Abstract
This cross-sectional study was designed to investigate correlations between muscle strength and regional bone mineral density (BMD) in sedentary postmenopausal women. Sixty-two women who ranged in age from 41 to 76 years were investigated. Hip and trunk muscle strength was measured by isokinetic dynamometry. Grip strength of the nondominant hand was measured using a hand-held dynamometer. Bone mineral density of the lumbar spine, femur, and distal radius was measured by dual-energy X-ray absorptiometry. Only the correlation between hip abductor strength and femoral BMD was significant (P=0.009, r=0.327). There was no correlation between trunk muscle strength and lumbar vertebral BMD or between grip strength and distal radius BMD. Subjects with osteoporosis (T score <−2.5) or osteopenia T (−2.5 to −1) and normal subjects (T>−1) exhibited similar isokinetic hip and trunk muscle strength. Women with osteoporotic distal radii had significantly lower grip strength than subjects who were osteopenic or normal at this site, but the osteoporotic group was also significantly older. In conclusion, our results indicate that the isokinetic strength of hip abductors weakly correlates with femoral BMD in postmenopausal women with and without osteoporosis. Trunk muscle strength did not correlate with lumbar vertebral BMD in either of these groups. The weaker handgrip we observed in the women with osteoporotic radii may be attributed to older age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advancing age is associated with profound changes in body composition such as decreased bone mineral density (BMD), sarcopenia, and hormonal fluctuations [1, 2, 3, 4]. After 30 years of age, BMD decreases at an average rate of 1% per year [5]. Muscular strength also declines during this time, with a gradual 10% loss from 25 to 50 years. After age 60, humans experience a 40% decline in lifetime muscle mass [4, 6]. These changes in BMD and muscle strength are accompanied by a decline in estrogen production from approximately 47 years onwards [7]. The loss of strength is more gradual and not significantly affected by the sudden hormonal decline, like bone loss.
Although the skeletal and muscular systems are structurally interdependent and both adapt to mechanical loading, some aspects of their relationship remain unclear. The threshold levels of loading or intensity required to elicit changes in these systems has been studied extensively, but the results are controversial. Some reports have documented positive correlations between muscle strength and bone mass [8, 9, 10, 11, 12, 13, 14]; other authors stated that the effect of muscle strength is systemic, not local, and still others found no link between muscle strength and bone mineral content [9, 15, 16, 17, 18].
The conflicting results concerning the relationship between strength and BMD may be due to confounding variables. Specifically, the effects of muscle strength on bone may be influenced by variables not necessarily investigated by the given research, and these elements may directly influence BMD as well. This theory seems to be valid, as BMD is influenced by many factors that may also affect each another [19]. In addition, disagreement in results may be partially attributed to study design and the different techniques for measuring bone density and muscle strength.
Experts continue to question whether exercise can be considered an integral part of osteoporosis prevention and treatment. However, it is widely agreed that disuse results in bone mass loss, that sedentary individuals tend to have less bone mass than those who exercise, and that exercise tends to produce a modest increase in bone mass and alone cannot compensate for estrogen loss [20]. There is a need for better understanding of the connection between muscle strength and bone mass, as this is one of the keys to rehabilitation outcome. The purpose of this study was to clarify relationships between BMD and muscle strength at three different body sites in a population of postmenopausal women with similar physical activity levels.
Materials and methods
Patients
The study involved 62 healthy postmenopausal women who ranged in age from 41–76 years and did not engage in regular exercise. All the subjects were sedentary and worked in the home. The exclusion criteria were (1) illness or drug treatments known to influence bone mass and (2) any abnormal finding in a set of standard laboratory tests consisting of complete blood count, serum protein electrophoresis, and serum levels of calcium, phosphorus, albumin, creatinine, total protein, and alkaline phosphatase. If even one of these results was outside the normal range, the candidate was excluded.
For each participant, body weight and height were measured, and body mass index (BMI) was calculated as weight (kg)/height (m)2. The time since menopause (TSM) was also recorded. Bone mineral density of the lumbar vertebrae, femur, and distal radius was measured by dual-energy X-ray absorptiometry (Hologic QDR-4500A S/N 45835). Based on the BMD findings (T score), the subjects were grouped as normal (T>−1), osteopenic (T −1 to −2.5), or osteoporotic (T<−2.5).
Method
A Cybex 770 NORM isokinetic dynamometer (Lumex, Ronkonkoma, N.Y., USA) was used to evaluate reciprocal concentric and isometric trunk flexion and extension muscle strength in all subjects. The TEF modular component attached to the NORM unit was used. The subjects were positioned and secured in the TEF modular component according to the manufacturer’s instructions. The axis of rotation was set at the point at which the midaxillary line intersects the lumbosacral junction, or approximately 3.5 cm below the top of the iliac crest. The lower body was stabilized with a slightly bent knee position (15° knee flexion) using tibial, popliteal, and thigh pads. The trunk was stabilized using pelvic, scapular, and chest pads. Vertical standing position was determined as the anatomic zero position. Isokinetic trunk muscle strength was measured at angular velocities of 60°/s, 90°/s, and 120°/s. Testing started with the trunk in full extension. During each trial, five submaximal warm-up repetitions preceded each test velocity recording. A rest period of 30 s was allowed between warm-up repetitions and the actual test session. In each test procedure, the subject performed five maximal concentric contractions. The mean values of five repetitions for each set were recorded. Isometric trunk flexor and extensor muscle strength was measured with the subjects in 60° trunk flexion for 30 s.
The same computerized isokinetic dynamometer was used for hip abductor and adductor muscle strength measurement. The subjects lay on the bench of the dynamometer in lateral recumbent position on the dominant side, leaving the nondominant side up for testing. The axis of the dynamometer was in line with the hip joint. The trunk was stabilized using pelvic belts. The side-lying position was determined as the anatomic zero position. Isokinetic hip abductor and adductor muscle strength was measured at angular velocities of 30°/s, 60°/s, and 90°/s. This testing started with the hip in full adduction. Each muscle strength test consisted of five repetitions preceded by five submaximal warm-up repetitions at each velocity, with a 30-s rest period after each set. The average of the five maximum peak torques was recorded. Grip strength of the nondominant hand was measured using a hand-held dynamometer, and the means of five attempts were recorded.
Statistical analysis
Pearson correlation coefficients were calculated to analyze relationships between torque values and BMD T scores. Analysis of variance was used for differences among mean values for the normal, osteopenic, and osteoporotic groups. P values of <0.05 were considered statistically significant.
Results
The mean age of the women was 62.6±9.2 years, and the mean BMI was 28.3±3.5 (range 18.4–34.7). The mean TSM for the entire group was 14.2±9.0 years (range 0.5–36). The anthropometric data and TSM findings for the subjects grouped according to regional BMD T scores are shown in Table 1. Time since menopause was significantly longer in the groups with osteoporosis in the femur and distal radius than in the groups that were osteopenic and normal in these regions (P=0.001 and P=0.002, respectively). The same trend was observed for the results in the lumbar spine, but the TSM for the group with osteoporosis at this site was not significantly different from the TSM results for the other two groups. Considering the groupings for each body region, the osteoporotic women were older than the other subjects in all cases (P<0.001 for all). There were no differences between the normal and osteopenic groups in each set with respect to mean age. There were also no significant differences in weight, height, or BMI within each set of three groups.
Table 2, Table 3, and Table 4 show the respective results for trunk muscle, hip muscle, and grip strength, with the women grouped according to BMD for the related region. Trunk and hip muscle strengths were similar in the subjects that had normal, osteopenic, and osteoporotic T scores in the lumbar spine and femur, respectively. Mean grip strength in the group with osteoporotic distal radii was significantly lower than in the groups with normal and osteopenic distal radii (P=0.006 and P=0.002, respectively). The groups with normal and osteopenic radii had similar grip strength.
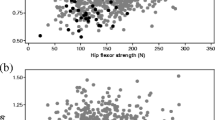
Grip strength negatively correlated with age (P=0.001, r=−0.416). There was no correlation between lumbar spine BMD and trunk muscle strength or between distal radius BMD and grip strength. There was a weak but statistically significant correlation between femur BMD and hip abductor muscle strength (P=0.009, r=0.327).
Discussion
Previous reports have documented strong correlations between muscle strength and regional BMD; however, our findings do not support this. As mentioned before, there is much discrepancy in the literature concerning relationships between muscle strength and bone mass. Multiple factors are involved in the pathogenesis of osteoporosis, and these may be partially responsible for much of the variation in results.
Research has shown that the drop in estrogen levels at the onset of menopause causes accelerated bone loss [21]. Further, it has been proven that many other factors, including activity level, body weight, height, BMI, smoking habit, and alcohol and dietary calcium intake, also influence peak bone mass in women [16, 19, 22, 23]. One study by Madsen et al. revealed highly significant correlations in women between quadriceps strength and BMD of both site-specific bone of the proximal tibia and distant bone of the distal forearm [24]. However, in more recent work on female subjects, Madsen and other coworkers demonstrated that bone mass is most closely linked to body weight and not related to age, disease duration, quadriceps strength, or current level of activity [25].
Ravn et al. reported that the BMD of the proximal femur is largely unchanged until menopause [26]. They investigated the factors of age, BMI, and TSM in relation to bone density, and they identified TSM as the strongest predictor of BMD in the first 10–15 years after onset of menopause. In line with this, we noted significantly longer TSM in the groups that were osteoporotic in the femur and distal radius regions than in the groups that were osteopenic and normal at these sites. Also, in our study, the osteoporotic women were older than the normal and osteopenic subjects in all cases, as would be expected; but our study group consisted of a number relatively small for drawing definite conclusions about osteoporosis/age/TSM relationships. With this small sample size, our results underline that confounding factors (TSM and others) must be considered when trying to relate a single variable, such as torque, to BMD.
One report in the literature notes significant correlations between hip flexor torque and BMD of the lumbar spine and proximal femur in postmenopausal women [27]. The interior of the femoral neck is composed of cancellous bone, with trabeculae organized in medial and lateral trabecular systems. It is likely that the lateral trabecular system resists the compressive forces that are placed on the femoral head when the hip abductor muscles contract [28]. We found a weak but statistically significant correlation between femoral BMD and hip abductor muscle strength in the women we studied. This is the only finding in our investigation that is in accordance with earlier results concerning the effect of muscular loads on bone.
Our assessments also showed that the women with osteoporotic distal radii had lower grip strength than women who were normal or osteopenic in this region. This is in line with a previous study that identified grip strength as a strong independent predictor of distal radius BMD in postmenopausal women [29]. Associations between grip strength and bone density at distant sites such as the spine and hip have also been reported [9]. However, considering each set of groupings for the three body regions in our study, we observed that the osteoporotic women were always significantly older than the normal and osteopenic groups. In addition to bone mineral loss, it is possible that the effects of age on muscle strength might be responsible for weaker grip in these women.
We found only one correlation between muscle strength and regional BMD—a weak relationship between hip abductor strength and femur BMD. One limitation of this study is that we did not have a control group of women who were actively engaged in an exercise program. This would have given us a chance to compare the relationships between muscle strength and BMD in exercising and nonexercising women and make a clearer conclusion about the effect of exercise on BMD.
Epidemiological evidence suggests that being active at higher age cuts the risk of hip fracture by almost one half [30]. This effect is probably multifactorial through positive effects on bone, muscle strength, balance, and joint flexibility. Avoidance of inactivity and participation in moderate exercise programs can be encouraged to promote mobility and coordination and prevent falls. However, the most beneficial type, duration, and intensity of exercise for this purpose have yet to be determined.
It is clear that, in contrast to vigorous aerobic weight-bearing exercise, walking or strolling has a negligible effect on BMD retention and cannot be relied upon as a therapeutic component of osteoporosis treatment [31, 32, 33]. The main benefit of muscle-strengthening exercises in osteoporosis may be in promoting mobility, not reducing bone mass. It should be kept in mind that exercise alone does not eliminate or compensate for the multiple factors involved in the pathogenesis of osteoporosis.
References
Dalsky GP, Stocke KS, Ehsani AA, Slotopolsky F, Lee WC, Birge SJ (1988) Weight-bearing exercise training and lumbar BMC in postmenopausal women. Ann Int Med 108:824–828
Kerr D, Morton A, Dick I, Prince R (1996) Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. J Bone Miner Res 11:218–225
McCartney N, Hicks AL, Martin J, Webber CE (1995) Long-term resistance training in the elderly: effect on dynamic strength, exercise capacity, muscle and bone. J Gerontol Biochem Sci Med Ser 50:97–104
Powers SK, Dodd SL (1996) Effects of aging and exercise on skeletal muscle. Res Q Exerc Sport 67:26–36
Riggs BL, Wahner HW, Melton LJ, Richelson LS, Judd HL, Offord KP (1986) Rates of bone loss in the appendicular and axial skeletons of women: evidence of substantial vertebral bone loss before menopause. J Clin Invest 77:1487–1491
Hakkinen KW, Kraemer WJ, Kallinen M, Linnamo V, Pastinen U, Newton RU (1996) Bilateral and unilateral neuromuscular function and muscle cross-sectional area in middle-aged and elderly men and women. J Geront A Biol Sci Med Sci 51:B21–B29
Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC (1993) Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci 84:95–98
Bevier WC, Wiswell RA, Pyka G, Kozak KC, Newhall KM, Marcus R (1989) Relationship of body composition, muscle strength and aerobic capacity to bone mineral density in older men and women. J Bone Miner Res 4:421–432
Kritz-Silverstein D, Barret-Connor E (1994) Grip strength and bone mineral density in older men and women. J Bone Miner Res 9:45–51
Nguyen T, Sambrook P, Kelly P, Jones G, Lord S, Freund J, Eisman J (1993) Prediction of osteoporotic fractures by postural instability and bone density. Br Med J 307:1111–1115
Pocock N, Eisman J, Gwinn T, Sambrook P, Kelly P, Freund J, Yeates M (1989) Muscle strength, physical fitness, and weight but not age predict femoral neck bone mass. J Bone Miner Res 4:441–448
Vico L, Pouget JF, Calmels P, Chatard JC, Rehaila M, Minaire P, Geyssant A, Alexandre C (1995) The relationship between physical ability and bone mass in women aged over 65 years. J Bone Miner Res 10:374–382
Vuori I, Heinonen H, Sievanen H, Kannus P, Pasanen M, Oja P (1994) Effects of unilateral strength training and detraining on bone mineral density and content in young women: a study of mechanical loading and deloading on human bones. Calcif Tissue Int 55:59–67
Beverly M, Rider T, Evans M, Smith R (1989) Local bone mineral response to brief exercise that stresses the skeleton. Br Med J 299:233–235
Tan J, Çubukçu S, Sepici V (1998) Relationship between bone mineral density of the proximal femur and strength of hip muscles in postmenopausal women. Am J Phys Med Rehab 77:477–482
Sinaki M, Fitzpatrick L, Ritchie C, Montesano A, Wahner H (1998) Site-specificity of bone mineral density and muscle strength in women: job-related physical activity. Am J Phys Med Rehab 77:470–476
Madsen K, Adams W, Van Loan M (1998) Effects of physical activity, body weight and composition and muscular strength on bone density in young women. Med Sci Sports Exerc 30:114–120
Duppe H, Gardsell P, Johnell O, Nilsson B, Ringsberg K (1997) Bone mineral density, muscle strength and physical activity. A population-based study of 332 subjects aged 15–42 years. Acta Orthop Scand 68:97–103
Bauer D, Browner W, Cauley J (1993) Factors associated with appendicular bone mass in older women. Ann Intern Med 118:657–665
Chesnut CH (1993) Bone mass and exercise. Am J Med 95:34–36
Hannson T, Roos B (1984) Age-related changes in the bone mineral density of the lumbar spine in normal women. Calcif Tis Int 38:328–332
Madsen KL, Adams WC, Van Loan MD (1998) Effects of physical activity, body weight and composition, and muscular strength on bone density in young women. Med Sci Sports Exerc 30:114–120
Uusi Rasi K, Sievanen H, Pasanen M, Oja P, Vuori I (2001) Maintenance of body weight, physical activity and calcium intake helps preserve bone mass in elderly women. Osteoporos Int 12:373–379
Madsen OR, Schaadt O, Bliddal H, Egsmose C, Sylvest J (1993) Relationship between quadriceps strength and bone mineral density of the proximal tibia and distal forearm in women. J Bone Miner Res 8:1439–1444
Madsen OR, Lauridsen UB, Sorensen OH (2000) Quadriceps strength in women with a previous hip fracture: relationships to physical ability and bone mass. Scand J Rehab Med 32:37–40
Ravn P, Hetland ML, Overgaard K, Christiansen C (1994) Premenopausal and postmenopausal changes in bone mineral density of the proximal femur measured by dual-energy X-ray absorptiometry. J Bone Miner Res 9:1975–1980
Zimmermann CL, Smidt GL, Brooks JS, Kinsey WJ, Eekhoff TL (1990) Relationship of extremity muscle torque and bone mineral density in postmenopausal women. Phys Ther 70:302–309
Nordin M, Frankel VH (1989) Biomechanics of the hip. In: Nordin M, Frankel VH, Forssen K (eds) (1989) Basic biomechanics of the musculoskeletal system. Lea and Febiger, Philadelphia, pp 135–152
Di Monaco M, Di Monaco R, Manca M, Cavanna A (2000) Handgrip strength is an independent predictor of distal radius bone mineral density in postmenopausal women. Clin Rheumatol 19:473–476
Rutherford OM (1999) Is there a role for exercise in the prevention of osteoporotic fractures? Br J Sports Med 33:378–386
Allen S (1994) Exercise considerations for postmenopausal women with osteoporosis. Arthritis Care Res 7:205–214
Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ (1994) Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA 272:1909–1914
Swezey RL (1996) Exercise for osteoporosis: is walking enough? The case for site specificity and resistive exercise. Spine Update 21:2809–2814
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayramoğlu, M., Sözay, S., Karataş, M. et al. Relationships between muscle strength and bone mineral density of three body regions in sedentary postmenopausal women. Rheumatol Int 25, 513–517 (2005). https://doi.org/10.1007/s00296-004-0475-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-004-0475-8