Abstract
We studied the genomic structure and RNA editing of mitochondrial cox1, cox2, cob and atp9 from the horsetail Equisetum arvense, a representative of an old fern lineage. Editing of cox1, cob and atp9 mRNAs occur only by C-to-U transitions. No changes were found in cox2 transcripts constituting one of the rare examples of unedited mitochondrial mRNA in land plants. From three intervening sequences in cox1, cox1i395 and cox1i624 are group IB introns homologous to the Marchantia polymorpha cox1 introns, and cox1i747 is a group IIA intron different to other introns found in plant mtDNA. The group II intron cox2i373 is very similar to other introns found in cox2 from vascular plants. While cob and atp9 have no introns and display the gene structure found in seed plants, various nucleotide substitutions abolish the only potential ORF, a LAGLIDADG endonuclease present in cox1i395. Thus, E. arvense mitochondria conserve two group I introns from non-vascular plants, probably inherited from a common ancestor with liverworts. Analogous to seed plants, E. arvense has no potential mitochondrial splicing factors encoded in these introns. This is the first report concerning the presence of vertically inherited group I introns in vascular plant mitochondria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Group I and II introns are present in all land plant mitochondrial genomes so far described (Chaw et al. 2008). Both intron types are characterized by a distinct and conserved RNA secondary structure (Waring et al. 1982; Michel et al. 1982), and differ by their splicing mechanism (Saldanha et al. 1993). Comparative analysis of complete genomes and phylogenetic studies of individual introns revealed that their occurrence in different genes varies by sporadic gain and loss during embryophyte evolution (Knoop 2004; Bonen 2008).
In mitochondria of vascular plants, all the introns belong to group II type except for a group I intron present in cox1 from Peperomia first described by Vaughn et al. (1995) and more recently identified in a relatively large number of flowering plants (Cho et al. 1998; Sanchez-Puerta et al. 2008). This intron seems to be acquired from independent horizontal transfers during angiosperm evolution. In contrast, non-vascular plants mitochondria present several group I introns. Beside 25 group II introns, 7 group I introns split mitochondrial genes in the liverwort Marchantia polymorpha (Oda et al. 1992). Six of them are present in cox1 considered the main group I intron reservoir, and one is found in nad5. The moss Physcomitrella patens has two group I introns, the counterparts of cox1 and nad5 introns from M. polymorpha (Terasawa et al. 2007). The nad5 group I intron is found in all liverworts and mosses investigated so far, but is absent in hornworts (Steinhauser et al. 1999; Duff 2006). Thus, it seems that a gradual loss of group I introns occur in non-vascular plants after the divergence between liverwort and mosses. While a small number of mitochondrial group II introns have been vertically transmitted between non-vascular and vascular plants during evolution, such inheritance has not been observed for group I introns (Bonen 2008).
Some ORFs encoded in mitochondrial introns are required for splicing and intron mobility (Dujon et al. 1986; Lambowitz and Zimmerly 2004). Group I and II intron-encoded proteins have different characteristics. Group I introns encode site-specific endonucleases with conserved LAGLIDADG or GIY-YIG motifs, while group II introns encode for proteins with homology to viral reverse transcriptase (RT), referred as maturase-reverse transcriptases (MAT-R). Several ORFs have been found in introns from bryophyta mitochondria where eight group II introns encode MAT-R proteins and two group I introns encode LAGLIDADG endonucleases (Oda et al. 1992). Physcomitrella mitochondrial genome (accession number AB251495) presents two maturase-encoding group II introns, which are different to Marchantia, while no group I intron-encoded endonucleases are detected. In seed plant, only one ORF related to fungal RT-maturases (mat-r), is highly conserved among species in the same group II intron of nad1 (Wahleithner et al. 1990). This ORF is absent in E. arvense nad1 (Dombrovska and Qiu 2004). The loss of the coding capacity of introns might be partially compensated by the transfer of maturase genes into the nuclear genome (Mohr and Lambowitz 2003; Nakagawa and Sakurai 2006).
The aim of this work was to investigate the status of selected mitochondrial introns in pteridophytes, using the horsetail E. arvense as a model. We were particularly interested in intron-encoded ORFs which are present in six mitochondrial genes from the liverwort M. polymorpha (Oda et al. 1992). Among vascular plants, horsetails, living ferns, and seed plants (euphylophytes) are assumed to be the sister group of hornworts and mosses (Groth-Malonek et al. 2005). Liverworts are proposed to be the basal clade to the whole embryophyte (Henrick and Crane 1997; Qiu et al. 2006). Up to date, no pteridophyte mitochondrial genome has been reported, and only partial cox1 gene sequence is known for four species (Sper-Whitis et al. 1996). Moreover, cox2 has been described only in the primitive vascular plant Psilotum nudum (Sper-Whitis et al. 1994), and neither cob nor atp9 gene sequence is available for that clade. The study of horsetails (equisetopsids) is important since they constitute an early branching group in the moniliformopses which are considered as the closest living relatives to seed plants (Pryer et al. 2001).
We report here the genomic structure of cox1, cox2, cob and atp9 from the horsetail E. arvense to elucidate the gain and loss of coding introns in pteridophyte. Two others genes bearing potential coding introns, rrn18 and atp1 have been described (Duff and Nickrent 1997; Wikström and Pryer 2005). By analyzing genomic and cDNA sequences, we verify the actual exon-joining boundaries and determine the RNA editing status of the respective coding regions. We found that E. arvense mitochondria have conserved group I introns from non-vascular plants, but similar to seed plants, have lost the coding capacity of introns. This work constitutes the first report concerning the presence of two group I introns in the mitochondrial genome of a vascular plant, probably inherited from a common ancestor with liverworts.
Materials and methods
Nucleic acid extraction
Equisetum arvense plants were field-collected near Bordeaux (France). Total DNA and RNA were extracted from fresh lateral shoots using the DNeasy Plant Mini Kit and the RNeasy Plant Mini Kit (Qiagen), respectively, following the protocol specified by the manufacturer.
Genomic and cDNA amplification, cloning and sequencing
Genomic fragments were amplified from total DNA by nested PCR to improve the signal and the specificity. The orthologous primers used are detailed below. Two different thermostable DNA polymerase were used: Advantage 2 Polymerase Mix (Clontech) in current PCR assay and Tfl DNA polymerase (Promega) to amplify products longer than 3 kbp. PCR amplification assays contained 10–100 ng of total DNA, 250 μM each dNTP, 10 μM primers and 2.5 units of DNA polymerase in a final volume of 50 μl. PCR reactions were performed essentially as described by the supplier. Hybridization temperature and elongation time were adapted according to the primer melting temperature and the fragment size as follows: for cox1, the hybridization temperature was 47 and 50°C for PCR1 and PCR2, respectively, and the elongation step was 5 min; for cox2, the hybridization temperature was 50 and 48°C for PCR1 and PCR2, respectively, and 2 min elongation; for cob, 40 and 44°C hybridization temperature for PCR1 and PCR2, respectively, and 1 min elongation; for atp9 hybridization was at 44°C and 1 min elongation. In all cases, the elongation temperature was 68°C.
cDNA synthesis was performed on total RNA with the Access RT-PCR kit (Promega) according to the protocol of the manufacturer, followed by a nested PCR performed as described for genomic PCR, using 1 μl of RT-PCR product. The results presented correspond, at least, to two independent experiments performed with different nucleic acids preparations.
PCR and RT-PCR products were purified with the GFX PCR DNA and Gel Band Purification Kit (GE Healthcare) and cloned into the pGEMT-Easy vector (Promega). PCR clones were purified with Wizard plus SV minipreps DNA purification kit (Promega) and sequenced on an ABI PRISM® 3130xl Genetic Analyzer (Applied Biosystems) using the Big Dye Terminator v1.1 cycle sequencing kit (Applied Biosystems). Data presented here are the result of the analysis of 20 genomic and at least 40 cDNA clones for each gene.
PCR and RT-PCR primers
Orthologous PCR primers were designed based on conserved 5′ and 3′ exonic sequences of homologous genes from M. polymorpha (Oda et al. 1992). The primers chosen cover about 85% of the coding sequence of the E. arvense genes compared to the M. polymorpha homologous genes. In some cases, the primers used for nested PCR (S2/AS2) were designed from the actual Equisetum sequence (Ea) after cloning and sequencing the first PCR product (S1/AS1 primers).
cox1-S1, GATATAGGTACTCTATATTT; cox1-AS1, AGGATTCTGTTCAACCGC; cox1-S2, CTATATTTAATCTTCGGTGC (E.a); cox1-AS2, AACCGCCGCCCAAGG (E.a); cox2-S1, CACTCCTATGATGCAAGG; cox2-AS1, ATACCCAAGAAACATAATCA; cox2-S2, AAGGAATAATTGACTTACATC; cox2-AS2, CAAAGAAACAGCTTCTA (E.a); cob-S1, CATTTGATAGATTATCC; cob-AS1, AGAATGGGCGTTAT; cob-S2, AGTTATTGGTGGGG; cob-AS2, GGCGAAATACAAGAA (E.a); atp9-S, AATGGAGCAGGAGC; atp9-AS, TATAAAAATGCCATCATT.
Sequence analysis and secondary structure prediction
The search for similar regions was done with the BLASTN 2.2.18+ program (http://www.ncbi.nlm.nih.gov/BLAST/). Nucleotidic sequences were aligned using Gene Jockey II software (Biosoft). Intron secondary structure prediction was obtained with the mfold software (Zuker 2003) (http://www.mfold.bioinfo.rpi.edu/), or by analogy with known homologous introns and adjusted manually according to the canonical group I and group II intron structures described by Michel et al. (1989).
Results
Genomic structure of cox1, cox2, cob and atp9
PCR amplification using cox1 orthologous primers generated a product of 4,345 bp length from total DNA preparation, while a RT-PCR analysis showed a cDNA product of 1,451 bp, suggesting the presence of large intronic sequences. In the case of cox2, the genomic PCR product is 2,029 bp in length, while the RT-PCR product has only 651 bp indicating the presence of intervening sequences in this gene. The genomic and cDNA amplification products from cob produced an identical 1,061-bp band; therefore, we conclude that cob is not interrupted by introns. Similar to cob, atp9 presents no introns. In all cases, the RT-PCR amplification products were obtained from specific mRNA, since no amplification products were obtained in the absence of RT which is a clear evidence that the RT-PCR products originated from the respective mRNAs.
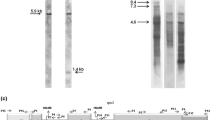
To define precisely the position of the putative introns, the genomic and cDNA amplification products of cox1 and cox2 were cloned and sequenced. To facilitate the comparison with the homologous M. polymorpha introns, thereafter the introns will be named according the nomenclature proposed by Dombrovska and Qiu (2004). cox1 presents three introns, intron 1 (cox1i395), intron 2 (cox1i624) and intron 3 (cox1i747) which are 1,126, 1,085 and 712 bp in length, respectively. In the case of cox2, an intron of 1,378 bp, cox2i373, interrupts the ORF. Sequence analysis of cDNA clones showed that the mitochondrial cob gene has not intervening sequences in the horsetail E. arvense. A diagram of the exon–intron structure of the three genes is depicted in Fig. 1b (for details, see Supplementary material).
Genomic organization of cox1, cox2, cob and atp9, and RNA editing of the mature transcripts from the horsetail Equisetum arvense. Gene structure was established after sequencing genomic and cDNA PCR products. Exons are represented by grey rectangles. Introns are depicted by split thin lines connecting exons. Intron names are according to the nomenclature suggested by Dombrovska and Qiu (2004), and intron size is indicated in parentheses. Missing information at exonic ends (white rectangles) is estimated to 18 and 30 codons at 5′ and 3′ ends of cox1, respectively, 30 and 4 codons at 5′ and 3′ ends of cox2, 29 and 36 codons at 5′ and 3′ ends of cob, and 7 and 4 codons for atp9 mRNAs based on comparison with Marchantia coding sequences. Vertical arrows indicate the localization of edited C residues
RNA editing of Equisetum arvense transcripts
RNA editing is a hallmark of land plant mitochondria characterized mainly by C-to-U, and in some species U-to-C changes. At least 40 independent cDNA clones were sequenced for each gene transcript and compared to the respective genomic sequences.
cox1 mRNA is extensively edited with 47 C-to-U changes distributed throughout the mature transcripts: 6 changes in exon1, 7 in exon 2, 6 in exon 3 and 28 C-to-U changes in exon 4 (Fig. 1c). A total of 13.7% of exonic C residues are edited, concerning 41 out of 463 codons in cox1 mRNA, but no silent editing was observed (Supplementary material S1). Thirty-six out of 41 codons are edited either at the first or second position. Four codons are edited (underlined) at two residues: codon 71 (CCU); codon 428 (CCC); codon 434 (CCA) and codon 86 (UCC). Finally, a Pro 242 (CCC) codon is changed to Phe (UUU) by triple editing. RNA editing is quite efficient for cox1 spliced mRNAs since 27 out of 41 codons were found edited in all cDNA clones, the remaining 14 codons were edited at frequencies from 76 to 98%. Twenty-six out of 50 cox1 mature mRNAs were fully edited, 22% present only one unedited codon and 16% have two unedited codons. The putative COX1 protein encoded in the E. arvense mitochondrial genome share 85.8% identity with the M. polymorpha and 85.3% with A. thaliana homologous protein. However, the protein encoded by the edited mRNA presents 94.6 and 94.2% identity with the M. polymorpha and A. thaliana homologous counterparts, respectively.
In the case of cob transcripts, 10 C-to-U conversions were detected after sequence analysis of 40 cDNA clones compared to the genomic sequence. The editing sites were formed two clusters with four editing sites located at the 5′ end and six sites at the 3′ end of the mRNA (Fig. 1c). RNA editing modifies 9 out of 322 codons (Supplementary material S2). Eight codons are modified at only one residue, three of them are in position 1 in the codon and seven in position 2. Only one, Pro 363 (CCC) codon was edited at two positions. Among the sequenced cDNA clones, 27 out of 40 corresponds to fully edited mRNAs, the others were partially edited presenting one (7/40), two (4/40), three (1/40) or six (1/40) unedited codons. Seven C residues are changed to Us in atp9 mRNA (Supplementary material S3). Different to cox1 and cox2 transcripts, the atp9 mRNAs were fully edited.
While several C-to-U changes were found in different transcripts, no U-to-C changes were observed in any the cDNA clones sequenced. It is interesting to note that no difference between cox2 genomic and cDNA nucleotide sequence was found, indicating that cox2 transcripts are correctly spliced but are not edited in E. arvense mitochondria. The sequence identity between the putative COX2 proteins of Equisetum and Marchantia is about 90%.
Secondary structure and coding capacity of Equisetum arvense cox1 and cox2 introns
The introns interrupting the horsetail cox1 and cox2 ORFs were compared to M. polymorpha counterparts (Ohta et al. 1993). E. arvense intron1 (cox1i395) and intron 2 (cox1i624) from cox1 are homologous to intron 4 (aI4) and intron 6 (aI6) from M. polymorpha cox1, respectively (Fig. 2). No sequence similar to cox1i747 was found in mitochondrial genomes available in data banks.
Comparison of group I introns from E. arvense cox1i395 and cox1i624 with the P. patens cox1i624 and M. polymorpha ai4 and ai6 homologues. a E. arvense cox1i395 (E. arv) is compared to the Marchantia ai4 intron (M. pol). The conserved LAGLIDADG endonuclease motif is indicated. b Sequence alignment of E. arvense cox1i624 with Physcomitrella (P. pat) cox1i624 and Marchantia ai6 introns. Nucleotides not shown in the alignment are signaled in parentheses. Intron sequences are in uppercase and exonic sequence junctions in lowercase letters. Numbers above alignments indicate the nucleotide position within each intron in Equisetum sequences. Nucleotide identity with Equisetum sequence is indicated by dots. Motifs P1–P9 (small rectangles) involved in base pairing in the secondary structure of group I introns were defined as reported by Ohta et al. (1993). Brackets indicate conserved core sequences P, Q, R and S. The premature stop codon in the putative encoded-ORF is underlined and signaled by an asterisk
The mitochondrial intervening sequences have canonical secondary structures with particular regions allowing to define them as type I or type II introns. cox1i395 and cox1i624 present highly conserved elements of secondary structure designated P1 through P9 and the conserved consensus sequence elements P, Q, R, and S characteristic of group I introns according to standard representation established by Burke et al. (1987). Both sequences can be folded in a secondary structure presenting a catalytic core made up of two extended helices, the P4–P6 domain formed by the stacking of P5, P4, P6, and P6a helices whereas the P3–P9 domain is formed by the stacking of P8, P3, P7, and P9 (see S4 and S5 of Supplementary material).
Marchantia polymorphaaI4 encodes for a LAGLIDADG endonuclease located in loop 8, fused to the preceding exon1. cox1i395, the Equisetum homologue of aI4, presents only one LAGLIDADG motif (Fig. 2a). The downstream region in loop 8 differs from the Marchantia sequence leading to the loss of the endonuclease reading frame. In contrast, Equisetumcox1i624, the homologue of aI6 from Marchantia, present high identity from P1 to P8 regions. The L8 loop is totally different in primary sequence and size compared to aI6, leading to a divergent secondary structure of the P9 region between the two species (Fig. 2b and accompanying Supplementary material). As MarchantiaaI6, cox1i624 is a non-encoding intron.
Intron 3 (cox1i747) presents all the characteristics of a group II intron. It can be folded into a canonical secondary structure with six conserved helical domains I–VI (D1–D6) linked to a central core (Michel et al. 1989). Moreover, some motifs such as the EBS1-IBS1 and EBS2-IBS2 base pair interactions between D1 and exon1 region, the A residue in a bulge on D6 involved in the first transesterification step and the tertiary structure interactions α–α′, ε–ε′ and γ–γ′ characteristic of functional group II introns are clearly distinguished (Fig. 3). Domain V differs from the consensus structure with a short distal helix and an unusual 8nt loop instead of the GAAA-tetraloop conserved motif. This variation has been observed in some other group II introns (Lang et al. 2007).
Secondary structure of the 710 nt cox1747 intron of the horsetail E. arvense. Roman numerals (I–VI) indicate the conserved domains characteristic of group II introns. Structural elements α–α′, ε–ε′, γ–γ′, EBS and IBS implicated in putative tertiary interactions are connected by dotted lines. The nucleotides in lowercase letters correspond to the 3′-end of exon 3 and the 5′-end of exon 4, respectively. The C→U editing event in IBS2 is indicated. Some characteristics defining cox1712 as group IIA intron are a bulging “A” (circled) in domain VI that is located 7 nt sequence YAY (γ′ region) according to Michel et al. (1989)
The location and the coding properties of cox 1 introns from different plant species is drawn in Fig. 4.
Diagram of introns inserted within cox1 ORF found in different land plants: Arabidopsis thaliana (A.tha) (Unseld et al. 1997) Peperomia polybotrya (P.pol) (Vaughn et al. 1995), Equisetum arvense (E.arv) (this study), Physcomitrella patens (P. pat) (Terasawa et al. 2007) and Marchantia polymorpha (M.pol) (Oda et al. 1992). Vertical arrows indicate the intron insertion sites. Arrows with open circles signal group I introns and arrows with filled circles indicate group II introns. M group II intron-encoded RT-maturase; E group I intron-encoded LAGLIDADG endonuclease. The name of introns found in E. arvense is indicated
As indicated above, E. arvense cox2 is interrupted by a 1,383 bp intron with no homologue in M. polymorpha mitochondrial genome. Gene bank blast analysis revealed that cox2i373 has high nucleotidic sequence identity with other group II introns of cox2 mitochondrial genes from many vascular plants located at identical insertion site. In fact, cox2i373 can be folded in a canonical secondary structure with tertiary interaction motifs characteristics of group II introns (for details see Supplementary material S6).
Discussion
The horsetail E. arvense cox1 and cox2 are interrupted by introns
In bryophytes, some mitochondrial genes present a complex organization. In the liverwort M. polymorpha, cox1, cox2, cob and atp9 are interrupted by nine, two, three and one introns, respectively (Oda et al. 1992). Nine of them correspond to group II and six to group I introns. Moreover, five out of these nine group II introns carry maturase-reverse transcriptase type ORFs and two group I introns encode for homing endonucleases of the LAGLIDADG type. This situation is radically modified in higher plants where all these introns, particularly the group I introns, are not present. This study focuses on the horsetail cox1, cox2, cob, and atp9 mitochondrial genes. E. arvense is one of the ancient ferns, placed at the interface between non-vascular and vascular plants in the conquest of land during evolution (Pryer et al. 2001). The structure of the four Equisetum genes is less complex than the homologous M. polymorpha counterparts. Three introns interrupt cox1 and one split cox2, while no introns were found in cob and atp9. Interestingly, cob and atp9 present the intronless configuration found in higher plants. E. arvense atp1 (Wikström and Pryer 2005) and rrn18 (Duff and Nickrent 1997; accession no. AF058663) have no intron. It should be noted that the data presented in this report concern the functional introns defined by the actual splicing sites and exon-joining boundaries resulting from comparison between the gene with the respective mature transcript cDNA sequences.
RNA editing involves C-to-U but no U-to-C conversions in Equisetum arvense mitochondria
The RNA editing process occurs by C-to-U modifications in all land plant organelles with the exception of the marchantiid subclass of liverwort (Malek et al. 1996). In addition, U-to-C RNA editing has been reported in hornworts (Steinhauser et al. 1999; Kugita et al. 2003), isoetes and ferns (Vangerow et al. 1999) where it modifies codons and suppresses genomic stop codons.
Sequence analysis of cDNA clones from E. arvense transcripts showed that cox1, cob and atp9 mRNAs are edited by C-to-U conversions at 47, 10, and 7 residues, respectively. In all cases, the codon changes produced by RNA editing lead to a higher identity of the encoded protein with plant and non-plant homologues (Gualberto et al. 1989; Bégu et al. 1990; Araya et al. 1994). The E. arvense cox1 mRNA is extensively edited as observed for the same transcript in some gymnosperms (Chaw et al. 2008), and higher than reported for angiosperms cox1 transcripts (Giegé and Brennicke 1999; Notsu et al. 2002; Handa 2003). For cob and atp9 mRNAs, editing level is similar to those disclosed for higher plants transcripts. No editing events were observed in mature cox2 transcripts, an uncommon situation already observed for a few transcripts in seed plants (Lu et al. 1998; Giegé and Brennicke 1999). The fact that U-to-C changes were not detected in none of the transcripts analyzed is a strong indication that this kind of editing event does not occur in this clade. Additionally, no stop codon interrupts the mitochondrial ORFs, a fact that could foresee such possibility. The results presented here validate the prediction, based on gene sequence analyses, that only C-to-U RNA editing occurs in this species (Malek et al. 1996; Dombrovska and Qiu 2004; Qiu et al. 2006).
Two group I introns are present in cox1
Equisetum cox1 introns cox1i395 and cox1i624, the homologues of the fourth and sixth M. polymorpha introns, respectively, are located at similar position in both species. It should be noted that cox1i395 is absent, but cox1i624 is conserved in the moss P. patens (Terasawa et al. 2007) (Figs. 2b, 4). Group I introns are present in bryophytes, but are absent in seed plants. In fact all mitochondrial introns described so far in vascular plants are group II introns, with one exception, a group I intron acquired by an angiosperm through horizontal transfer from a fungal donor (Cho et al. 1998). Both, cox1i395 and cox1i624 introns can be drawn in a secondary structure characteristic of group I introns (Ohta et al. 1993; Vicens and Cech 2006). The elements involved in intron splicing are clearly identified: (a) the conserved G-U pair in P1 in the 5′ splice site involved in the first transesterification step, (b) the P10 sequence, complementary to the first residues of the downstream exon, required for the second transesterification step, (c) the conserved sequence GACU in P7 which forms the GTP binding site, (d) the domains P1, P3, P4, P5, P6, P7, and P8 forming the catalytic core.
While the first intron has a high identity with the M. polymorpha intron 4, cox1i624 sequence is conserved only at regions spanning from P2 to P8 base pair segments, but diverge in their L8 loop and P9 region (Fig. 2b). Interestingly, the P. patens, homologous cox1 intron (Terasawa et al. 2007) also presents a divergent sequence after P8 region, exactly at the same position where the Marchantia and Equisetum sequences diverge (Fig. 2b). This situation results in a different stem-loop structure of the L9–P9 domain (see accompanying Supplementary material). The situation of cox1i624 suggests that a rearrangement might have occurred during transfer of the introns in these species. These regions may be a useful species marker for phylogenetic studies. This is the first evidence of group I introns probably transferred from non-vascular plant ancestors to a vascular plant mitochondrial genome. Considering the basal position of Equisetum in moniliformopses, it should be interesting to know whether these mitochondrial group I introns are conserved in other ferns, and transmitted to lycophytes.
The group II intron, cox1i747, is found only in Equisetum mitochondria
The third intron of cox1, cox1i747, is a group II intron of the subclass A as revealed by the canonical secondary structure that can be drawn from the nucleotide sequence (Fig. 3). The different domains D1–D6 radiating from a central wheel, and the tertiary structure interactions are clearly identified following the rules proposed by Michel et al. (1989). With the exception of some restricted conserved motifs common to group II introns, no sequences related to cox1i747 are found in M. polymorpha. Moreover, it is inserted 18 downstream compared to the insertion position of ai7, a group I intron in M. polymorpha cox1 (Fig. 4). Interestingly, a group II intron, referred as i10, in the moss P. patens also differs of E. arvense and Marchantia intron in sequence and insertion site (Terasawa et al. 2007). Contrary to the description given by the authors, i10 has no homologue with any cox1 intron from M. polymorpha, but corresponds to a group II intron encoding for a putative maturase-like protein. No intron from either fungi or protist was found at this insertion site in cox1 sequences present in databases. However, a group I intron is found 7 nt upstream the i747 in cox1 of several fungi (Gonzalez et al. 1998). It is striking that a restricted region of cox1 has been targeted by unrelated intervening sequences in different species. In contrast, no introns were found in 25 different plants, including one fern, one psilophyte, and two lycophytes, by analyzing a region of cox1 encompassing the insertion site (Sper-Whitis et al. 1996). Moreover, analysis of gene data bank indicates that no such intron is present in land plant mitochondrial genomes described so far. This suggests that cox1i747 is a new intron acquired by horizontal transfer during evolution of Equisetaceae, but was not transmitted to related pteridophytes.
cox2i373 is highly conserved among land plants
Marchantia mitochondria share one group II intron with angiosperms, and nine are common with the moss P. patens introns (Chaw et al. 2008; Terasawa et al. 2007). Equisetumcox2i373 is conserved in P. patens and several vascular plants (Terasawa et al. 2007). Remarkable is the strong conservation of this intron throughout evolution. Indeed, the primary and secondary structure, even in the complex domain I, are very close to wheat mitochondria cox2 intron (Farré and Araya 2002; Supplementary material S6). The main difference was found in domain IV which may vary from 212 in Zea mays (Covello and Gray 1990) up to 1,647 nt in Huperzia lucidula (Qiu et al. 2006 and accession no. DQ677486). Another group II intron (cox2i696) has been found in some angiosperms (reviewed by Bonen 2008), the lycophyte H. lucidula (accession no. DQ677486) and the moss P. patens (Terasawa et al. 2007). Our results clearly show that this intron is absent in cox2 from horsetail E. arvense mitochondria.
It should be noted that ambiguous responses can be obtained when comparing either the cox1 group I introns or the cox2 group II intron of horsetails with other plant species. In the first case, E. arvense will be placed close to liverworts, while in the second, Equisetum will be closer to angiosperms. This particular situation point out that it is important to use a multifaceted approach in phylogenetic studies. Our results suggest that the cox2 intron was acquired very early during vascular plant evolution.
Intron-encoded ORFs have been lost in the horsetail Equisetum arvense
Intron-encoded proteins are required for splicing (Saldanha et al. 1993). In Marchantia, ten ORFs were found in introns from six genes: two code for endonucleases and two for maturases in cox1, two encode for maturase-like protein in atp1, and one maturase-like ORF is present in cox2, cob, atp9 and rrn18 introns (Oda et al. 1992). In all higher plant mitochondria investigated so far, a single Mat-r like ORF is encoded in domain IV of one nad1 intron (Bégu et al. 1998; Zhu et al. 2007). The endonucleases encoded for by group I introns have two LAGLIDADG motif characteristics of active fungal endonucleases (Colleaux et al. 1986). Equisetum cox1i395 has only one such motif. Translation of all three reading frames is prevented by the deletion of four nucleotides at the very beginning of the intron, creating a premature stop codon (Fig. 2a). In some plant species, the presence of stop codons inside ORFs may be solved by U-to-C RNA editing (Malek et al. 1996). This is not the case since, as earlier discussed, E. arvense does not undergo U-to-C editing at least for the four transcripts studied here.
In many instances, maturase-related ORFs are found associated to group II introns and seem to be required for splicing (Lazowska et al. 1980). Group II introns from E. arvense cox1 and cox2 do not bear mat-r ORFs, a situation also found in three group II introns that interrupt nad1 (Dombrovska and Qiu 2004). Moreover, no introns were found in cob and atp9 (this work), atp1 (Wikström and Pryer 2005) and rrn18 (Duff and Nickrent 1997, accession no. AF058663). Taken together, these results indicate that compared to liverwort, E. arvense mitochondrial genome lacks of a large part of the introns, in particular the coding introns, and that the remaining ones are devoid of coding capacity. This situation raises the question of how mitochondrial introns are excised in the absence of intron ORFs. In higher plants, the transfer of mitochondrial genetic information into the nucleus is an ongoing process (Nugent and Palmer 1991). Interestingly, four ORFs presenting a relevant homology with the mat-r like proteins encoded in group II introns, cox2i250 and cobi824 of Marchantia are nuclear encoded in the seed plant A. thaliana, and potentially targeted to mitochondria (Mohr and Lambowitz 2003). One of them has been demonstrated to be involved in the splicing of a mitochondrial transcript (Nakagawa and Sakurai 2006). Moreover, a nuclear-encoded gene is responsible for the splicing of the unique group I intron present in land plant chloroplast (Asakura and Barkan 2007). It is tempting to propose that transfer from mitochondria to the nucleus of intron might have already occurred in the sphenophyte clade. If the evidence presented here support the hypothesis of a loss of coding introns in pteridophyte, it remains an open question until the complete sequence of the mitochondrial genome will be available.
References
Araya A, Bégu D, Litvak S (1994) RNA editing in plants. Physiol Plant 91:543–550
Asakura Y, Barkan A (2007) A CRM domain protein functions dually in group I and group II intron splicing in land plant chloroplasts. Plant Cell 19:3864–3875
Bégu D, Graves PV, Domec C, Arselin G, Litvak S, Araya A (1990) RNA editing of wheat mitochondrial atp synthase subunit 9: direct protein and cDNA sequencing. Plant Cell 2:1283–1290
Bégu D, Mercado A, Farré JC, Moenne A, Holuigue L, Araya A, Jordana X (1998) Editing status of mat-r transcripts in mitochondria from two plant species: C-to-U changes occur in putative functional RT and maturase domains. Curr Genet 33:420–428
Bonen L (2008) Cis- and trans-splicing of group II introns in plant mitochondria. Mitochondrion 8:26–34
Burke JM, Belfort M, Cech TR, Davies RW, Schweyen RJ, Shub DA, Szostak JW, Tabak HF (1987) Structural conventions for group I introns. Nucl Acids Res 15:7217–7221
Chaw S-M, Shih AC-C, Wang D, Wu Y-W, Liu S-M, Chou T-Y (2008) The mitochondrial genome of the gymnosperm Cycas taitungensis contains a novel family of short interspersed elements, BpU sequences, and abundant RNA editing sites. Mol Biol Evol 25:603–615
Cho Y, Qiu YL, Kuhlman P, Palmer JD (1998) Explosive invasion of plant mitochondria by a group I intron. Proc Natl Acad Sci USA 95:14244–14249
Colleaux L, d’Auriol L, Betermier M, Cottarel G, Jacquier Agalibert F, Dujon B (1986) Universal code equivalent of a yeast mitochondrial reading frame is expressed into E. coli as a specific double stranded endonuclease. Cell 44:521–533
Covello PS, Gray MW (1990) Differences in editing at homologous sites in messenger RNAs from angiosperm mitochondria. Nucleic Acids Res 18:5189–5196
Dombrovska O, Qiu Y-L (2004) Distribution of introns in the mitochondrial gene nad1 in land plants: phylogenetic and molecular evolutionary implications. Mol Phylogenet Evol 32:246–263
Duff RJ (2006) Divergent RNA editing frequencies in hornwort mitochondrial nad5 sequences. Gene 366:285–291
Duff RJ, Nickrent DL (1997) Characterization of mitochondrial small-subunit ribosomal RNAs from holoparasitic plants. J Mol Evol 45:631–639
Dujon B, Colleaux L, Jacquier A, Michel F, Monteilhet C (1986) Mitochondrial introns as mobile genetic elements: the role of intron-encoded proteins. In: Wickner RB, Hinnebush A, Lambowitz AM, Gunsalus IC, Hollaender A (eds) Extrachromosomal elements in lower eukaryotes. Plenum Press, New York, pp 5–27
Farré J-C, Araya A (2002) RNA splicing in higher plant mitochondria: determination of functional elements in group II intron from a chimeric cox II gene in electroporated wheat mitochondria. Plant J 29:203–214
Giegé P, Brennicke A (1999) RNA editing in Arabidopsis mitochondria effects 441 C to U changes in ORFS. Proc Natl Acad Sci 96:15324–15329
Gonzalez P, Barroso G, Labarère J (1998) Molecular analysis of the split cox1 gene from the Basidiomycota Agrocybe aegerita: relationship of its introns with homologous Ascomycota introns and divergence levels from common ancestral copies. Gene 220:45–53
Gualberto J-M, Lamattina L, Bonnard G, Weil J-H, Grienenberger J-M (1989) RNA editing in wheat mitochondria results in the conservation of protein sequences. Nature 341:660–662
Groth-Malonek M, Pruchner D, Grewe F, Knoop V (2005) Ancestors of trans-splicing mitochondrial intron support serial sister group relationships of hornworts and mosses with vascular plants. Mol Biol Evol 22:160–174
Handa H (2003) The complete nucleotide sequence and RNA editing content of the mitochondrial genome of rapeseed (Brassica napus L): comparative analysis of the mitochondrial genomes of rapeseed and Arabidopsis thaliana. Nucleic Acids Res 31:5907–5916
Henrick P, Crane PR (1997) The origin and early evolution of plants on land. Nature 389:33–39
Knoop V (2004) The mitochondrial DNA of land plants: peculiarities in phylogenetic perspective. Curr Genet 46:123–139
Kugita M, Yamamoto Y, Fujikawa T, Matsumoto T, Yoshinaga K (2003) RNA editing in hornwort chloroplasts makes more than half of the genes functional. Nucleic Acids Res 31:2417–2423
Lambowitz AM, Zimmerly S (2004) Mobile group II introns. Annu Rev Genet 38:1–35
Lang BF, Laforest M-J, Burger G (2007) Mitochondrial introns: a critical view. Trends Genet 23:119–125
Lazowska J, Jacq C, Slonimski PP (1980) Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell 22:333–348
Lu MZ, Szmidt AE, Wang XR (1998) RNA editing in gymnosperms and its impact on the evolution of the mitochondrial cox1 gene. Plant Mol Biol 37:225–234
Malek O, Lättig K, Hiesel R, Brennicke A, Knoop V (1996) RNA editing in bryophytes and a molecular phylogeny of land plants. EMBO J 15:1403–1411
Michel F, Jacquier A, Dujon B (1982) Comparison of fungal mitochondrial introns reveals extensive homologies in RNA secondary structure. Biochimie 64:867–881
Michel F, Umesono K, Oseki H (1989) Comparative and functional anatomy of group II catalytic intron—a review. Gene 82:5–30
Mohr G, Lambowitz AM (2003) Putative proteins related to group II intron reverse transcriptase/maturases are encoded by nuclear genes in higher plants. Nucleic Acids Res 32:647–652
Nakagawa N, Sakurai N (2006) A mutation in At-nMat 1a, which encodes a nuclear gene having similarity to group II intron maturase, causes impaired splicing of mitochondrial NAD4 transcript and altered carbon metabolism in Arabidopsis thaliana. Plant Cell 47:772–783
Notsu Y, Masood S, Nishikawa T, Kubo N, Akiduki N, Nakazono M, Hirai A, Kadowaki K (2002) The complete sequence of the rice (Oryza sativa L.) mitochondrial genome: frequent DNA acquisition and loss during the evolution of flowering plants. Mol Genet Genomics 268:434–445
Nugent JM, Palmer JD (1991) RNA-mediated transfer of the gene coxII from the mitochondrion to the nucleus during flowering plant evolution. Cell 66:473–481
Oda K, Yamato K, Ohta E, Nakamura Y, Takemura M, Nozato N, Akashi K, Kanegae T, Ogura Y, Kohchi T, Ohyama K (1992) Gene organization deduced from the complete sequence of liverwort Marchantia polymorpha mitochondrial DNA. J Mol Biol 223:1–7
Ohta E, Oda K, Yamato K, Nakamura Y, Takemura M, Nozato N, Akashi K, Ohyama K, Michel F (1993) Group I introns in the liverwort mitochondrial genome: the gene coding for subunit 1 of cytochrome oxidase shares 5 intron positions with its fungal counterparts. Nucleic Acids Res 21:1297–1305
Pryer KM, Schneider H, Smith AR, Cranfilll R, Wolf PG, Hunt JS, Sipes SD (2001) Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature 409:618–622
Qiu YL, Li L, Wang B, Chen Z, Knoop V, Groth-Malonek M, Dombrovska O, Lee J, Kent L, Rest J, Estabrook GF, Hendry TA, Taylor DW, Testa CM, Ambros M, Crandall-Stotler B, Duff RJ, Stech M, Frey W, Quandt D, Davis CC (2006) The deepest divergences in land plants inferred from phylogenomic evidence. Proc Natl Acad Sci USA 103:15511–15516
Saldanha R, Mohr G, Belfort M, Lambowitz AM (1993) Group I and group II introns. FASEB J 7:15–24
Sanchez-Puerta MV, Cho Y, Mower JP, Alverson AJ, Palmer JD (2008) Frequent, phylogenetically local horizontal transfer of the cox1 group I intron in flowering plant mitochondria. Mol Biol Evol 25:1762–1777
Sper-Whitis GL, Russell AL, Vaughn JC (1994) Mitochondrial RNA editing of cytochrome-c-oxidase subunit II (coxII) in the primitive vascular plant Psilotum nudum. Biochim Biophys Acta 1218:218–220
Sper-Whitis GL, Moody JL, Vaughn JC (1996) Universality of mitochondrial RNA editing in cytochrome-c oxidase subunit I (coxI) among land plants. Biochim Biophys Acta 1307:301–308
Steinhauser S, Beckert S, Capesius I, Malek O, Knoop V (1999) Plant mitochondrial RNA editing. J Mol Evol 48:303–312
Terasawa K, Odahara M, Kabeya Y, Kikugawa T, Sekine Y, Fujiwata M, Sato N (2007) The mitochondrial genome of the moss Physcomitrella patens sheds a new light on mitochondrial evolution in land plants. Mol Biol Evol 19:24–38
Unseld M, Marienfeld JR, Brandt P, Brennicke A (1997) The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat Genet 15:57–61
Vangerow S, Teerkorn T, Knoop V (1999) Phylogenetic information in the mitochondrial nad5 gene of pteridophytes: RNA editing and intron sequences. Plant Biol 1:235–243
Vaughn JC, Mason MT, Sper-Whitis GL, Kulman P, Palmer JD (1995) Fungal origin by horizontal transfer of a plant mitochondrial group I intron in the chimeric cox1 gene of Peperomia. J Mol Evol 41:563–572
Vicens Q, Cech TR (2006) Atomic level architecture of group I introns revealed. Trends Biol Sci 31:41–51
Wahleithner JA, Macfarlane JL, Wolstenholme DR (1990) A sequence encoding a maturase-related protein in a group-II intron of a plant mitochondrial nad1 gene. Proc Natl Acad Sci USA 87:548–552
Waring RB, Davies RW, Scazzochio C, Brown TA (1982) Internal structure of a mitochondrial intron of Aspergillus nidulans. Proc Natl Acad Sci USA 79:6332–6336
Wikström N, Pryer KM (2005) Incongruence between primary sequence data and the distribution of a mitochondrial atp1 group II intron among fern and horsetails. Mol Phylogenet Evol 36:484–493
Zhu X-Y, Chase MW, Qiu Y-L, Kong H-Z, Dilcher DL, Li J-H, Chen Z-D (2007) Mitochondrial matR sequences help to resolve deep phylogenetic relationships in rosids. BMC Evol Biol 7:217–231
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Acknowledgments
The authors acknowledge Christophe Hubert for helpful assistance in sequence analysis, and are greatly indebted to Simon Litvak for constant encouragements and support. This research was supported by the Centre National de la Recherche Scientifique, the Université Victor Segalen Bordeaux 2, France and the Ministère de l’Enseignement Supérieur et de la Recherche. DNA Sequencing was performed at the Genotyping and Sequencing Facility of Bordeaux (grants from the Conseil Régional d’Aquitaine no. 20030304002FA and 20040305003FA and from the European Union, FEDER no. 2003227).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Tomaska.
Nucleotide sequence data reported are available in GEN Bank databases under the accession numbers FJ376598 (cox1), FJ376799 (cox2), FJ376600 (cob) and FJ389746 (atp9).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bégu, D., Araya, A. The horsetail Equisetum arvense mitochondria share two group I introns with the liverwort Marchantia, acquired a novel group II intron but lost intron-encoded ORFs. Curr Genet 55, 69–79 (2009). https://doi.org/10.1007/s00294-008-0225-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-008-0225-7