Abstract
Many proteobacteria modulate a suite of catabolic genes using the second messenger cyclic 3′, 5′-AMP (cAMP) and the cAMP receptor protein (CRP). Together, the cAMP-CRP complex regulates target promoters, usually by activating transcription. In the canonical model, the phosphotransferase system (PTS), and in particular the EIIAGlc component for glucose uptake, provides a mechanistic link that modulates cAMP levels depending on glucose availability, resulting in more cAMP and activation of alternative catabolic pathways when glucose is unavailable. Within the Vibrionaceae, cAMP-CRP appears to play the classical role in modulating metabolic pathways; however, it also controls functions involved in natural competence, bioluminescence, pheromone signaling, and colonization of animal hosts. For this group of marine bacteria, chitin is an ecologically relevant resource, and chitin’s monomeric sugar N-acetylglucosamine (NAG) supports robust growth while also triggering regulatory responses. Recent studies with Vibrio fischeri indicate that NAG and glucose uptake share EIIAGlc, yet the responses of cAMP-CRP to these two carbon sources are starkly different. Moreover, control of cAMP levels appears to be more dominantly controlled by export and degradation. Perhaps more surprisingly, although CRP may require cAMP, its activity can be controlled in response to glucose by a mechanism independent of cAMP levels. Future studies in this area promise to shed new light on the role of cAMP and CRP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The second messenger cyclic 3′, 5′-AMP (cAMP) together with the cAMP receptor protein (CRP) constitutes an archetype bacterial global regulator that contributes to the phenomenon of catabolite repression, wherein the lack of a preferred carbon source (e.g., glucose) induces catabolic pathways for growth on alternative substrates. Some of these catabolic pathways require cAMP-CRP for transcriptional activation (Gorke and Stulke 2008; Meadow et al. 1990; Postma et al. 1993; Botsford and Harman 1992). In the absence of glucose, cAMP levels are high and bind to CRP to alter its conformation, and together the cAMP-CRP duo binds DNA at specific palindromic sequences in target promoters to affect transcription of catabolic genes (Kolb et al. 1993; Busby and Ebright 1999).
E. coli as a model of CRP- and cAMP-mediated regulation
The control of cAMP-CRP and its role in catabolite repression have been studied most thoroughly in Escherichia coli, where the paradigm model is that cAMP-CRP activity is controlled largely by cAMP levels. In E. coli, adenylate cyclase (CyaA) generates cAMP (Yang and Epstein 1983), and CyaA activity is connected to glucose by this sugar’s phosphotransferase system (PTS) EIIAGlc component, which when phosphorylated stimulates CyaA to synthesize cAMP (Levy et al. 1990). When glucose is available, it is transported into the cell by the PTS EIIC domain and phosphorylated by the EIIB domain, which receives the phosphate from EIIAGlc, thus leaving less phosphorylated EIIAGlc to stimulate cAMP production by CyaA (Amin and Peterkofsky 1995; Bettenbrock et al. 2007; Feucht and Saier 1980; Peterkofsky et al. 1995; Takahashi et al. 1998; Levy et al. 1990; Saier et al. 1976). Homeostasis of cAMP is also maintained by counterbalancing CyaA activity with cAMP turnover via the cAMP phosphodiesterase CpdA (Imamura et al. 1996) and/or with cAMP export (Saier et al. 1975). Recent studies, including one combining global “omics” measurements with mathematical modeling, suggest that cAMP-CRP activity is tied to availability of metabolic precursors and “metabolic need” (You et al. 2013), although the mechanisms involved remain elusive. As discussed below, our studies with Vibrio fischeri have in many ways paralleled the model derived from E. coli; however, neither “metabolic need” nor EIIAGlc’s role in cAMP synthesis fully explain our observations.
Roles of CRP within the Vibrionaceae
Within the Vibrionaceae, which like E. coli are gamma-proteobacteria, the CRP protein is ~95 % identical to CRP in E. coli, and crp can functionally complement between these genera (Lyell et al. 2013). There is ample evidence that CRP plays a classical role in regulating catabolic genes in the Vibrionaceae. For example, bioinformatic predictions of CRP-binding sites indicate they are often located upstream of catabolic operons, and experimental data have suggested or validated the role of cAMP-CRP in some of these catabolic pathways (Adin et al. 2008; Kim et al. 2011). Recently, phenotype microarray screening validated the role of crp in the utilization of several carbon sources by Vibrio cholerae (Chen et al. 2013). Furthermore, the observation that crp mutants grow relatively well on glucose but poorly if at all on other carbon sources is consistent with a role of cAMP-CRP in directing catabolism of non-glucose carbon sources (Colton et al. 2015; Lyell et al. 2013).
As is the case in other bacteria, cAMP-CRP can also direct regulatory changes associated with virulence both in V. cholerae [for a review, see (McDonough and Rodriguez 2012)] and Vibrio vulnificus (Kim et al. 2013). In addition to a long-established role in regulating virulence factors (Skorupski and Taylor 1997), cAMP-CRP has more recently been implicated in these Vibrio pathogens as regulating such varied activities as motility (Liang et al. 2007), biofilm formation (Fong and Yildiz 2008), pheromone signaling (Kim et al. 2012; Liang et al. 2007, 2008), natural competence (Antonova et al. 2012; Blokesch 2012), osmotolerance (Lee and Choi 2006), resistance to bacteriophages (Zahid et al. 2010), and integrase activity (Baharoglu et al. 2012). Although some of these behaviors, notably the uptake of foreign DNA, could be interpreted as nutritional responses, these observations illustrate the pervasive rethinking of cAMP-CRP as a means to not only link nutritional status to nutritional responses but also to regulate appropriate behaviors based on the nutrient profile in a particular environment. This line of thinking reflects a larger trend wherein the regulatory responses to second messengers and phenomena like catabolite repression are being viewed more broadly, beyond the more narrowly defined behaviors initially described [e.g., (Bordeleau and Burrus 2015; Yang and Lan 2015)].
Sweetness and light: glucose, bioluminescence, and CRP in the symbiont Vibrio fischeri
We became interested in CRP in the bioluminescent light-organ symbiont V. fischeri, because of its reported role in regulating the lux genes underlying pheromone signaling and bioluminescence. The symbiosis between V. fischeri and the Hawaiian bobtail squid, Euprymna scolopes, has become a powerful model for studying persistent and mutualistic animal-bacteria associations (Stabb and Visick 2013; Stabb 2006), and bioluminescence is a colonization factor required for symbiotic persistence (Bose et al. 2008; Visick et al. 2000; Koch et al. 2014). How bioluminescence benefits the colonizing bacteria is unknown (Stabb 2005; Walker et al. 2006), but studies of its regulation in response to environmental cues could suggest conditions where it is advantageous (Bose et al. 2007; Lyell et al. 2010, 2013; Septer et al. 2010, 2013, 2014; Septer and Stabb 2012; Stabb and Flores-Cruz 2013; Cao et al. 2012; Whistler and Ruby 2003).
It is well established that bioluminescence is repressed by glucose (Friedrich and Greenberg 1983), and several studies suggested the involvement of cAMP and CRP (Dunlap 1989; Dunlap and Greenberg 1985, 1988; Shadel et al. 1990). We recently validated and extended these studies in V. fischeri ES114, showing that the two acyl-homoserine lactone pheromone-signaling systems that control bioluminescence are both activated by cAMP-CRP (Lyell et al. 2013). Bioluminescence is induced early in symbiotic colonization, and we recently showed that cAMP-CRP is active in this time period and also contributes to symbionts ability to colonize the host (Colton et al. 2015). We therefore became interested in validating a model of how cAMP and CRP functioned, based upon the E. coli paradigm described above.
Control of cAMP-CRP activity in V. fischeri is the same only different
Upon comparing cAMP-CRP activity and cAMP levels in V. fischeri mutants and wild-type under different growth conditions, a model (Fig. 1) emerged that is both similar and different from our original hypotheses (Colton et al. 2015). It should be noted that as in all studies, our results are only valid under the specific set of conditions we tested. The relative importance of various mechanisms controlling cAMP-CRP could well be context dependent, and our interpretations cannot be extrapolated to all conditions. Similarly, it remains unclear the extent to which our data reflect differences between E. coli and V. fischeri as opposed to differences in the conditions of specific experiments. Studying cAMP-CRP also poses some experimental difficulties. For example, transcriptional reporters of cAMP-CRP-regulated genes are generally also controlled by other regulators, a complication that we avoided by generating a synthetic cAMP-CRP-dependent promoter (Colton et al. 2015). On the other hand, we found no workable solution for our observation that cAMP present in yeast extract interfered with attempts to measure Vibrio-derived extracellular cAMP when cells were grown in our complex and rich media amended with various carbon sources. Given the many ways cells can be grown and the various measurements that can or cannot be made in vivo or in vitro, with their respective limitations interpreting, and reconciling the literature in this field can be challenging.
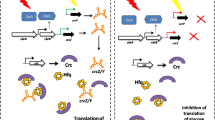
Working model of cAMP-CRP control in Vibrio fischeri. CRP (purple) combines with cAMP and then binds DNA to regulate target promoters. Generation of cAMP (green) is accomplished by CyaA, which is stimulated by phosphorylated EIIAGlc. Turnover of cAMP (red) involves intracellular degradation by CpdA, extracellular degradation by CpdP, and excretion by unknown transporters. Glucose and NAG are transported via dedicated PTS systems, EIIBC (PtsG) and NagE1, respectively, which apparently share substrate phosphorylation by EIIAGlc. Three unresolved mysteries include: First (? #1), availability of glucose can modulate CRP activity independently of CyaA-determined cAMP levels, possibly by post-translational modification. Second (? #2), like glucose, NAG requires EIIAGlc to be utilized, and NAG is presumably quickly processed to glucose-6-P, yet NAG and glucose have opposite effects on cAMP-CRP activity. Third, (? #3) cAMP is transported either into the periplasm or out of the cell, and the amount of extracellular cAMP seems to vary with growth substrate, but no mechanisms for transport or its control have been identified. Inner membrane (IM) and outer membrane (OM)
With those caveats in mind, in certain respects, our data were consistent with our understanding of cAMP-CRP’s function. Most importantly, cAMP-CRP activity was relatively low in media supplemented with glucose. Our data were also consistent with a model whereby CyaA was responsible for cAMP generation, EIIAGlc could enhance cAMP production, CpdA consumed intracellular cAMP, and some cAMP was exported from the cell. Each of these findings is consistent with the model described above derived from our understanding of E. coli.
We also knew that V. fischeri is endowed with a periplasmic cAMP phosphodiesterase, named CpdP (Dunlap and Callahan 1993; Callahan et al. 1995), and this effectively prevented the accumulation of extracellular cAMP (Colton et al. 2015). Although this role for CpdP was not unexpected, it does cast the function of CpdP in a new light. Initially discovered and characterized in the context of enabling growth on exogenous cAMP as a sole source of carbon and energy, Dunlap and Callahan (1993) also speculated that CpdP might separate cAMP production and turnover into different compartments. Our data indicate that CpdP does not replace the cytoplasmic turnover of cAMP by CpdA; however, it could provide a way to turn over and recycle endogenously produced cAMP that other bacteria would lose upon exporting it.
Despite similarities to the model based on E. coli, the relative contributions of the components shown in Fig. 1 were unexpected. In particular, the influence of EIIAGlc, which in theory is the main connection between carbon source and cAMP-CRP activity, was subtle and only evident in cpdA mutants, which have elevated cAMP. Instead, it appears that intracellular levels of cAMP were determined more by its export and turnover, and that export of cAMP is somehow influenced by carbon source (Fig. 1). Our data indicated that while more cAMP was generated by cells growing on glycerol, more of it was exported too (Colton et al. 2015).
By far the most startling deviation from the paradigm model was our observation that intracellular cAMP levels were lower in cells grown on glycerol relative to cells grown with glucose, even though our cAMP-CRP-dependent reporter indicated higher activity under the same conditions (Colton et al. 2015). The latter observation is more consistent with all of our other data, which suggest a classical role of increased cAMP-CRP activity when glucose is unavailable. Moreover, using a CRP* variant, which does not require cAMP for activity, in a background lacking cyaA (and presumably cAMP), we still saw modulation of CRP* activity in response to glucose. Other experiments suggested this response is not due to transcriptional regulation of crp or changes in CRP levels as described in E. coli (Ishizuka et al. 1993). As discussed below, we hypothesize that CRP might be post-translationally regulated (Fig. 1). Thus, taken together, our data suggest that cAMP levels in V. fischeri are modulated in ways that are both understood and not; however, it seems likely that cAMP-CRP activity can be altered in response to glucose via a cAMP-independent mechanism.
Other NAGging questions
Within the Vibrionaceae, which are predominately marine bacteria, the oligosaccharide chitin is an ecologically relevant resource, as is chitin’s monomeric sugar N-acetylglucosamine (NAG). Chitin is a major structural component of many marine invertebrates, and the pathways for chitin and NAG utilization appear widespread and conserved within the Vibrionaceae (Hunt et al. 2008). In V. fischeri, it appears that chitin oligosaccharides and NAG play a role in nutrient provisioning from host to symbiont that is initially absent but ultimately develops and becomes part of the diurnal cycle of the adult host squid (Miyashiro et al. 2011; Schwartzman et al. 2015; Sun et al. 2015; Wier et al. 2010). Like glucose, NAG supports robust growth of many Vibrio species while also triggering regulatory responses. It is certainly true that NAG supports rapid growth of V. fischeri, and NAG resembles glucose in largely restoring growth to crp or cya mutants of V. fischeri (Colton et al. 2015; Lyell et al. 2013). As a carbon source, NAG seems no less “preferred” than glucose.
Pan et al. recently showed that the EIIAGlc component of glucose transport is shared by, and required for, NAG import and utilization in V. fischeri (Pan et al. 2015), which is consistent with our own observations under different conditions. Moreover, EIIAGlc and NAG appeared to function in the phenomenon of “inducer exclusion,” an aspect of catabolite repression, whereby uptake of alternative carbon sources is attenuated by protein–protein interactions in the presence of a preferred carbon source. Yet despite the parallels between glucose and NAG, we found that the activity of cAMP-CRP was high in cells grown on NAG or glycerol, in contrast to low activity during growth on glucose (Colton et al. 2015) and (Fig. 2). Neither nutritional status nor the shared EIIAGlc seems to account for the difference between NAG and glucose with respect to cAMP-CRP activity.
cAMP-CRP activity in V. fischeri grown on media supplemented with glycerol, glucose, or NAG. Activity of the cAMP-CRP activated gfp transcriptional reporter on pDC85 (Colton et al. 2015) reported as green (GFP) fluorescence normalized against the red fluorescence of a constitutive mCherry on the same plasmid. The fluorescence ratios are averaged between three independent experiments for each condition, and bars indicate standard error. Cells were grown on plates of SWTO medium supplemented with glycerol, glucose, or NAG (Colton et al. 2015). The reporter activity in cells grown on glucose is significantly different from those grown on media supplemented with glycerol or NAG (p < 0.01). There is no significant difference in reporter activity in cells grown on media supplemented with NAG or glycerol (p > 0.5)
Perhaps, in certain members of the Vibrionaceae, glucose and NAG are both preferred carbon sources that share features such as the PTS component, EIIAGlc, yet they represent different environments and niches and therefore elicit different regulatory responses. For example, NAG-specific regulation by transcriptional regulator NagC can mediate some differences in gene regulation between glucose- and NAG-grown cells (Ghosh et al. 2011). If the global effect of glucose was preserved in the cAMP-CRP response, it would make sense to evolve a system of cAMP-CRP control that de-emphasized the role of EIIAGlc, so that the glucose response could remain distinct from NAG. How cAMP-CRP activity became connected to glucose but not NAG availability remains to be discovered.
Future directions
Chitin and NAG are important resources for Vibrio species, and additionally glucose is known to underpin many key regulatory responses in these bacteria. The recent studies discussed above and others suggest that much remains to be learned about cAMP and CRP in the Vibrionaceae. Although catabolite repression, the effects of cAMP-CRP, and responses to glucose are known to involve more than EIIAGlc and cAMP levels (You et al. 2013; Ishizuka et al. 1993; Magasanik 1961), the recent results in V. fischeri are intriguing and suggest that this organism might be a good model for uncovering new connections between carbon source, cAMP, and CRP activity.
One priority for future experimentation should be to investigate the possible post-translational modification of CRP. As we discussed elsewhere (Colton et al. 2015), a potential mechanism for such control would be lysine acetylation. In E. coli, CRP can be acetylated (Kuhn et al. 2014), and the acetylation levels of metabolic enzymes can depend on whether cells are growing on glucose or other carbon sources (Wang et al. 2010). If the analysis of the CRP protein harvested from cells grown on different carbon sources does show differences in mass, this would raise several questions, potentially including whether the decorations are added enzymatically or directly through acetyl-phosphate, whether they affect DNA binding or interactions with RNAP polymerase, and whether they act in a promoter-specific fashion.
Another unresolved question is how cAMP is exported. This phenomenon seems to be a common feature of bacteria that remains poorly understood. Although there is a report that the outer membrane channel protein TolC mediates cAMP export (Hantke et al. 2011), tolC did not influence extracellular cAMP in V. fischeri. Moreover, the results in E. coli seem open to other interpretations. We attempted without success to isolate mutants of V. fischeri that failed to export cAMP, but perhaps more sensitive or clever high-throughput genetic screens could be developed.
Given that NAG and glucose promote growth similarly and both require the EIIAGlc PTS component, yet have radically different effects on cAMP-CRP activity, it should be interesting to investigate further the similarities and differences between utilization of these two sugars. Metabolic, transcriptomic, and proteomic profiles of wild-type and crp mutant cells grown on NAG versus glucose might provide valuable insight into both the different regulatory responses to these carbon sources and the mechanisms underpinning those responses. Such approaches could be particularly powerful if combined with follow-up experiments in select mutants, to test specific mechanistic hypotheses.
Investigations of regulatory responses to other carbon sources could also be useful. Notably, there are intriguing links to CRP activity and utilization of cellobiose, which is a dimer of beta-linked glucose units. V. fischeri can utilize cellobiose, there is a CRP-binding site upstream of the cel operon for cellobiose catabolism, and glucose leads to lowered expression from this promoter (Adin et al. 2008). The cellobiose PTS has its own EIIA component (CelA), the cel operon also encodes a glucokinase, and cellobiose utilization does not require EIIAGlc, yet results suggest that the breakdown of cellobiose into glucose units leads to lowered expression from the cel promoter reminiscent of growth on glucose (Adin et al. 2008). Thus, cellobiose may represent a mechanism for affecting CRP activity with the carbon entering the cells through a different path than monomeric glucose.
Finally, the roles of EIIAGlc seem to include both modulating cAMP levels and inducer exclusion, but the extent of its function remains to be explored. Notably, mutants disrupting the crr gene that encodes EIIAGlc have brighter bioluminescence than wild-type (Visick et al. 2007), and nothing in our understanding of cAMP-CRP, EIIAGlc, or luminescence regulation seems to explain this phenomenon.
These studies and others in V. fischeri along with the ongoing investigations of CRP-mediated regulation and catabolite repression in other members of the Vibrionaceae, promise to expand our view of this global regulatory system.
References
Adin DM, Visick KL, Stabb EV (2008) Identification of a cellobiose utilization gene cluster with cryptic beta-galactosidase activity in Vibrio fischeri. Appl Environ Microbiol 74:4059–4069
Amin N, Peterkofsky A (1995) A dual mechanism for regulating cAMP levels in Escherichia coli. J Biol Chem 270:11803–11805
Antonova ES, Bernardy EE, Hammer BK (2012) Natural competence in Vibrio cholerae is controlled by a nucleoside scavenging response that requires CytR-dependent anti-activation. Mol Microbiol 86:1215–1231
Baharoglu Z, Krin E, Mazel D (2012) Connecting environment and genome plasticity in the characterization of transformation-induced SOS regulation and carbon catabolite control of the Vibrio cholerae integron integrase. J Bacteriol 194:1659–1667
Bettenbrock K, Sauter T, Jahreis K, Kremling A, Lengeler JW, Gilles ED (2007) Correlation between growth rates, EIIACrr phosphorylation, and intracellular cyclic AMP levels in Escherichia coli K-12. J Bacteriol 189:6891–6900
Blokesch M (2012) Chitin colonization, chitin degradation and chitin-induced natural competence of Vibrio cholerae are subject to catabolite repression. Environ Microbiol 14:1898–1912
Bordeleau E, Burrus V (2015) Cyclic-di-GMP signaling in the Gram-positive pathogen Clostridium difficile. Curr Genet. doi:10.1007/s00294-015-0484-z
Bose JL, Kim U, Bartkowski W, Gunsalus RP, Overley AM, Lyell NL, Visick KL, Stabb EV (2007) Bioluminescence in Vibrio fischeri is controlled by the redox-responsive regulator ArcA. Mol Microbiol 65:538–553
Bose JL, Rosenberg CS, Stabb EV (2008) Effects of luxCDABEG induction in Vibrio fischeri: enhancement of symbiotic colonization and conditional attenuation of growth in culture. Arch Microbiol 190:169–183
Botsford JL, Harman JG (1992) Cyclic AMP in prokaryotes. Microbiol Rev 56:100–122
Busby S, Ebright RH (1999) Transcription activation by catabolite activator protein (CAP). J Mol Biol 293:199–213
Callahan SM, Cornell NW, Dunlap PV (1995) Purification and properties of periplasmic 3′:5′-cyclic nucleotide phosphodiesterase. A novel zinc-containing enzyme from the marine symbiotic bacterium Vibrio fischeri. J Biol Chem 270:17627–17632
Cao X, Studer SV, Wassarman K, Zhang Y, Ruby EG, Miyashiro T (2012) The novel sigma factor-like regulator RpoQ controls luminescence, chitinase activity, and motility in Vibrio fischeri. mBio 3:e00285–e00311
Chen B, Liang W, Wu R, Liang P, Kan B (2013) Phenotype microarray screening of carbon sources used by Vibrio cholerae identifies genes regulated by the cAMP receptor protein. Can J Microbiol 59:472–478
Colton DM, Stoudenmire JL, Stabb EV (2015) Growth on glucose decreases cAMP-CRP activity while paradoxically increasing intracellular cAMP in the light-organ symbiont Vibrio fischeri. Mol Microbiol (in press)
Dunlap PV (1989) Regulation of luminescence by cyclic AMP in cya-like and crp-like mutants of Vibrio fischeri. J Bacteriol 171:1199–1202
Dunlap PV, Callahan SM (1993) Characterization of a periplasmic 3′:5′-cyclic nucleotide phosphodiesterase gene, cpdP, from the marine symbiotic bacterium Vibrio fischeri. J Bacteriol 175:4615–4624
Dunlap PV, Greenberg EP (1985) Control of Vibrio fischeri luminescence gene expression in Escherichia coli by cyclic AMP and cyclic AMP receptor protein. J Bacteriol 164:45–50
Dunlap PV, Greenberg EP (1988) Control of Vibrio fischeri lux gene transcription by a cyclic AMP receptor protein-LuxR protein regulatory circuit. J Bacteriol 170:4040–4046
Feucht BU, Saier MH Jr (1980) Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol 141:603–610
Fong JC, Yildiz FH (2008) Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation. J Bacteriol 190:6646–6659
Friedrich WF, Greenberg EP (1983) Glucose repression of luminescence and luciferase in Vibrio fischeri. Arch Microbiol 134:87–91
Ghosh S, Rao KH, Sengupta M, Bhattacharya SK, Datta A (2011) Two gene clusters co-ordinate for a functional N-acetylglucosamine catabolic pathway in Vibrio cholerae. Mol Microbiol 80:1549–1560
Gorke B, Stulke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624
Hantke K, Winkler K, Schultz JE (2011) Escherichia coli exports cyclic AMP via TolC. J Bacteriol 193:1086–1089
Hunt DE, Gevers D, Vahora NM, Polz MF (2008) Conservation of the chitin utilization pathway in the Vibrionaceae. Appl Environ Microbiol 74:44–51
Imamura R, Yamanaka K, Ogura T, Hiraga S, Fujita N, Ishihama A, Niki H (1996) Identification of the cpdA gene encoding cyclic 3′,5′-adenosine monophosphate phosphodiesterase in Escherichia coli. J Biol Chem 271:25423–25429
Ishizuka H, Hanamura A, Kunimura T, Aiba H (1993) A lowered concentration of cAMP receptor protein caused by glucose is an important determinant for catabolite repression in Escherichia coli. Mol Microbiol 10:341–350
Kim BS, Hwang J, Kim MH, Choi SH (2011) Cooperative regulation of the Vibrio vulnificus nan gene cluster by NanR protein, cAMP receptor protein, and N-acetylmannosamine 6-phosphate. J Biol Chem 286:40889–40899
Kim SP, Kim CM, Shin SH (2012) Cyclic AMP and cyclic AMP-receptor protein modulate the autoinducer-2-mediated quorum sensing system in Vibrio vulnificus. Curr Microbiol 65:701–710
Kim YR, Lee SE, Kim B, Choy H, Rhee JH (2013) A dual regulatory role of cyclic adenosine monophosphate receptor protein in various virulence traits of Vibrio vulnificus. Microbiol Immunol 57:273–280
Koch EJ, Miyashiro TI, McFall-Ngai MJ, Ruby EG (2014) Features governing symbiont persistence in the squid-vibrio association. Mol Ecol 23:1624–1634
Kolb A, Busby S, Buc H, Garges S, Adhya S (1993) Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem 62:749–795
Kuhn ML, Zemaitaitis B, Hu LI, Sahu A, Sorensen D, Minasov G, Lima BP, Scholle M, Mrksich M, Anderson WF, Gibson BW, Schilling B, Wolfe AJ (2014) Structural, kinetic and proteomic characterization of acetyl phosphate-dependent bacterial protein acetylation. PLoS One 9:e94816
Lee JH, Choi SH (2006) Coactivation of Vibrio vulnificus putAP operon by cAMP receptor protein and PutR through cooperative binding to overlapping sites. Mol Microbiol 60:513–524
Levy S, Zeng GQ, Danchin A (1990) Cyclic AMP synthesis in Escherichia coli strains bearing known deletions in the pts phosphotransferase operon. Gene 86:27–33
Liang W, Pascual-Montano A, Silva AJ, Benitez JA (2007) The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology 153:2964–2975
Liang W, Sultan SZ, Silva AJ, Benitez JA (2008) Cyclic AMP post-transcriptionally regulates the biosynthesis of a major bacterial autoinducer to modulate the cell density required to activate quorum sensing. FEBS Lett 582:3744–3750
Lyell NL, Dunn AK, Bose JL, Stabb EV (2010) Bright mutants of Vibrio fischeri ES114 reveal conditions and regulators that control bioluminescence and expression of the lux operon. J Bacteriol 192:5103–5114
Lyell NL, Colton DM, Bose JL, Tumen-Velasquez MP, Kimbrough JH, Stabb EV (2013) Cyclic AMP receptor protein regulates pheromone-mediated bioluminescence at multiple levels in Vibrio fischeri ES114. J Bacteriol 195:5051–5063
Magasanik B (1961) Catabolite repression. Cold Spring Harb Symp Quant Biol 26:249–256
McDonough KA, Rodriguez A (2012) The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol 10:27–38
Meadow ND, Fox DK, Roseman S (1990) The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Ann Rev Biochem 59:497–542
Miyashiro T, Klein W, Oehlert D, Cao X, Schwartzman J, Ruby EG (2011) The N-acetyl-d-glucosamine repressor NagC of Vibrio fischeri facilitates colonization of Euprymna scolopes. Mol Microbiol 82:894–903
Pan M, Schwartzman JA, Dunn AK, Lu Z, Ruby EG (2015) A single host-derived glycan impacts key regulatory nodes of symbiont metabolism in a coevolved mutualism. Mbio (in press)
Peterkofsky A, Seok YJ, Amin N, Thapar R, Lee SY, Klevit RE, Waygood EB, Anderson JW, Gruschus J, Huq H, Gollop N (1995) The Escherichia coli adenylyl cyclase complex: requirement of PTS proteins for stimulation by nucleotides. Biochemistry 34:8950–8959
Postma PW, Lengeler JW, Jacobson GR (1993) Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev 57:543–594
Saier MH Jr, Feucht BU, McCaman MT (1975) Regulation of intracellular adenosine cyclic 3′:5′-monophosphate levels in Escherichia coli and Salmonella typhimurium. Evidence for energy-dependent excretion of the cyclic nucleotide. J Biol Chem 250:7593–7601
Saier MH Jr, Feucht BU, Hofstadter LJ (1976) Regulation of carbohydrate uptake and adenylate cyclase activity mediated by the enzymes II of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Biol Chem 251:883–892
Schwartzman JA, Koch E, Heath-Heckman EA, Zhou L, Kremer N, McFall-Ngai MJ, Ruby EG (2015) The chemistry of negotiation: rhythmic, glycan-driven acidification in a symbiotic conversation. Proc Natl Acad Sci USA 112:566–571
Septer AN, Stabb EV (2012) Coordination of the arc regulatory system and pheromone-mediated positive feedback in controlling the Vibrio fischeri lux operon. PLoS One 7:e49590
Septer AN, Bose JL, Dunn AK, Stabb EV (2010) FNR-mediated regulation of bioluminescence and anaerobic respiration in the light-organ symbiont Vibrio fischeri. FEMS Microbiol Lett 306:72–81
Septer AN, Lyell NL, Stabb EV (2013) The iron-dependent regulator Fur controls pheromone signaling systems and luminescence in the squid symbiont Vibrio fischeri ES114. Appl Environ Microbiol 79:1826–1834
Septer AN, Bose JL, Lipzen A, Martin J, Whistler C, Stabb EV (2014) Bright luminescence of Vibrio fischeri aconitase mutants reveals a connection between citrate and the Gac/Csr regulatory system. Mol Microbiol 95:283–296
Shadel GS, Devine JH, Baldwin TO (1990) Control of the lux regulon of Vibrio fischeri. J Biolumin Chemilumin 5:99–106
Skorupski K, Taylor RK (1997) Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc Nat Acad Sci USA 94:265–270
Stabb EV (2005) Shedding light on the bioluminescence “paradox”. ASM News 71:223–229
Stabb EV (2006) The Vibrio fischeri-Euprymna scolopes light organ symbiosis. In: Thompson FL, Austin B, Swings J (eds) The Biology of Vibrios. ASM Press, Washington, DC, pp 204–218
Stabb EV, Flores-Cruz Z (2013) Who turned on the lights: what the regulation of bacterial bioluminescence tells us about this and other bacterial group behaviours. The Biochemist 35:18–23
Stabb EV, Visick KL (2013) Vibrio fischeri: a bioluminescent light-organ symbiont of the bobtail squid Euprymna scolopes. In: Rosenberg E, DeLong EF, Stackebrandt E, Lory S, Thompson F (eds) The Prokaryotes. Springer-Verlag, Berlin Heidelberg, pp 497–532
Sun Y, Verma SC, Bogale H, Miyashiro T (2015) NagC represses N-acetyl-glucosamine utilization genes in Vibrio fischeri within the light organ of Euprymna scolopes. Front Microbiol (in press)
Takahashi H, Inada T, Postma P, Aiba H (1998) CRP down-regulates adenylate cyclase activity by reducing the level of phosphorylated IIA(Glc), the glucose-specific phosphotransferase protein, in Escherichia coli. Mol Gen Genet 259:317–326
Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG (2000) Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol 182:4578–4586
Visick KL, O’Shea TM, Klein AH, Geszvain K, Wolfe AJ (2007) The sugar phosphotransferase system of Vibrio fischeri inhibits both motility and bioluminescence. J Bacteriol 189:2571–2574
Walker EL, Bose JL, Stabb EV (2006) Photolyase confers resistance to UV light but does not contribute to the symbiotic benefit of bioluminescence in Vibrio fischeri ES114. Appl Environ Microbiol 72:6600–6606
Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP (2010) Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327:1004–1007
Whistler CA, Ruby EG (2003) GacA regulates symbiotic colonization traits of Vibrio fischeri and facilitates a beneficial association with an animal host. J Bacteriol 185:7202–7212
Wier AM, Nyholm SV, Mandel MJ, Massengo-Tiasse RP, Schaefer AL, Koroleva I, Splinter-Bondurant S, Brown B, Manzella L, Snir E, Almabrazi H, Scheetz TE, Bonaldo Mde F, Casavant TL, Soares MB, Cronan JE, Reed JL, Ruby EG, McFall-Ngai MJ (2010) Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc Natl Acad Sci USA 107:2259–2264
Yang JK, Epstein W (1983) Purification and characterization of adenylate cyclase from Escherichia coli K12. J Biol Chem 258:3750–3758
Yang N, Lan L (2015) Pseudomonas aeruginosa Lon and ClpXP proteases: roles in linking carbon catabolite repression system with quorum-sensing system. Curr Genet. doi:10.1007/s00294-015-0499-5
You C, Okano H, Hui S, Zhang Z, Kim M, Gunderson CW, Wang YP, Lenz P, Yan D, Hwa T (2013) Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 500:301–306
Zahid MS, Waise TM, Kamruzzaman M, Ghosh AN, Nair GB, Mekalanos JJ, Faruque SM (2010) The cyclic AMP (cAMP)-cAMP receptor protein signaling system mediates resistance of Vibrio cholerae O1 strains to multiple environmental bacteriophages. Appl Environ Microbiol 76:4233–4240
Acknowledgments
The authors wish to thank the National Science Foundation for support under research under grants OCE-0929081 and IOS-1121106, and several colleagues, particularly Julia A. Schwartzman, Alan J. Wolfe, and Edward G. Ruby, for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Colton, D.M., Stabb, E.V. Rethinking the roles of CRP, cAMP, and sugar-mediated global regulation in the Vibrionaceae . Curr Genet 62, 39–45 (2016). https://doi.org/10.1007/s00294-015-0508-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-015-0508-8