Abstract
Studies on a yeast cell cycle checkpoint that can delay mitosis depending on whether cells have built a bud have identified a “sensor” that seems to recognize the organization of filament-forming septin proteins. Innovative work applying correlative light and platinum replica electron microscopy suggests that the informative septin organization involves parallel alignment of septin filaments, and another striking study shows that septin filaments prefer to populate membranes that have positive micron-scale curvature. Together, these findings suggest a model for how cells may monitor aspects of their own shape to influence cell behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell shape is thought to influence many aspects of cell physiology. For example, experiments constraining mammalian cell shape using micro-patterned adhesive surfaces indicated that the shape can dictate whether a cell proliferates, differentiates, or dies (Chen et al. 1997). But how do the cells know what shape they are?
Over 20 years ago, research on the budding yeast Saccharomyces cerevisiae suggested that yeast cells, too, know what shape they are: specifically, they seem to know whether or not they have begun to build a bud (Fig. 1a) (Lew and Reed 1995). Bud formation is normally followed by nuclear division, but many environmental stresses can delay bud emergence. When that happens, a pathway called the morphogenesis checkpoint delays nuclear division to compensate for the delay in budding (Howell and Lew 2012). The delay in nuclear division is dependent on the Wee1-family kinase Swe1, which delays mitosis by inhibiting the cyclin-dependent kinase Cdc28. Research over the intervening years uncovered an intricate Swe1-regulatory pathway that is localized to the mother-bud neck by the septin cytoskeleton. These findings led to the suggestion that septins “know” whether or not a bud is present, and that they somehow relay that information to Swe1 (Fig. 1a) (Howell and Lew 2012). But how do the septins know whether or not there is a bud? And how does that information reach Swe1? Recent findings suggest appealing answers to these questions, which may apply to animal as well as fungal cells.
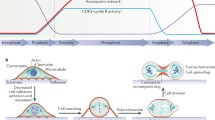
Septins and cell shape. a Unbudded yeast cells make a septin ring that becomes a septin collar following bud emergence. Proteins localized to the septin collar (but not the septin ring) promote inactivation of Swe1. b The septin collar consists of a dense parallel array of septin filaments oriented along the mother-bud axis. c Curvatures associated with different shapes. Red negative curvature (concave). Blue positive curvature (convex). Purple zero curvature (flat). d Localization of the Swe1-regulatory kinase Elm1 depends on local cell shape, while localization of Hsl1 does not. e Mammalian septins are concentrated at the base of primary cilia and dendritic spines
Septins are filament-forming proteins first discovered in yeast but later found across several eukaryotic phyla (Nishihama et al. 2011). Individual septins assemble into rod-shaped complexes that polymerize end-to-end to form non-polar filaments usually associated with the plasma membrane (Oh and Bi 2011). In budding yeast, septin filaments assemble at the polarity site in unbudded cells, forming a ring that morphs into a collar at the mother-bud neck upon bud emergence (Fig. 1a). In a tour de force using correlative light and platinum replica electron microscopy, Ong and colleagues showed that the collar consists of a dense parallel “picket fence” array of short septin filaments (Fig. 1b) (Ong et al. 2014). As yet, we do not understand how the septins come to be arranged in such a striking pattern. However, another recent breakthrough suggests that bud emergence could lead to the picket fence structure simply as a result of the change in cell shape (Bridges et al. 2016).
Bridges and colleagues noticed that septins at branch sites of the filamentous fungus Ashbya gossypii accumulated in a manner that was correlated with the local micron-scale curvature of the plasma membrane (Bridges et al. 2016). By measuring the association of purified septins with artificial membrane bilayers in vitro, they demonstrated that septins had an innate preference for convex membranes with positive curvature (Bridges et al. 2016). This curvature preference has profound implications for septin organization in cells, and it suggests that septins may arrange themselves in different ways depending on the cell shape.
Consider the curvature associated with a few simple shapes (Fig. 1c). Inside a sphere (1), the concave membrane has negative and isotropic curvature (i.e., the curvature is the same in every direction). But inside a cylinder (2), the curvature is asymmetric: zero (flat) along the axis and negative (concave) around the circumference. For a round cell with a protrusion (3), the curvature around the circumference of the protrusion is highly negative while that of the cell body is mildly negative, but where the protrusion meets the cell body, the curvature along the axis leading from the main body to the protrusion is positive. If septin filaments were free to undergo rotational diffusion at the membrane until they found the most positive curvature available, then different cell shapes would lead to different septin organizations. In particular, shapes with asymmetric curvature (more positive in one direction than another) would promote alignment of septin filaments in parallel along the direction with more positive curvature, potentially explaining the parallel picket fence oriented along the mother-bud axis (Fig. 1b).
These findings suggest a simple mechanism by which certain cell shapes (like the presence or absence of a protrusion) could lead to different arrangements of septin filaments. But how would that difference lead to downstream changes in cell behavior? Returning to the morphogenesis checkpoint in yeast, recent findings suggest that the most upstream “sensing” components of the checkpoint are two protein kinases, Elm1 and Hsl1, that both localize to the septin collar (Kang et al. 2016). By manipulating yeast cell shape using mating pheromones, it became apparent that whereas Hsl1 would bind septin rings formed in any cell geometry, Elm1 bound much better to septin structures that formed inside a projection (asymmetric curvature) than those that did not (symmetric curvature) (Fig. 1d). Combined with the findings discussed above, this suggests a simple model in which Elm1 preferentially binds to parallel septin filaments, which develop where there is asymmetric curvature to align the septin filaments. Although still speculative, this hypothesis is testable and provides a clear mechanism to monitor cell shape.
Might similar mechanisms apply outside of yeast? Mammalian septins display similar curvature preferences to those of yeast septins (Bridges et al. 2016). And mammalian septins are notably concentrated at sites that display asymmetric curvature. For example, septins have been found at the bases of primary cilia (Ghossoub et al. 2013; Hu et al. 2010) and dendritic spines in neurons (Ewers et al. 2014; Tada et al. 2007), both of which are projections with similar curvature asymmetry to yeast buds (Fig. 1e). Just as yeast cells monitor whether or not they have a bud, one can imagine that mammalian cells may find it useful to know whether or not they have a primary cilium, or that neurons may productively count the number of spines along a dendrite, using a strategy similar to that of the yeast morphogenesis checkpoint. Septins are also concentrated at the cleavage furrow in dividing cells (Oh and Bi 2011), another location with highly asymmetric curvature that changes as furrow ingression proceeds. The septin curvature preference would suggest that septin filaments become aligned perpendicular to the cleavage furrow, and proteins recognizing parallel septin arrays could be useful either for signaling furrow progression or for providing structural input to other elements (like actin and myosin filaments) in the furrow.
Conclusion
Recent studies indicate that septins associate with cell membranes in a manner that is responsive to the curvature of those membranes. Cell shapes that involve asymmetric curvature would, therefore, provide a preferred orientation for septin filaments, yielding parallel septin arrays consistent with those found at the yeast mother-bud neck. Proteins that bind preferentially to parallel septin filaments could then provide readouts dependent on local cell shape. Such sensing may underlie the functioning of the yeast morphogenesis checkpoint, and could also occur in other contexts where cells may wish to watch their figures.
References
Bridges AA, Jentzsch MS, Oakes PW, Occhipinti P, Gladfelter AS (2016) Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J Cell Biol 213:23–32
Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE (1997) Geometric control of cell life and death. Science (New York, NY) 276:1425–1428
Ewers H, Tada T, Petersen JD, Racz B, Sheng M, Choquet D (2014) A septin-dependent diffusion barrier at dendritic spine necks. PLoS One 9:e113916
Ghossoub R, Hu Q, Failler M, Rouyez MC, Spitzbarth B, Mostowy S, Wolfrum U, Saunier S, Cossart P, Jamesnelson W, Benmerah A (2013) Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J Cell Sci 126:2583–2594
Howell AS, Lew DJ (2012) Morphogenesis and the cell cycle. Genetics 190:51–77
Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ (2010) A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science (New York, NY) 329:436–439
Kang H, Tsygankov D, Lew DJ (2016) Sensing a bud in the yeast morphogenesis checkpoint: a role for Elm1. Mol Biol Cell 27:1764–1775
Lew DJ, Reed SI (1995) A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J Cell Biol 129:739–749
Nishihama R, Onishi M, Pringle JR (2011) New insights into the phylogenetic distribution and evolutionary origins of the septins. Biol Chem. 392:681–687
Oh Y, Bi E (2011) Septin structure and function in yeast and beyond. Trends Cell Biol 21:141–148
Ong K, Wloka C, Okada S, Svitkina T, Bi E (2014) Architecture and dynamic remodelling of the septin cytoskeleton during the cell cycle. Nat Commun 5:5698
Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M (2007) Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol 17:1752–1758
Acknowledgments
Work in the authors’ lab is supported by NIH/NIGMS Grants GM62300 and GM103870 to DJL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Kang, H., Lew, D.J. How do cells know what shape they are?. Curr Genet 63, 75–77 (2017). https://doi.org/10.1007/s00294-016-0623-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-016-0623-1