16]. However, in those studies infliximab “survival rate” was not reported. A previous report by our group found that infliximab survival was 89% after 2 years of treatment [14].
The efficacy and safety of infliximab and its survival in the present study of patients with AS is greater than expected. Loss of infliximab survival may be explained by the generation of human antichimeric antibodies (HACA). These antibodies appeared to be associated with lower serum infliximab concentrations, and may be related to a shorter duration of response after repeated infliximab doses, as reported for patients with RA. Concomitant administration of MTX appears to reduce the formation of HACA. However, in our study only three patients were taking MTX. The absence of serious adverse events is probably attributed to the strict inclusion criteria used. Another issue to take into consideration is related to lower infusion reactions observed in our study. This is probably related to high doses used (5 mg/kg weight) since infusion reactions are shown in those patients receiving lower doses [25]. A limitation on our study is that our results are based on a limited number of patients and that this is an open label study. On the other hand, the present study is one of the longest with a 4-year follow-up in AS patients.
As regard the dosage of infliximab used in AS patients, we used 5 mg/kg weight according to the recommendation of the Canadian Rheumatology Association [26]. However, other studies suggest that a lower dose of 3 mg/kg weight may be efficacious in patients with AS [25].
The results of this 4-year observational study suggest that infliximab treatment is effective, safe, and well tolerated in patients with AS. A significant number of patients continued to have beneficial effects after the fourth year of treatment, with an infliximab survival of 77.9%.
References
Gran JT, Husby G (1993) The epidemiology of ankylosing spondylitis. Semin Arthritis Rheum 22:319–334
Alamanos Y, Papadopoulos NG, Voulgari PV, Karakatsanis A, Siozos C, Drosos AA (2004) Epidemiology of ankylosing spondylitis in Northwest Greece, 1983–2002. Rheumatology (Oxford) 43:615–618
Braun J, Sieper J, Bollow M (2000) Imaging of sacroiliitis. Clin Rheumatol 19:51–57
van der Horst-Bruinsma IE, Clegg DO, Dijkmans BA (2002) Treatment of ankylosing spondylitis with disease modifying antirheumatic drugs. Clin Exp Rheumatol 20 (6 Suppl 28):S67–S70
Brandt J, Haibel H, Cornely D, Golder W, Gonzalez J, Reddig J, Thriene W, Sieper J, Braun J (2000) Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor alpha monoclonal antibody infliximab. Arthritis Rheum 43:1346–1352
Van Den Bosch F, Kruithof E, Baeten D, Herssens A, de Keyser F, Mielant H, Veys EM (2002) Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthopathy. Arthritis Rheum 46:755–765
Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, Gromnica-Ihle E, Kellner H, Krause A, Schneider M, Sorensen H, Zeidler H, Thriene W, Sieper J (2002) Treatment of active ankylosing spondylitis with infliximab: a randomized controlled multicentre trial. Lancet 359(9313):1187–1193
Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, Golder W, Gromnica-Ihle E, Kellner H, Schneider M, Sorensen H, Zeidler H, Reddig J, Sieper J (2003) Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum 48:2224–2233
Breban M, Vignon E, Claudepierre P, Devauchelle V, Wendling D, Lespessailles E, Euller-Ziegler L, Sibilia J, Perdriger A, Mezieres M, Alexandre C, Dougados M (2002) Efficacy of infliximab in refractory ankylosing spondylitis: results of a six-month open-label study. Rheumatology (Oxford) 41:1280–1285
[No authors listed] (2005) TNF antagonists for ankylosing spondylitis. Drug Ther Bull 43:19–22
Braun J, Baraliakos X, Brandt J, Sieper J (2005) Therapy of ankylosing spondylitis. Part II: biological therapies in the spondyloarthritides. Scand J Rheumatol 34:178–190
De Keyser F, Baeten D, Van den Bosch F, Kruithof E, Verbruggen G, Mielants H, Veys EM (2004) Structure-modifying capacity of anti-tumour necrosis factor-alpha therapy in ankylosing spondylitis. Drugs 64:2793–2811
Temekonidis TI, Alamanos Y, Nikas SN, Bougias DV, Georgiadis AN, Voulgari PV, Drosos AA (2003) Infliximab therapy in patients with ankylosing spondylitis: an open label 12 month study. Ann Rheum Dis 62:1218–1220
Nikas SN, Alamanos Y, Voulgari PV, Pliakou XI, Papadopoulos CG, Drosos AA (2005) Infliximab treatment in ankylosing spondylitis: an observational study. Ann Rheum Dis 64:940–942
Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, Gromnica-Ihle E, Kellner H, Schneider M, Sorensen H, Zeidler H, Sieper J (2005) Two year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitis. Ann Rheum Dis 64:229–234
Braun J, Baraliakos X, Brandt J, Listing J, Zink A, Alten R, Burmester G, Gromnica-Ihle E, Kellner H, Schneider M, Sorensen H, Zeidler H, Sieper J (2005) Persistent clinical response to the anti-TNF-alpha antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology (Oxford) 44:670–676
Gossec L, Le Henanff A, Breban M, Vignon E, Claudepierre P, Devauchelle V, Wendling D, Lespessailles E, Euller-Ziegler L, Sibilia J, Perdriger A, Alexandre C, Dougados M et al (2006) Continuation of treatment with infliximab in ankylosing spondylitis: 2-yr open follow-up. Rheumatology (Oxford) 45:859–862
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 21:2286–2291
Brandt J, Listing J, Sieper J, Rudwaleit M, van der Heijde D, Braun J (2004) Development and preselection of criteria for short term improvement after anti-TNF alpha treatment in ankylosing spondylitis. Ann Rheum Dis 63:1438–1444
Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, Jenkinson T (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 21:2281–2285
Anderson JJ, Baron G, van der Heijde D, Felson DT, Dougados M (2001) Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum 44:1876–1886
Braun J, Brandt J, Listing J, Rudwaleit M, Sieper J (2003) Biologic therapies in the spondyloarthritis: new opportunities, new challenges. Curr Opin Rheumatol 15:394–407
Robinson DM, Keating GM (2005) Infliximab: in ankylosing spondylitis. Drugs 65:1283–1294
Keeling S, Oswald A, Russell AS, Maksymowych WP (2006) Prospective observational analysis of the efficacy and safety of low-dose (3 mg/kg) infliximab in ankylosing spondylitis: 4-year followup. J Rheumatol 33:558–561
Maksymowych WP, Inman RD, Gladman D, Thomson G, Stone M, Karsh J, Russell AS; Spondyloarthritis Research Consortium of Canada (SPARCC) (2003) Canadian Rheumatology Association Consensus on the use of anti-tumor necrosis factor-alpha directed therapies in the treatment of spondyloarthritis. J Rheumatol 30:1356–1363
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venetsanopoulou, A.I., Voulgari, P.V., Alamanos, Y. et al. Persistent clinical response of infliximab treatment, over a 4-year period in ankylosing spondylitis. Rheumatol Int 27, 935–939 (2007). https://doi.org/10.1007/s00296-007-0330-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-007-0330-9