Abstract
The effects of a new generation bisphosphonate, incadronate, in established adjuvant arthritis rats were evaluated according to the arthritis index, hind paw volume, and radiological and histopathological examinations. Incadronate suppressed the radiological and histopathological changes of hind paws, as well as the joint swelling in a dose-dependent manner. In contrast, the arthritis control rats showed drastic joint inflammation, marked destruction of bone and articular cartilage. The remains of articular cartilage lost Safranin O staining, and were attached with numerous TRAP-positive multinuclear cells. Some of resorption lacunas could be seen at the cartilage matrix nearby the TRAP-positive multinuclear cells. As regards the chondroprotective effects of bisphosphonates, we speculate that it is probably concerned with the inhibition of the chondroclasts. These data indicate that bisphosphonates may be a class of effective agent that can be considered for treatment of various arthritic conditions, including human rheumatoid arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is characterized by chronic inflammation of the synovium, progressive erosion of the articular cartilage through pannus formation, and joint destruction. However, the etiology and pathogenesis of RA are not clearly understood. The degree to which bone involvement in RA can be controlled is also unsatisfactory, as the currently available disease-modifying antirheumatic drugs have little overall effect on the course of bone destruction. Thus, agents capable of suppressing both bone resorption and disease activity would be ideal candidates for the management of RA.
Bisphosphonates are a class of synthetic analogs of pyrophosphate that contain nonhydrolyzable P–C–P bonds, have a high affinity for bone minerals, and inhibit bone resorption by osteoclasts [1]. These compounds have become the drugs of choice for the treatment of skeletal disorders involving excessive osteoclast-mediated bone resorption, such as Paget’s disease [2, 3], tumor-induced osteolysis and hypercalcemia [4, 5], postmenopausal osteoporosis [6], and juxtaarticular and generalized osteoporosis in RA [7, 8]. Early studies demonstrated that osteoclasts played a crucial part in the pathogenesis of bone and articular cartilage destruction in RA [9, 10]. From these data, we speculate that inhibition of osteoclast activity might limit bone and cartilage destruction in RA patients.
It has been reported that bisphosphonates, pamidronate and alendronate could improve the clinical and laboratory parameters in RA [8, 11, 12]. In addition, clodronate and risedronate were shown to inhibit the joint inflammation and the bone destruction in rat adjuvant arthritis (AA), an animal model used in RA [13, 14]. More recently, zoledronate was shown to cause partial chondroprotective effect in the carrageenan-induced arthritis in rabbits [15]. Our earlier studies also showed that incadronate, a new generation bisphosphonate, inhibited joint inflammation, as well as bone and articular cartilage destruction in rat with AA [16, 17]. But the mechanisms by which these compounds inhibit articular cartilage destruction are not yet completely clear.
In this study, we evaluated the effects of incadronate on joint inflammation and bone and articular cartilage destruction in established rat AA. In addition, the mechanisms by which this compound inhibits bone and articular cartilage destruction were clarified partly. This is more relevant to clinical therapy of RA.
Materials and methods
Animals
A total of 40 female Lewis rats (Charles River Japan, Kanagawa, Japan), 6 weeks of age and weighing 135–145 g, were randomly allocated into 5 groups of 8 animals each, before being acclimatized for 1 week under standard laboratory conditions. They were fed ad libitum with standard laboratory chow and water. All procedures were approved by the Committee of the Ethics on Animal Experiment in Faculty of Medicine, Kyushu University.
Induction of AA
Freund’s complete adjuvant was prepared by suspending heat-killed Mycobacterium butyricum (Lot 104320JA; Difco Laboratories, Detroit MI, USA) in mineral oil (Nacalai Tesque, Inc., Kyoto, Japan) at a concentration of 10 mg/ml. Two 0.05-ml aliquots were injected intradermally twice into the proximal 1/4 of the tail on the first day (termed day 0) of the experiment, following a method described in [16].
Administration of the test compounds
Incadronate (YM175; disodium dihydrogen cycloheptylamino methylene bisphosphonate monohydrate) was kindly provided by Yamanouchi Pharmaceutical Co., Ltd. (Tokyo, Japan) and dissolved in phosphate buffer solution (PBS). The rats were used for the study at 7 weeks of age and were treated as shown in Table 1. The experiment was conducted over a 42-day period. Each rat immunized with the adjuvant was given either incadronate (0.1, 0.5 and 1 mg/kg/day) or PBS in a volume of 1 ml/kg/day by a single subcutaneous injection beginning on day 15 and continuing until the day before sacrifice.
Clinical measurements
Clinical measures, including measurements of body weight, an arthritis index, and hind paw volume, were assessed respectively on day 0, 10, 15, 20, 25, 30, 35, and 42. An arthritis index was assigned to every paw by a single observer, who remained blind to the animal’s treatment group, using a standard method, as described by Kinne et al. [18]. Briefly, according to the extent of erythema and edema of the periarticular tissues, the severity of arthritis of each paw was scored using a scale of 0–4. The total of the score for each rat was designated by the arthritis index (maximum possible index per animal = 16). The arthritis index of each rat on day 0 was determined to be 0. Hind paw volume was measured with a volumetric apparatus (MK-550; Muromachi-kikai Co., Ltd., Tokyo, Japan), as described in [16]. The arthritis index and hind paw volume were used as the measurement parameters of inflammation.
Radiological analysis
Radiographs were taken with an X-ray apparatus (C-SM, Softex Co., Ltd., Tokyo, Japan) and industrial X-ray film (FUJI Photo Film Co., Ltd., Tokyo, Japan). The X-ray apparatus was operated at 30 kV peak, 3 mA and 10-s exposure with a 45 cm tube-to-film distance for lateral projections. Radiographs of the hind limbs were obtained from all the animals on day 42. The severity of bone and joint destruction was scored blindly by the same person for each hind limb according to the method described by Barbier et al. [13], with some modifications. Briefly, according to the extent of osteoporosis, osteophytes, calcification, joint spaces and joint structure, the severity of bone destruction of each paw was scored using a scale of 0–4. The radiological score was termed radiological index.
Histological examination
On day 42, 24 h after the final injection of incadronate or PBS, the animals were anesthetized by intraperitoneal injection of pentobarbital (Nembutal; Dainabot, Co., Ltd., Osaka, Japan) and fixed by intracardiac perfusion with 4% paraformaldehyde solution (pH 7.4). After perfusion, the hind paws were removed from the distal part of the femurs and radiographs were taken as described above, before further immersing the hind paws in the same fixative at 4°C for 48 h. After being rinsed several times with PBS, these specimens were decalcified in 10% ethylenediamine tetracetic acid (EDTA) solution (PH 7.2) at 4°C for 6 weeks, dehydrated and then embedded in paraffin. Longitudinal sections (5?μm in thickness) through the entire foot/ankle were stained with Safranin O and Fast Green, and tartrate-resistant acid phosphate (TRAP) using a leukocyte acid phosphatase kit (Sigma Chemical Co., St. Louis, Mo), at 37°C for 20 min in a moist chamber, before counterstaining with hematoxylin.
Histopathological examination, including inflammatory infiltration, articular cartilage and bone destruction in the tibiotarsal region, was assessed respectively in 8 sections from 8 different specimens in each group. We estimated the histopathological changes under a microscope (AX70TRF, Olympus Optical Co., Ltd., Japan), using ×100 magnification. Grading of the inflammatory infiltration, articular cartilage and bone destruction were scored by 2 observers who were blinded to the experimental outcome. The grading scheme used was described by Kinne et al. [18]. Briefly, to evaluate the degree of inflammatory infiltration, a subjective semiquantitative score of 0–3 was used, where 0 = no infiltration, 1 = mild, 2 = moderate, 3 = severe infiltration. To evaluate the degree of cartilage and bone destruction, a semiquantitative score of 0–4 was used, where 0 = no erosion of cartilage or bone, 1 = unequivocal erosion ≤10% of cartilage or bone cross-sections, 2 = erosion of <50%, 3 = erosion of 50–90%, and 4 = erosion of >90% of cartilage and bone cross-sections. The degree of inflammatory infiltration was termed inflammation score. The degree of cartilage and bone destruction was termed joint destruction score. The inflammation score and joint score were used as the measurement parameters of histopathology.
Statistical analysis
All measurements are shown as mean ± SEM. Statistical analyses were performed using Macintosh Statview software (Abacus Concepts, Berkeley, CA, USA). The Mann–Whitney U-test was used to analyze each set of variables. Differences were considered significant when P<0.05.
Results
Clinical evaluation
A quite typical manifestation of AA was induced in all the rats by day 15 after injection of adjuvant. These arthritic animals showed erythema and swelling in their extremities. Administration of incadronate to rats with AA resulted in a significant decrease in disease severity, as evaluated by arthritis index and hind paw volume.
The arthritic index of the PBS groups peaked on day 20 (Fig. 1a). Incadronate significantly decreased the arthritic index at all the time points studied (P<0.05 or P<0.01), except that incadronate at a dose of 0.1 mg/kg produced only a mild inhibition of inflammatory reaction on day 20. There was no significant difference between group of 0.5 mg/kg and group of 1 mg/kg from day 20 to day 42.
Time course of changes in clinical parameters. a Time course of changes in arthritis index (number of rats per group = 8). b Time course of changes in hind paw volume (number of hind paws per group = 16). c Time course of changes in body weight (number of rats per group = 8). Values are expressed as the mean ± SEM. *P<0.05 and **P<0.01 (the incadronate-treated groups versus the PBS-treated group at the same time points). Mann–Whitney U-test was used
The hind paw volume of the PBS group showed a progressive increase, with it peaking on day 25 and then slowly decreasing thereafter, although it still remained large until day 42 (Fig. 1b). In contrast, the maximum swelling of incadronate-treated animals was observed on day 20, after which the hind paw volume rapidly decreased as compared with that in the PBS group. At all the dose levels we examined, the hind paw volume of incadronate-treated animals showed a significant dose-dependent inhibition (P<0.01). Incadronate at a dose of 0.1, 0.5 and 1 mg/kg inhibited the hind paw volumes to 65, 62 and 60%, respectively, compared to the PBS group (termed 100%) on day 42.
The loss of body weight in incadronate-treated arthritic groups was the same as the PBS-treated group (Fig. 1c). A significant difference was not observed in all the arthritic groups.
Radiological evaluation of the hind paw
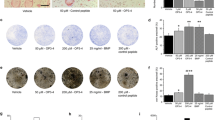
Representative radiographs of the hind limbs from each group were shown in Fig. 2. Compared to the radiographs of normal rats, the PBS-treated group exhibited severe bone destruction, with active osteophytosis. This was characterized by reduced bone density, deranged bone trabecula, focal areas of excessive bone resorption and no visible joint spaces. The severity of the bone destruction in the hind limbs of all the arthritic groups was scored as described in the Materials and methods (Fig. 3). The radiological index for each of the treatment groups was significantly lower than that for the positive control group, with the high dose (1.0 mg/kg) group displaying the lowest degree of joint destruction. On day 42, in the end of the experiment, incadronate at a dose of 0.1, 0.5 and 1 mg/kg inhibited the radiological score to 60, 47 and 39%, respectively, compared to that of the arthritis control (termed 100%) in a dose-dependent manner.
Radiographical appearances of rat hind limbs on day 42. a Normal control rats. b PBS-treated positive control rats. The hind limb shows severe bone destruction with activity osteophytosis, reduced bone density, focal areas of bone resorption and no visible joint spaces. Significant aggravation of joint destruction is apparent in the PBS-treated rats compared with the normal control rats. c–e Arthritic rats treated respectively with 0.1, 0.5 and 1 mg/kg/day of incadronate. c shows mild bone enlargement with stabilized osteophytosis in the distal metaphysis of the tibia and minor joint space alterations. d, e shows almost normal findings. In contrast to the PBS group, there is increased bone density in the distal metaphysis of the tibia, and obvious preservation of joint structure in the incadronate-treated groups
Histopathological evaluation of the hind paw
Representative histopathological findings of the peritarsal region were shown in Fig. 4. In the PBS-treated group, the bone and joint were severely affected and the normal structure had been partially replaced by fibrous connective tissue (Fig. 4b). Comparison of the PBS-treated arthritic group with the incadronate-treated arthritic groups, revealed a significant reduction in inflammatory infiltration in the tibiotarsal region after 4 weeks with incadronate treatment. Icadronate suppressed synovium hyperplasia, fibrous ankylosis and pannus formation in the ankle joints, in a dose-dependent manner.
Histopathological appearances of the peritarsal region on day 42. Sections were stained with TRAP, before being counterstained with hematoxylin. Original magnification ×20. a Normal control rats. b PBS-treated positive control rats. No normal bone or joint structures. Severe destruction of the articular cartilage and replacement with dense fibrous connective tissue producing a fibrous ankylosis. c–e Arthritic rats treated respectively with 0.1, 0.5 and 1 mg/kg/day of incadronate. d, e show mild synovial proliferation around the joints. The bone and joint structures are well preserved in the incadronate-treated rats
On the other hand, marked differences were seen in the articular cartilage of talus between the incadronate-treated group and the PBS group (Fig. 5). The positive control group showed variable degenerative changes, characterized by fibril formation (Fig. 5b). The supertalar cartilage showed extensive disorganization and absence of Safranin O staining. The subchondral bone of talus was lost completely. Incadronate prevented destruction of the supertalar cartilage and bone in talus. In the incadronate-treated 0.1 mg/kg group, the supertalar cartilage showed some loss of Safranin O staining (Fig. 5c). Whereas, in the incadronate-treated 1 mg/kg group, the Safranin O staining was similar to normal control group (Fig. 5d). Incadronate partially prevented some of these degenerative changes and ameliorated the decrease in Safranin O staining of the articular cartilage, in a dose-dependent manner.
Histopathological appearances of the tibiotarsal region on day 42. ae, bf, cg, and dh are serial sections, respectively. a, b, c and d were stained with Safranin O and Fast Green. e, f, g and h were stained for TRAP before counterstaining with hematoxylin. Original magnification ×100. a, e Normal control. b, f PBS-treated positive control. Multiple layers of synovial cells occupy the tibiotarsal region. Loss of Safranin O staining is evident in the articular cartilage. The subchondral bone of talus was lost completely. There is marked infiltration of TRAP positive cells in the marrow cavity. cg and dh Arthritic rats treated with 0.1 and 1 mg/kg/day of incadronate, respectively. Preservation of Safranin O staining of articular cartilage is observed, and the number of TRAP positive cells in the marrow cavity is markedly decreased. The cartilage and bone structures are well preserved
On day 42, as shown in Fig. 6, incadronate (at a dose of 0.1, 0.5 and 1 mg/kg) inhibited the inflammatory infiltration to 58, 50 and 46%, the bone and articular cartilage destruction to 69, 62 and 48% (termed 100% of the arthritis control). Incadronate suppressed the pathological changes seen in AA described above in a dose-dependent manner. Histopathological evaluation of ankle joints from arthritic rats revealed that incadronate inhibited the degree of histological lesions.
Histopathological index of the tibiotarsal region on day 42. a Inflammation score in hind paws. b Joint destruction score in hind paws. (number of rats per group = 8). Values are expressed as the mean ± SEM (number of sections per group = 8). **P<0.01 (the incadronate-treated groups versus the PBS-treated group). Mann–Whitney U-test was used
Appearance of osteoclasts in AA
The TRAP-positive reaction was a marker of osteoclasts and preosteoclasts or precursors. Generally, the multinuclear TRAP-positive cells were identified as osteoclasts.
In the PBS-treated group, striking infiltration of numerous TRAP-positive cells was observed in the bone marrow space as well as on the surface of the bone trabeculae (Figs. 5f, 7a). A little of TRAP-positive cells were also seen in the bone marrow cavities by the bone trabecular surface in the incadronate-treated 0.1 mg/kg group (Figs. 5g, 7b). In contrast, the TRAP-positive cells were rarely seen in the incadronate-treated 1 mg/kg group (Fig. 5h). The treatment of incadronate reduced the number of TRAP-positive cells in the marrow cavities of talus.
Cytomorphological appearances of osteoclasts. Sections were stained for TRAP before counterstaining with hematoxylin. Original magnification ×200. a PBS-treated positive control rats. TRAP-positive multinuclear cells are adjacent to the trabecular bone. b Arthritic rats treated with 0.1 mg/kg/day of incadronate. The TRAP-positive multinuclear cells showed apoptotic changes, with condensation and magnification of heterochromatins. These cells changed to round cells and detached from trabecular bone
The TRAP-positive cells showed some different appearance between the PBS-treated group and incadronate-treated groups (Fig. 7). In the PBS-treated group, there were many TRAP-positive multinuclear cells with marked polarity to be seen near the bone trabecula surface. TRAPase histochemical reaction products were accumulated clearly in the bone matrix juxtaposed to these osteoclasts. It was certified that they were very active in bone destruction (Fig. 7a). In contrast, the TRAP-positive multinuclear cells displayed unable cytological characteristics, such as lost polarity, scaled off the bone trabeculae, and contracted spheroid body in the marrow cavity in incadronate-treated arthritic rats (0.1 mg/kg). These cells showed apoptotic changes, showing condensation and magnification of heterochromatin (Fig. 7a).
Figure 8 showed the histopathological appearance of a disorganized intermetatarsal joint in the PBS-treated group. The remains of articular cartilage lost Safranin O staining (data not shown), and were attached with numerous TRAP-positive multinuclear cells. Some of resorption lacunas could be seen at the cartilage matrix nearby the TRAP-positive multinuclear cells. These TRAP-positive multinuclear cells seemed to be osteoclasts.
Histopathological appearances of the articular cartilage destruction in the PBS-treated positive control rats. Sections were stained for TRAP before counterstaining with hematoxylin. a Original magnification ×100. The remains of articular cartilage were attached with numerous TRAP-positive multinuclear cells. b High-power magnification view of the boxed region in a. Original magnification ×400. These TRAP-positive multinuclear cells displayed the cytologic characteristics of maturative osteoclast, such as multiple nuclei; resorption lacunas at the cartilage matrix attached nearby these cells
Discussion
This report shows that incadronate inhibited not only bone destruction but also joint inflammation and articular cartilage destruction in rats with established AA; these results were confirmed both histologically and radigraphically.
Treatment of established AA rats with incadronate by a single subcutaneous injection per day (over a 4-week period) resulted in marked inhibition of bone destruction. Bisphosphonates are well-known inhibitors of osteoclastic bone resorption. Several studies have shown that incadronate could inhibit multinuclear TRAP-positive cell formation in mouse bone marrow cultures [19, 20], and facilitate apoptosis of osteoclasts in normal rats which were injected intravenously with 1 mg/kg [21]. In our previous study, incadronate also decreased the number of multinuclear TRAP-positive cells in AA rats [16]. In this study, the morphological changes of osteoclasts indicated their functional differences (Fig. 7). The ability of osteoclasts was inhibited with bisphosphonate in incadronate-treated groups. As shown in Fig. 7b, the osteoclasts had lost polarity, contracted spheroid body, and scaled off the bone trabeculae. Some of these osteoclasts seemed to be apoptosis or preapoptosis. Thus, incadronate suppressed the bone resorption and protected bone structures in AA rats.
Administration of incadronate to rats with established AA significantly inhibited articular cartilage destruction, as shown in Fig. 5. These obtained similar results to our previous study for prophylactic treatment of incadronate to AA rats [17]. In contrast, other investigators have reported that zoledronate suppressed articular cartilage from degradation in a rabbit model of inflammatory arthritis induced with carrageenan [15]. These suggested that bisphosphonates may have chondroprotective abilities. But, the mechanisms underlying chondroprotective abilities of bisphosphonates are not yet completely clarified.
It is well known that type II collagen is almost exclusively localized in cartilage. The fragments derived from the type II collagen should represent a specific index for cartilage degradation. Recently, some reports demonstrated that alendronate, ibandronate, and zoledronate decreased the urinary excretion of type II C-telopeptide degradation products (CTX-II), a new marker of cartilage degradation, in patients with Paget’s disease or in healthy postmenopausal [22, 23]. Both pamidronate and alendronate could reduce the product of MMP-3 and MMP-13 which were correlated with degradation of type II collagen in supernatant of human tumorous cell lines [24]. In addition, pamidronate and risedronate have been shown to prevent dexamethasone-induced growth retardation and apoptosis of chondrocytes in vitro [25]. All of these findings just showed us some indirect evidence of chondroprotective effects of bisphosphonates. The prevention of articular cartilage destruction in incadronate-treated groups may be also due to the same mechanisms.
In the present study, we found that TRAP-positive multinuclear cells could destroy the degenerating articular cartilage directly (Fig. 8). As previously described by Bromley et al. [10], these TRAP-positive multinuclear cells were chondroclasts, and they were morphologically and histochemically similar to osteoclasts. If so, bisphosphonates may also suppress the function of chondroclasts directly. As regards the protection of articular cartilage of bisphosphonate, we speculate that it was probably concerned with the inhibition of the chondroclasts. As shown in Fig. 8, a little of new insight has been given in this animal model. This effect is required to be confirmed further.
Besides its effective inhibition of bone and articular cartilage destruction, incadronate also had a beneficial effect on inflammation in established rat AA, as shown by arthritis index, hind paw volume and inflammation score. In our earlier studies, we obtained similar results for the inhibition of joint inflammation after incadronate injection to arthritic rats [17]. Macrophages are known to be involved in the inflammatory response by, for instance, secreting mediators of inflammation such as prostaglandins, cytokines and various neutral proteases, and are found in large numbers within inflamed joints [26]. We had shown that incadronate decreased the number of ED1 (a mouse anti-rat macrophage antibody) positive cells infiltrating the synovial tissue, and inhibited the chemotaxis of the mouse macrophage-like cells (RAW 264.7) induced with macrophage chemoattractant protein (MCP)-1 in vitro [17]. Luckman et al. [27] had reported that incadronate and other nitrogen-containing bisphosphonates could cause apoptosis in mouse J774 macrophages in vitro by inhibiting posttranslational modification of proteins. The anti-inflammatory effect of incadronate was very likely by inducing apoptosis of macrophages resident in synovium. In addition, one study indicated that oral alendronate therapy in early RA could significantly decrease the serum interleukin 1 beta (IL-1beta), IL-6, tumor necrosis factor alpha (TNF-alpha), erythrocyte sedimentation rate, and C-reactive protein [8]. It was possible that incadronate could exert its anti-inflammatory effect by modulating some preinflammatory cytokines. In contrast, other investigators have reported that incadronate exacerbated the arthritis induced in mice by the co-injection of type II collagen and an adjuvant [28]. The discrepancy in the effects may be due to differences in the methods of administration or the animal models used. At present, the exact mechanism behind the anti-inflammtory effect of incadronate has not yet been fully understood.
Further, compared with the PBS-treated positive control group, no loss of body weight was observed in the rats receiving incadronate in the present study, although we noted that body weight tended to decrease when a high daily dose of incadronate was given before the onset of arthritis. Regarding the adverse effects of bisphosphonates on bone, administration of high doses of alendronate and pamidronate has been shown to inhibit bone turnover and cause cumulative microdamage in vivo [29, 30]. Komatsubara et al. [31] indicated that 3-year incadronate treatment also increased accumulation of microdamage in dog vertebra, but this was not necessarily associated with vertebral fragility because of compensated increase of bone mass and improved microarchitecture. Although no microdamage accumulation was observed in the present study, this possibility will need to be addressed in long-term studies with histological and radiological monitoring to confirm the safety of long-term bisphosphonate use for human patients.
Taken together, these observations clearly indicate that incadronate protected bone and articular cartilage structures, suppressed joint inflammation in established rat AA. On the basis of these results, incadronate may be an effective agent that can be considered for treatment of various arthritic conditions, including human RA.
References
Rodan GA, Fleisch HA (1996) Bisphosphonates: mechanisms of action. J Clin Invest 97:2692–6
Harinck HI, Papapoulos SE, Blanksma HJ, Moolenaar AJ, Vermeij P, Bijvoet OL (1987) Paget’s disease of bone: early and late responses to three different modes of treatment with aminohydroxypropylidene bisphosphonate (APD). Br Med J 295:1301–1305
Thiebaud D, Jaeger P, Gobelet C, Jacquet AF, Burckhardt P (1988) A single infusion of the bisphosphonate AHPrBP (APD) as treatment of Paget’s disease of bone. Am J Med 85:207–212
Body JJ, Mancini I (2003) Treatment of tumor-induced hypercalcemia: a solved problem? Expert Rev Anticancer Ther 3:241–246
Body JJ (2000) Current and future directions in medical therapy: hypercalcemia. Cancer 88(Suppl):3054–3058
Storm T, Thamsborg G, Steiniche T, Genant HK, Sorensen OH (1990) Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. N Engl J Med 322:1265–1271
Eggelmeijer F, Papapoulos SE, van Paassen HC, Dijkmans BA, Breedveld FC (1994) Clinical and biochemical response to single infusion of pamidronate in patients with active rheumatoid arthritis: a double blind placebo controlled study. J Rheumatol 21:2016–2020
Cantatore FP, Acquista CA, Pipitone V (1999) Evaluation of bone turnover and osteoclastic cytokines in early rheumatoid arthritis treated with alendronate. J Rheumatol 26:2318–2323
Havdrup T, Hulth A, Telhag H (1976) The subchondral bone in osteoarthritis and rheumatoid arthritis of the knee. A histological and microradiographical study. Acta Orthop Scand 47:345–350
Bromley M, Woolley DE (1984) Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum 27:968–975
Eggelmeijer F, Papapoulos SE, van Paassen HC, Dijkmans BA, Valkema R, Westedt ML, Landman JO, Pauwels EK, Breedveld FC (1996) Increased bone mass with pamidronate treatment in rheumatoid arthritis. Results of a three-year randomized, double-blind trial. Arthritis Rheum 39:396–402
Maccagno A, Di GE, Roldan EJ, Caballero LE, Perez LA (1994) Double blind radiological assessment of continuous oral pamidronic acid in patients with rheumatoid arthritis. Scand J Rheumatol 23:211–214
Barbier A, Breliere JC, Remandet B, Roncucci R (1986) Studies on the chronic phase of adjuvant arthritis: effect of SR 41319, a new diphosphonate. Ann Rheum Dis 45:67–74
Osterman T, Kippo K, Lauren L, Hannuniemi R, Sellman R (1994) Effect of clodronate on established adjuvant arthritis. Rheumatol Int 14:139–147
Podworny NV, Kandel RA, Renlund RC, Grynpas MD (1999) Partial chondroprotective effect of zoledronate in a rabbit model of inflammatory arthritis. J Rheumatol 26:1972–1982
Zhao H, Shuto T, Hirata G, Iwamoto Y (2000) Aminobisphosphonate (YM175) inhibits bone destruction in rat adjuvant arthritis. J Orthop Sci 5:397–403
Matsuo A, Shuto T, Hirata G, Satoh H, Matsumoto Y, Zhao H, Iwamoto Y (2003) Antiinflammatory and chondroprotective effects of the aminobisphosphonate incadronate (YM175) in adjuvant induced arthritis. J Rheumatol 30:1280–1290
Kinne RW, Schmidt-Weber CB, Hoppe R, Buchner E, Palombo-Kinne E, Nurnberg E, Emmrich F (1995) Long-term amelioration of rat adjuvant arthritis following systemic elimination of macrophages by clodronate-containing liposomes. Arthritis Rheum 38:1777–1790
Nishikawa M, Akatsu T, Katayama Y, Yasutomo Y, Kado S, Kugal N, Yamamoto M, Nagata N (1996) Bisphosphonates act on osteoblastic cells and inhibit osteoclast formation in mouse marrow cultures. Bone 18:9–14
Nishikawa M, Yamamoto M, Murakami T, Akatsu T, Kugai N, Nagata N (1998) A third-generation bisphosphonate, YM175, inhibits osteoclast formation in murine cocultures by inhibiting proliferation of precursor cells via supporting cell-dependent mechanisms. J Bone Miner Res13:986–995
Ito M, Amizuka N, Nakajima T, Ozawa H (1999) Ultrastructural and cytochemical studies on cell death of osteoclasts induced by bisphosphonate treatment. Bone 25:447–452
Lehmann HJ, Mouritzen U, Christgau S, Cloos PA, Christiansen C (2002) Effect of bisphosphonates on cartilage turnover assessed with a newly developed assay for collagen type II degradation products. Ann Rheum Dis 61:530–533
Garnero P, Christgau S, Delmas PD (2001) The bisphosphonate zoledronate decreases type II collagen breakdown in patients with Paget’s disease of bone. Bone 28:461–464
Teronen O, Heikkila P, Konttinen YT, Laitinen M, Salo T, Hanemaaijer R, Teronen A, Maisi P, Sorsa T (1999) MMP inhibition and downregulation by bisphosphonates. Ann N Y Acad Sci 878:453–465
Van Offel JF, Schuerwegh AJ, Bridts CH, Stevens WJ, De Clerck LS (2002) Effect of bisphosphonates on viability, proliferation, and dexamethasone-induced apoptosis of articular chondrocytes. Ann Rheum Dis 61:925–928
Johnson WJ, DiMartino MJ, Hanna N (1986) Macrophage activation in rat models of inflammation and arthritis: determination of markers of stages of activation. Cell Immunol 103:54–64
Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ (1998) Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 13:581–589
Nakamura M, Ando T, Abe M, Kumagai K, Endo Y (1996) Contrast between effects of aminobisphosphonates and non-aminobisphosphonates on collagen-induced arthritis in mice. Br J Pharmacol 119:205–212
Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB (2001) Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone 28:524–531
Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB (2000) Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 15:613–620
Komatsubara S, Mori S, Mashiba T, Ito M, Li J, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H (2003) Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J Bone Miner Res 18:512–520
Acknowledgments
The authors thank Yamanouchi Pharmaceutical CO., Ltd., for kindly providing incadronate (YM175), and Mr. Takaaki Kanemaru, Mr. Masaharu Magara and Miss Naoko Tanaka for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H., Liu, S., Huang, D. et al. The protective effects of incadronate on inflammation and joint destruction in established rat adjuvant arthritis. Rheumatol Int 26, 732–740 (2006). https://doi.org/10.1007/s00296-005-0061-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-005-0061-8