Abstract
We characterized two developmental mutants of Coprinopsis cinerea, Apa56 and Sac29, newly isolated from a homokaryotic fruiting strain, 326 (Amut Bmut pab1-1), after restriction enzyme-mediated integration (REMI) mutagenesis. Both Apa56 and Sac29 exhibited slower mycelial growth than the parental wild-type strain and failed to initiate fruiting when grown on standard malt extract–yeast extract–glucose medium under 12 h light/12 h dark cycle. Both mutants exhibited unusual differentiation in aerial hyphae: differentiated hyphae lacked clamp connections and exhibited irregular shapes. The differentiated hyphae were similar to the component cells of hyphal knots, but did not form hyphal knots: they spread as dense mycelial mats. When the carbon source (glucose) in the medium was substituted with sucrose or galactose, both strains formed as many hyphal knots as the parental wild type. The hyphal knots formed, however, did not develop into fruiting-body initials, but developed into sclerotia. Molecular genetic analysis revealed that the gene, designated Cc.rmt1, is disrupted by REMI mutagenesis and is responsible for the phenotypes in both mutants. Cc.rmt1 is predicted to encode a putative protein arginine methyltransferase, some homologs of which have been shown to be involved in the regulation of gene expression in eukaryotes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual reproduction in homobasidiomycetes is characterized by the formation of relatively large multicellular structures, fruiting bodies or mushrooms, for the efficient production and dispersal of basidiospores. In Coprinopsis cinerea, the developmental sequence of fruiting has been described in detail (Moore 1981; Kües 2000; Kamada 2002). Briefly, fruiting starts with the formation of hyphal aggregates less than 0.2 mm in diameter, called hyphal knots, on the mycelial colony of the dikaryon. Some of hyphal knots subsequently develop into fruiting-body initials with cap and stipe tissues differentiated in 1 day. The initials attain to fruiting-body primordia about 1 cm in height in a few days, which culminate in mature fruiting bodies with expanded caps bearing basidiospores and elongated stipes in one night.
To date, mutational analyses in C. cinerea have identified several genes that regulate various processes during fruiting-body formation. They include cfs1 involved in the initiation of fruiting (Liu et al. 2006), ich1 involved in differentiation of the cap tissue (Muraguchi and Kamada 1998), eln2 (Muraguchi and Kamada 2000) and eln3 (Arima et al. 2004) involved in stipe elongation, and exp1 involved in cap expansion and autolysis (Muraguchi et al. 2008). In other mushroom species than C. cinerea, on the other hand, gene expression analyses have identified a number of genes that show developmentally specific expression (Leung et al. 2000; Miyazaki et al. 2005; Yamada et al. 2006; Joh et al. 2007; Chum et al. 2008). These gene expression studies suggest that several metabolic pathways and signal transduction cascades are involved in fruiting-body formation. However, molecular mechanisms regulating fruiting-body development in basidiomycetous mushrooms remain to be elucidated.
We have been isolating mutants defective in the initiation of fruiting from strain 326 (Amut Bmut pab1-1) of C. cinerea after restriction enzyme-mediated integration (REMI) mutagenesis. Strain 326 is a homokaryon carrying mutations in both A and B mating-type genes and forms fruiting bodies without the need of mating. In this study, we characterize two newly isolated REMI mutants, Apa56 and Sac29. Phenotypic analyses revealed that Apa56 and Sac29 exhibited slower mycelial growth than the parental wild-type strain and failed to initiate fruiting on standard malt extract–yeast extract–glucose medium. In both strains, the hyphae exhibited unusual differentiation: they were irregular in shape and lacked clamp connections such as the component cells of hyphal knots, but did not form hyphal knots. When glucose in the medium was substituted with sucrose or galactose, the mutant phenotypes were reduced and both mutants formed as many hyphal knots as the parental wild type. The hyphal knots formed, however, developed into sclerotia instead of fruiting-body initials. Molecular genetic analysis revealed that in both Apa56 and Sac29, the mutant phenotypes directly result from the disruption of a gene, designated Cc.rmt1, which encodes a protein showing high similarities to protein arginine methyltransferases.
Materials and methods
Strains, culture conditions and genetic techniques
Strains of C. cinerea used in this study are shown in Table 1. MY-glucose medium (1% malt extract, 0.4% yeast extract and 0.4% glucose) solidified with 2% (w/v) agar in 9 cm Petri dishes was used for routine mycelial cultures and fruiting. Slants of MY-glucose medium were used for fruiting. Plates of MY-sucrose medium (1% malt extract, 0.4% yeast extract and 1% sucrose) and MY-galactose medium (1% malt extract, 0.4% yeast extract and 0.4% galactose) were also used for examination of mycelial growth and fruiting. Minimum (MM) medium was that of Shahriari and Casselton (1974) modified by Binninger et al. (1987). Cultures were maintained at 28°C under 12 h light/12 h dark cycle unless otherwise stated.
Genetic analyses were performed as described previously (Inada et al. 2001).
REMI mutagenesis
REMI mutagenesis using plasmid pPHT1 (Cummings et al. 1999) was performed as described previously (Inada et al. 2001) except that ApaI or SacI was used as the restriction enzyme: ApaI and SacI were used for Apa56 and Sac29, respectively.
Transformation
Transformation of C. cinerea was performed as described by Binninger et al. (1987) except that protoplasts were prepared from vegetative mycelia because the recipient strains, Apa56 and Sac29, did not produce sufficient oidia for protoplasts (data not shown). For mycelial protoplasts, vegetative mycelia grown on MY-glucose plates overlaid with cellophane were macerated in a Waring blender, incubated in MY-glucose liquid medium in a 9 cm Petri dish at 28°C for 2 days and then treated by lytic enzymes. Protoplasts released from the mycelia were purified by filtration through a sheet of gauze before use for transformation.
Microscopy
Mycelia from the colonies of C. cinerea strains were harvested as described by Kamada and Tsuru (1993) for microscopic observation: briefly, a small piece of mycelia cultured on MY-glucose was excised from the inner part of the colony using a surgical knife and squashed between the slide glass and coverslip. Micrographs were collected by using a Zeiss Axiophoto microscope (Carl Zeiss AG, Germany).
Plasmid rescue
Plasmid rescues were performed as described by Makino and Kamada (2004): briefly, the genomic DNAs of Apa65 and Sac29 were digested by BamHI and EcoRI, respectively. They were then self-ligated and introduced into Escherichia coli DH10B (Life Technologies, California, USA) cells. Plasmids were then isolated from the ampicillin-resistant transformants. Genomic DNAs from the C. cinerea strains were prepared as described by Zolan and Pukkila (1986).
5 ′- and 3 ′-RACE experiments
The Cc.rmt1 cDNA was amplified from an existing cDNA library (Muraguchi and Kamada (1998) using two sets of nested gene-specific primers and the nested primers for the adaptors (AP-1 and AP-2) of cDNA clones (Table 2 and Fig. 2a). Amplified products were sequenced after cloning in pBluescript KS+ (Stratagene, La Jolla, CA).
Vector construction
To construct a binary vector, designated pPAB2-rmt1, carrying the C. cinerea pab1 and Cc.rmt1 genes, a 3.5 kb genomic region containing the gene CC1G_03287.2 predicted by the Broad Institute (http://www.broadinstitute.org/annotation/genome/coprinus_cinereus/MultiHome.html) was first amplified using the primers, Cc.rmt1 forward 1 and Cc.rmt1 reverse 1 (Table 2). The amplified product was digested with BamHI and then inserted into the BamHI site of plasmid pPAB2 carrying the pab1 gene (Granado et al. 1997). Plasmid pPAB2-rmt1 was linearized by digestion with SphI before use for complementation experiments.
Results
Isolation and genetic analysis of the REMI mutants, Apa56 and Sac29, defective in fruiting initiation
We newly isolated mutant strains, Apa56 and Sac29, defective in fruiting initiation among hygromycin-B resistant transformants of homokaryotic fruiting strain 326 (Amut Bmut pab1-1) after REMI mutagenesis. Apa56 was found among 1545 transformants after mutagenesis using restriction enzyme ApaI, while Sac29 was found among 673 transformants after mutagenesis using restriction enzyme SacI. Both strains formed fruiting bodies normally when mated with a monokaryotic strain, KF3#2 (A91 B91), indicating that the mutations were both recessive. We then analyzed the F1 progenies from the matings and found that in both matings, the defect in fruiting cosegregated with hygromycin-B resistance in the four Amut Bmut progenies from the mating between Apa56 and KF3#2, three were hygromycin-B sensitive and normal in fruiting, and the remaining one was hygromycin-B resistant and defective in fruiting. Of the 14 progenies from the mating between Sac29 and KF3#2, seven were hygromycin-B sensitive and normal in fruiting and the remaining seven were hygromycin-B resistant and defective in fruiting. These results suggested that the mutations in Apa56 and Sac29 were both likely direct results of insertion of plasmid pPHT1 used for REMI.
Phenotypes of Apa56 and Sac29
Under our standard culture conditions (MY-glucose plate 9 cm in diameter at 28°C under the 12 h light/12 h dark cycle), in the parental wild type inoculated at the center of the plate, the mycelial colony covered the whole surface of the plate and formed hyphal knots, the first visible structure of fruiting in a week. Some of the hyphal knots then underwent a series of developmental processes and formed mature fruiting bodies within 2 weeks after inoculation. Under the same culture conditions, strain Apa56 exhibited slower mycelial growth and formed a less fluffy and denser mycelial colony than the parental wild type (Fig. 1a). The mycelial colony formed did not produce a fruiting body after prolonged culture for a month. Microscopic observation revealed that in the mutants, most of the hyphae excised from the surface of the colony, except the marginal 1–2 mm region, differentiated into cells lacking clamp connections and exhibiting irregular shapes (Fig. 1b, panels 1 and 2) in contrast to slender hyphal cells with clamp connections from the inner parts of the colony of the parental wild type (Fig. 1b, panels 3 and 4). The marginal part of the mutant colony comprised normal vegetative hyphae with clamp connections (data not shown). The differentiated hyphae are like the component cells of hyphal knots, which are much shorter and thicker than vegetative hyphae and often have projections (Kamada and Tsuru 1993), but do not form a hyphal knot.
Hyphal growth and differentiaton in Apa56 and 326. a Mycelial colonies of Apa56 and 326 (parental wild type) 5 days after inoculation. b Phase-contrast micrographs of aerial cells of Apa56 (panels 1 and 2) and 326 (panels 3 and 4). Note that the hyphae of Apa56 lack clamp cells. Arrows indicate clamp cells. Scale bar 10 µm. c Panel 1 A colony of Apa56 cultured on MY-sucrose for 2 weeks. Scale bar 2 cm. Panel 2 Low magnification of the colony shown in panel 1, showing abundant sclerotia with pigmented rinds. Scale bar 1 mm
Colony formation and hyphal differentiation of another mutant strain, Sac29, were similar to those of Apa56 (data not shown).
Carbon source influences hyphal differentiation of Apa56 and Sac29
Because it has been reported that fruiting initiation in C. cinerea is inhibited by glucose and glucose analogs (for a review, see Kües 2000), it is possible that the phenotypes of the mutants may be different on media with other carbon sources than glucose. To test this possibility, we substituted sucrose or galactose for glucose in MY-glucose medium. We found that on MY-sucrose or MY-galactose medium, strain Apa56 exhibited faster mycelial growth and formed a less dense mycelial colony and as many hyphal knots as the parental wild type. However, none of the hyphal knots formed developed into fruiting bodies, but many developed into sclerotia with pigmented rinds (Fig. 1c). Similar responses to the carbon sources were also observed in strain Sac29. In addition, the production of sclerotia in Apa56 and Sac29 was not affected by light (data not shown), although sclerotial production has been shown to be suppressed by light in an Amut Bmut strain (Boulianne et al. 2000). The parental wild type normally produced mature fruiting bodies on both MY-sucrose and MY-galactose media and produced no sclerotia (data not shown).
Gene disrupted in Apa56 and Sac29 encodes a putative protein arginine methyltransferase
On the basis of the results of genetic analysis described above, we performed plasmid rescues to identify the genes disrupted in the REMI mutants, Apa56 and Sac29. We could clone 9 kb and 2.5 kb genomic fragments adjacent to plasmid pPHT1 from strains Apa56 and Sac29, respectively. Partial sequencing of the genomic fragments suggested that pPHT1 is integrated within the hypothetical gene CC1G_03287.2 predicted in the Coprinopsis cinerea (Coprinus cinereus) genome database (http://www.broadinstitute.org/annotation/genome/coprinus_cinereus/MultiHome.html) in both Apa56 and Sac29. PCR amplifications of the genomic DNA regions from the mutants followed by sequencing revealed that: in Apa56, a single copy of pPHT1 was inserted between 967 and 968 bp downstream of the translational start site of the predicted gene; in Sac29, a truncated copy of the plasmid was inserted 1,440 downstream of the translational start site and 10 bp just downstream of the insertion site was deleted (Fig. 2a).
The Cc.rmt1 gene disrupted in Apa56 and Sac29 encodes a putative protein arginine methyltransferase. a Structure of the Cc.rmt1 gene. This gene is located on chromosome III (Supercontig 3 in the C. cinerea genome database). The thick lines, open boxes and solid boxes indicate the regions transcribed, a deduced ORF and the introns, respectively. Vertical lines connected to triangular boxes indicate the sites where plasmid pPHT1 was inserted in strains Apa56 and Sac29. Horizontal arrows indicate the primers for 5′- and 3′-RACE experiments and for the genomic fragment for complementation. b Predicted structure of the Cc.Rmt1 protein. Pfam motif-finding software (Finn et al. 2008) indicated that the Cc.Rmt1 protein contains a protein arginine methyltransferase domain at the N-terminal region (the striped box). Vertical arrows indicate the sites where the Cc.Rmt1 protein is disrupted by pPHT1 in Apa56 and Sac29, respectively
To examine whether gene CC1G_03287.2 complements the mutations in Apa56 and Sac29, we transformed these mutants with plasmid pPAB2-rmt1, which carries a 3.5 kb genomic fragment (supercontig 3: 2762382-2759026) (http://www.broadinstitute.org/annotation/genome/coprinus_cinereus/MultiHome.html) containing the whole length of the gene CC1G_03287.2 and the C. cinerea pab1 gene as a selectable marker. As described in “Materials and methods”, we transformed protoplasts prepared from vegetative mycelia in this study because the mutants did not produce a sufficient number of oidia for protoplasts (data not shown). We found that both defects (fruiting initiation and hyphal growth) of strain Apa56 were rescued by pPAB2-rmt1 in 53% (19/36) of pab+ transformants. When we transformed Apa56 with plasmid pPAB2 carrying the pab1 gene only as a control, the defects were not rescued (0/16). Plasmid pPAB2-rmt1 also rescued the defects of strain Sac29 [56% (9/16) of pab+ transformants], while pPAB2 did not [0% (0/14)]. On the basis of these results, we conclude that the mutant phenotypes in Apa56 and Sac29 are direct results of insertion of pPHT1 within the predicted gene CC1G_03287.2.
ORF determination of the Cc.rmt1 gene disrupted in the mutants and characterization of the presumptive Cc.RMT
We performed 5′- and 3′-RACE experiments, sequenced cDNAs and compared the full-length cDNA sequence with the genomic DNA sequence from the C. cinerea database (URL is shown above), which identified an ORF interrupted by nine introns encoding a protein of 349 amino acids (Fig. 2). The full-length cDNA and deduced amino acid sequences have been deposited with the DDBJ/EMBL/GenBank data libraries under accession no. AB550661. The 5′ splice sites agree with the consensus sequence GTRNGT found for filamentous fungi and the 3′ splice sites with the consensus sequence YAG (Gurr et al. 1987), except that the first, second, third and sixth 5′ splice sites are GTTGCG, GTGCGC, GTCCGT and GTAGGC, respectively. Blast searches revealed that the predicted protein had high similarities to protein arginine methyltransferases (Rmt) and hence the gene was designated Cc.rmt1. Cc.Rmt1 is highly conserved in basidiomycetes: it exhibits the highest similarities to a Laccaria bicolor Rmt [NCBI Gene ID, 6073503 PRMT16202; score = 603, E value = 1e−170, identities = 285/340 (83%)], a Cryptococcus neoformans var. neoformans Rmt (NCBI Gene ID, 3255710 CNBB2180) [score = 507; E value = 7e–142, identities = 244/340 (71%)] and a Ustilago maydis hypothetical protein (NCBI Gene ID, 3634033 UM05900.1) [score = 501; E value = 4e−140, identities = 238/327 (72%)].
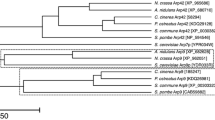
Searches on the genome databases of C. cinerea (URL is shown above), the filamentous ascomycete Aspergillus nidulans (http://www.broadinstitute.org/annotation/genome/aspergillus_group/MultiHome.html), the fission yeast Schizosaccharomyces pombe (http://www.sanger.ac.uk/Projects/S_pombe/) and the budding yeast Saccharomyces cereviae (http://www.yeastgenome.org/) indicated that C. cinerea, A. nidulans, and S. pombe have three genes for Rmts, while S. cerevisiae has two. Phylogenetic analysis of the predicted proteins by UPGMA (unweighted pair-group method with arithmetic mean) showed that Cc.Rmt1 is the closest to A. nidulans RmtA, S. pombe Rmt1 and S. cerevisiae Hmt1p (Fig. 3).
A phylogenic tree of Cc.Rmt1 and ten other fungal protein arginine methyltransferases in S. pombe, S. cerevisiae, A. nidulans and C. cinerea drawn by the UPGMA (unweighted pair-group method with arithmetic mean) using the CLC Free Workbench 4 software. The number shown in the figure is the distance matrix. Accession nos. that are not described in the text are as follows: S. cerevisiae Rmt2p (NCBI Gene ID, 3634033 NP_009590.1), S. pombe Rmt2 (NCBI Gene ID, 2542686 NP_594160.2), S. pombe Rmt3 (NCBI Gene ID, 2541248 NP_595572.1), A. niduans RmtA (GenBank/EMBL/DDBJ accession no. AY272042), A. nidulans RmtB (GenBank/EMBL/DDBJ accession no. AY375304) and A. nidulans RmtC (GenBank/EMBL/DDBJ accession no. AY375305). The amino acid sequences of hypothetical C. cinerea arginine methyltransferases are available from the C. cinerea genome database
Discussion
Here, we have characterized two newly isolated REMI mutants, Apa56 and Sac29, defective in fruiting initiation. Both Apa56 and Sac29 exhibited slower mycelial growth than the parental wild type and did not form hyphal knots, the first visible sign of fruiting, under the culture conditions that normally promote fruiting, i.e. on MY-glucose medium and under 12 h light/12 h dark cycle. Hyphae in the inner parts of mycelial colonies of both mutants exhibited unusual differentiation as the colonies grew. The differentiated hyphae lacked clamp connections and exhibited irregular shapes like the component cells of hyphal knots (Kamada and Tsuru 1993), but they spread as mycelia mats instead of forming distinctive hyphal knots. On MY-sucrose medium, in which the carbon source (glucose) in MY-glucose medium was substituted with sucrose, both mutants formed as many hyphal knots as the wild type. However, many of the hyphal knots developed into sclerotia and none of these developed into the fruiting-body initial even if light was given. Similar developmental processes were also observed when glucose in the medium was substituted with another carbohydrate, galactose. Because it has been reported that increasing glucose in the medium represses hyphal knot formation in an Amut Bmut strain of C. cinerea (Boulianne et al. 2000) and that light promotes the development of hyphal knots into fruiting-body initials and suppresses the development into sclerotia (Kües et al. 1998), both Apa56 and Sac29 might have altered the developmental responses to external signals such as carbon source and light. This hypothesis might be supported by the fact that the mutants produce much smaller number of oidia than the parental wild-type strain 326 under 12 h light/12 h dark cycle (data not shown), because oidia production is promoted by light in strains such as the parental wild type in which the A-regulated pathway is switched on (Kertesz-Chaloupková et al. 1998).
We found that gene Cc.rmt1, mutations of which are responsible for the phenotypes in both Apa56 and Sac29, encodes a putative protein arginine methyltransferase. Furthermore, we revealed that Cc.Rmt1 has high similarities to A. nidulans RmtA, S. pombe Rmt1 and S. cerevisiae Hmt1p. All of these four proteins exhibit significant sequence similarities to PRMT1, the predominant arginine methyltransferase in mammalian cells (Bedford and Clarke 2009). Biochemical studies on A. nidulans arginine methyltransferases have revealed that RmtA methylates histone H4 (Trojer et al. 2004; Bauer et al. 2010). Histone H4 has also been shown to be a target of protein arginine methyltransferases CARM1 and PRMT1, which act as transcriptional coactivators for nuclear receptors in mammalian cells (Chen et al. 1999; Koh et al. 2001; Strahl et al. 2001). S. cerevsiae Hmt1 was shown to be involved in mRNA export into the cytoplasm for subsequent translation (McBride et al. 2000). S. pombe Rmt1 is involved in poly(A) tail length control (Perreault et al. 2007). Taken together, it is suggested that Cc.Rmt1 is involved in the control of gene expression via transcriptional and/or posttranscriptional regulations, which in turn suggests that fruiting-body initiation in C. cinerea is a complex process involving sophisticated regulation of gene expression. Future studies will include identification of the target protein(s) of Cc.Rmt1, which may provide information useful for the elucidation of molecular mechanism underlying developmental regulation in basidiomycetous mushrooms.
References
Arima T, Yamamoto M, Hirata A, Kawano S, Kamada T (2004) The eln3 gene involved in fruiting body morphogenesis of Coprinus cinereus encodes a putative membrane protein with a general glycosyltransferease domain. Fungal Genet Biol 41:805–812
Bauer I, Graessle S, Loidl P, Hohenstein K, Brosch G (2010) Novel insights into the functional role of three protein arginine methyltransferases in Aspergillus nidulans. Fungal Genet Biol 47:551–561
Bedford MT, Clarke SG (2009) Protein arginine methylation in mammals: who, what, and why. Mol Cell 33:1–13
Binninger DM, Skrzynia C, Pukkila PJ, Casselton LA (1987) DNA-mediated transformation of the basidiomycete Coprinus cinereus. EMBO J 6:835–840
Boulianne RP, Liu Y, Aebi M, Lu BC, Kües U (2000) Fruiting body development in Coprinus cinereus: regulated expression of two galectins secreted by a non-classical pathway. Microbiology 146:1841–1853
Chen D, Ma H, Hong H, Koh SS, Huang S-M, Schurter BT, Aswad DW, Stallcup MR (1999) Regulation of transcription by a protein methyltransferase. Science 284:2174–2177
Chum WWY, Ng KTP, Shih RSM, Au CH, Kwan HS (2008) Gene expression studies of the dikaryotic mycelium and primordium of Lentinula edodes by serial analysis of gene expression. Mycol Res 112:950–964
Cummings WJ, Celerin M, Crodian J, Brunick LK, Zolan ME (1999) Insertional mutagenesis in Coprinus cinereus: use of a dominant selectable marker to generate tagged, sporulation-defective mutants. Curr Genet 36:371–382
Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, Ceric G, Forslund K, Eddy SR, Sonnhammer EL, Bateman A (2008) The Pfam protein families database. Nucleic Acid Res Database Issue 36:D281–D288
Granado JD, Kertesz-Chaloupková K, Aebi M, Kües U (1997) Restriction enzyme-mediated DNA integration in Coprinus cinereus. Mol Gen Genet 256:28–36
Gurr SJ, Unkle SE, Kinghorn JR (1987) The structure and organization of nuclear genes of filamentous fungi. In: Kinghorn JR (ed) Gene structure in eukaryotic microbe. IRL press, London, pp 93–139
Inada K, Morimoto Y, Arima T, Murata Y, Kamada T (2001) The clp1 gene of the mushroom Coprinus cinereus is essential for A-regulated sexual development. Genetics 157:133–140
Joh J-H, Lee S-H, Lee J-S, Kim K-H, Jeong S-J, Youn W-H, Kim N-K, Son E-S, Cho Y-S, Yoo Y-B, Lee C-S, Kim B-G (2007) Isolation of genes expressed during the developmental stages of the oyster mushroom, Pleurotus ostreatus, using expressed sequence tags. FEMS Microbiol Lett 276:19–25
Kamada T (2002) Molecular genetics of sexual development in the mushroom Coprinus cinereus. BioEssays 24:449–459
Kamada T, Tsuru M (1993) The onset of the helical arrangement of chitin microfibrils in fruit-body development of Coprinus cinereus. Mycol Res 97:884–888
Kertesz-Chaloupková K, Walser PJ, Granado JD, Aebi M, Kües U (1998) Blue light overrides repression of asexual sporulation by mating-type genes in the basidiomycete Coprinus cinereus. Fungal Genet Biol 23:95–109
Koh SS, Chen D, Lee YH, Stallcup MR (2001) Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem 276:1089–1098
Kües U (2000) Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol Mol Biol Rev 64:316–353
Kües U, Granado JD, Hermann R, Boulianne RP, Kertesz-Chaloupková K, Aebi M (1998) The A mating type and blue light regulate all known differentiation processes in the basidiomycete Coprinus cinereus. Mol Gen Genet 260:81–91
Leung GS, Zhang M, Xie WJ, Kwan HS (2000) Identification by RNA fingerprinting of genes differentially expressed during the development of the basidiomycete Lentinula edodes. Mol Gen Genet 262:977–990
Liu Y, Srivilai P, Loos S, Aebi M, Kües U (2006) An essential gene for fruiting body initiation in the basidiomycete Coprinopsis cinerea is homologous to bacterial cyclopropane fatty acid synthase genes. Genetics 172:873–884
Makino R, Kamada T (2004) Isolation and characterization of mutations that affect nuclear migration for dikaryosis in Coprinus cinereus. Curr Genet 45:149–156
McBride AE, Weiss VH, Kim HK, Hogle JM, Silver PA (2000) Analysis of the yeast arginine methyltransferase Hmt1p/Rmt1p and its in vivo function. J Biol Chem 275:3128–3136
Miyazaki Y, Nakamura M, Babasaki K (2005) Molecular cloning of developmentally specific genes by representational difference analysis during the fruiting body formation in the basidiomycete Lentinula edodes. Fungal Genet Biol 42:493–505
Moore D (1981) Developmental genetics of Coprinus cinereus: genetic evidence that carpophores and sclerotia share a common pathway of initiation. Curr Genet 3:145–150
Muraguchi H, Kamada T (1998) The ich1 gene of the mushroom Coprinus cinereus is essential for pileus formation in fruiting. Development 125:3133–3141
Muraguchi H, Kamada T (2000) A mutation in the eln2 gene encoding a cytochrome P450 of Coprinus cinereus affects mushroom morphogenesis. Fungal Genet Biol 29:49–59
Muraguchi H, Fujita T, Kishibe Y, Konno K, Ueda N, Nakahori K, Yanagi SO, Kamada T (2008) The exp1 gene essential for pileus expansion and autolysis of the inky cap mushroom Coprinopsis cinerea (Coprinus cinereus) encodes an HMG protein. Fungal Genet Biol 45:890–896
Perreault A, Lemieux C, Bachand F (2007) Regulation of the nuclear poly(A)-binding protein by arginine methylation in fission yeast. J Biol Chem 282:7552–7562
Shahriari H, Casselton LA (1974) Suppression of methionine mutants in Coprinus. I. Complementation and allele specificity as criteria of suppressor gene action. Mol Gen Genet 134:85–92
Strahl BD, Briggs SD, Brame CJ, Caldwell JA, Koh SS, Ma H, Cook RG, Shabanowitz J, Hunt DF, Stallcup MR, Allis CD (2001) Methylation of histone H4 at arginine 3 occurs in vivo and is mediated by the nuclear receptor coactivator PRMT1. Curr Biol 11:996–1000
Trojer P, Dangl M, Bauer I, Graessle S, Loidl P, Brosch G (2004) Histone methyltransferase in Aspergillus nidulans: evidence for a novel enzyme with a unique substrate specificity. Biochemistry (Mosc.) 43:10834–10843
Yamada M, Sakuraba S, Shibata K, Taguchi G, Inatomi S, Okazaki M, Shimosaka M (2006) Isolation and analysis of genes specifically expressed during fruiting body development in the basidiomycete Flammulina velutipes by fluorescence differential display. FEMS Microbiol Lett 254:165–172
Zolan ME, Pukkila PJ (1986) Inheritance of DNA methylation in Coprinus cinereus. Mol Cell Biol 6:195–200
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Research Fellowships of the Japan Society for the Promoting of Science (JSPS) for Young Scientists [08J00332].
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Kues.
Rights and permissions
About this article
Cite this article
Nakazawa, T., Tatsuta, Y., Fujita, T. et al. Mutations in the Cc.rmt1 gene encoding a putative protein arginine methyltransferase alter developmental programs in the basidiomycete Coprinopsis cinerea . Curr Genet 56, 361–367 (2010). https://doi.org/10.1007/s00294-010-0307-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-010-0307-1