Abstract
Polypropylene (PP) and high-density polyethylene (HDPE) are the recyclable and easily processed thermoplastics which are commonly used as cable insulation materials. The improvement of thermal and structural properties of PP and HDPE is very important for aging processes. For this purpose, powdered waste urea–formaldehyde was added to PP and HDPE at 5 wt%, 10 wt%, 20 wt% and 30 wt% ratios. The thermal, structural and morphological properties of the produced samples were observed by using thermogravimetric differential thermal analyser, oxygen induction time tests, fourier transform infrared spectrophotometry, X-ray diffractometer and scanning electron microscopy. By the results of the relevant study, the thermal and oxidative stability of the composites was improved by the incorporation of WUF into the polymer matrices. In conclusion, powdered urea–formaldehyde can be a cost-effective and good filler for PP and HDPE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introductıon
In recent years, studies on polymer blends show a rapid increase. Mixing of two or more polymers or copolymers can be performed more rapidly and it is cheaper than realization of new polymer chemistry including development of monomer synthesis and polymerization technology [1]. The main goal in the field of polymer composites has been the search for better performing materials with desired properties such as lightweight, high strength and ease of processability, etc. In today’s competitive world, companies have to reduce production costs significantly and manage the increased amount of polymer waste [2]. In order to achieve these goals, recycling, reduction of waste and raw material as well as process costs are essential. In many applications, thermoset plastics are cross-linked. The current economic crisis has led to increased competition among companies that have to significantly reduce production costs to maintain competitiveness [3]. These reductions should be found in raw materials, waste reduction, process optimization, etc.
The dependence on oil prices, as well as the economic crisis, has led many companies to use recycled materials to obtain raw materials at a more stable price. In addition, environmental benefits are obtained by reusing waste materials [4].
In many applications, thermoset plastics are the preferred materials for long-term use thanks to they are insoluble and infusible high-density networks. In recent years, the increased production of thermoset composites has greatly increased the amount of waste material. The recycling of thermoset polymers is considered one of the urgent problems that must be solved because of its technological difficulty. However, since they are difficult to degrade in nature, they must be recycled [5].
Urea formaldehyde (UF) is produced from urea and formaldehyde. UF accounts for about 15% of the total thermoset resin production. The good thermal, chemical and mechanical stability of urea formaldehyde is a great advantage, but it is also a difficult plastic to recycle. One of its major applications is in molded objects, including electrical appliances (electric switches and sockets, group sockets, accessories, low-voltage switchgear products, fuse boxes, electronic electricity meters, automated meter reading systems, smart house and building automation systems, led lighting products, professional hand tools, solar energy panels, video surveillance systems and fire detection systems), dinnerware, cosmetic caps and bottles UF powder is lighter and cheaper than other traditional fillers and can be used effectively in composites with thermosets [6]. UF is used as a plywood and particle board adhesive. Urea formaldehyde resins are poly-condensation products of urea and formaldehyde in an alkaline or neutral or acid or alkaline/acid environment. Urea formaldehyde are heat-hardened polymers that make up more than 80% of amino resins, mostly used as molding material, adhesive and protective coating components. Low price, good technological property, lack of color in cured polymer, low curing temperature, resistance to microorganisms and abrasion, hardness, good thermal properties and ease of application are the superior qualities of urea formaldehyde [7,8,9].
PP is widely used in many fields such as automobiles, household appliances and electrical parts owing to its excellent formability, high corrosion resistance, balanced mechanical properties and low cost [10, 11]. It exhibits an attractive combination of filled polypropylene, low-cost, low-weight materials and better properties [12,13,14,15].
Without adding a compatibilizer, the properties of the composites are usually lower because the different polymers are not compatible. Compatibilizer increases the contact area between particles and the polymer matrix, increase dispersion, and improve the adhesion of the two components to the interface. etc. [5]. For example, maleic anhydride-grafted polypropylene (MAPP) is one of the most common compatibilizers. In this study, MAPP is used as a compatibilizer to produce PP/WUF/MAPP composites.
HDPE is widely used with high tonnage production on account of its unique mechanical and physical properties. Its application has been limited in many areas because of its low toughness weather resistance, and environmental stress cracking resistance as compared to engineering polymers. In order to improve these disadvantages, they are strengthened by adding fillers such as WUF.
Bliznakov et al. [8], investigated the mechanical properties of HDPE/UFG polymer composites. In their study, urea–formaldehyde powder were evaluated in the range of 0–23% of filler by volume and ethylene–acrylic acid (EAA) copolymers and an ionomer based on EAA were evaluated as compatibilizers. As the UFG concentration was increased, both the tensile strength and elongation at break decreased. The study showed that recycled thermosetting filler, UFG (urea–formaldehyde grit) could be a cost-effective and appropriate filler for HDPE.
The present study also shows the ways of using recycled materials to obtain raw materials at a more stable price. Environmental benefits are also obtained by reusing waste materials. Papers relating to PP/WUF and HDPE/WUF polymer composites are limited and from the open literature, there have not been any studies reporting thermomechanical properties using, TG/DTA, Oxygen Induction Time (OIT) Tests.
This study was focused on two main objectives: investigating the effects of waste urea formaldehyde ratio on thermal, structural, and morphological properties of (1) PP/WUF/MAPP, (2) HDPE/WUF polymer composites. PP and HDPE are widely used in wires and cables for insulation and sheathing. So, PP and HDPE are chosen as polymer matrices. As it is known that using thermoset filler as reinforcement into thermoplastic matrices is hard work. There are very few studies in the literature about the recycling of urea–formaldehyde resin scrap, which is especially used in industry. The use of urea formaldehyde powders in this study also enables this waste product to be reused at almost zero cost. Compared to other additives, it also reduces the cost of the resulting mixture.
Experimental study
Materials
Ten different polymer blends were prepared. Compositions of PP/WUF/MA-g-PP polymer blends and HDPE/WUF that were formed are given in Table 1. Maleic anhydride grafted polypropylene (MA-g-PP) was evaluated as compatibilizer by the addition of 5 wt%. MA-g-PP) under the trade name Bondyram 1001 CN procured from Polyram Plastic Industries LTD, Israel, is used as the coupling agent. Its density is 0.90 g/cm3, MFI value is 100 g/10 min (D-1238, 190 °C/2.16 kg) and its melting point is 160 °C.
Urea formaldehyde (UF) is a nontransparent thermosetting resin or polymer formed by the polycondensation reaction of urea (U) and formaldehyde (FA) [16]. It was seen that the WUF used in the study consisted of particles ranging from 10 to 660 µm in the mastersizer (Malvern Hydro 2000MU, United Kingdom) measurement and its density was 1.39 g/cm3 in the helium pycnometer (Ultrapyc 1200e Helium Pycnometer; Quantachrome Instruments) measurement.
PP (Moplen EP 3307) supplied by Lyondell Basell. Its density is 0.900 g/cm3, MFI value is 15 g/10 min (230 °C, 2.16 kg) and its head deflection temperature (0.45 MPa, unannealed) is 95.0 °C. High-density polyethylene (HDPE) (I-668 UV) was supplied by Petkim (Izmir-Turkey). Specific gravity is 0,970 g/cm3. Melt flow rate is 5.2 g/10 min (190 °C–2.16 kg). Yield strength is 28 MPa and notched Izod impact (23 °C) is 12 kJ/m2.Waste ure formaldehyde was supplied Viko by Panasonic Company (Istanbul-Turkey).
Sample preparation
UF was dried in a Yamato vacuum oven ADP-31 (Yamato/VWR Scientific Products, Japan) at 105 °C for 24 h before being blended with PP. Mechanical premixing of solid compositions was done using a LB-5601 liquid-solids blender (The Patterson-Kelley Co., Inc.East Stroudsburg, PA-USA) brand batch blender for 15 min. Samples with various proportions of PP or HDPE/WUF polymer composites were produced between 180 and 220 °C at 20–30 bar pressure, and a rotation rate of 25 rpm, with a Microsan co-rotating twin-screw extruder (Microsan Instrument Inc. Kocaeli- Turkey). L/D ratio is 30, ø = 25 mm, Polymer composites were also dried in vacuum oven at 105 °C for 24 h after extrusion. Subsequently, test samples were molded in injection molding machine. Extrusion and injection conditions are given in Table 2.
In order for Urea–Formaldehyde (UF) scraps to be dispersed homogeneously in PP and HDPE polymers, they must be ground to the smallest possible grain (powder) size. Waste urea foraldehyde dry grinded with Siemens simatic C7-621 control system device to obtain unsegregated powders. The size of urea formaldehyde particles varied between 10 and 80 µm.
Thermal gravimetric analysis (TGA)
Thermal stability of the samples was measured in EXSTAR TG/DTA 6300 (Seiko, Tokyo, Japan). 5–10 mg of samples were placed in aluminum crucible under nitrogen atmosphere (flow rate 200 mL/min) and heated from room temperature to 550 °C at a heating rate of 10 °C/min.
Oxidation induction times (OIT) tests
OIT tests were performed using the EXSTAR TG/DTA 6300 (Seiko, Tokyo, Japan). Sample was heated up under a nitrogen atmosphere (flow rate 200 mL/min), typically to 200 °C at a heating rate of 5 °C/min. Then, oxygen was introduced to the sample cell and the sample was kept at 200 °C for 50 min under oxygen atmosphere (flow rate 200 mL/min) and the length of time before the onset of degradation was measured when an exothermic process was observed in the TGA trace.
Fourier transform infrared (FTIR) spectroscopy
The structural characteristics of the tested materials were evaluated by FTIR spectroscopy was performed using a FT-IR Spectrum100 (Perkin Elmer, Waltham, MA). The samples were scanned range the wave numbers 600–4000 cm−1 in the transmittance mode using a resolution of 2 cm−1 and 16 scans.
X‑ray diffraction (XRD) analysis
A Panalytical X’Pert PRO X-ray diffractometer (Malvern, United Kingdom) with CuKa (λ = 1.5418 Å) radiation of XRD system operated at 45 kV and 40 mA at room temperature was used for investigated the structural characterizations of composites.
Surface morphology
SEM images were taken to examine the effect of filler content, interfacial bond strength, and the distribution of microstructure by using the JEOL-JSM 5910 LV (JEOL Ltd., Tokyo, Japan) scanning electron microscopy (SEM) at an acceleration voltage of 5–20 kV. The fractured surfaces of the samples were coated with thin layer (approximately 10 nm) of gold (Au) (80%)/palladium (Pd) (20%) by Polaron SC 7620 (Gala Instrumente GmbH, Bad Schwalbach-Germany).
Results and discussion
Insulation materials are used to prevent the conductive wires used in electrical transmission from coming into contact with each other, short circuits, and to protect the cables from external factors. Different insulation materials are used according to the area and place of use of the cable. PP and HDPE are one of them [17, 18]. These materials are not used directly in the cable insulation system because of their low thermal and mechanical strength. For this reason, PP and HDPE are blended with different materials to improve their thermal and mechanical properties [18]. In this study, thermal and structural properties of PP and HDPE blended with different ratios of WUF (0–30 wt%) were investigated, and it was aimed to develop these properties with low cost. The surface resistances of the produced composites were measured with the Twintex LCR-T610 high-precision meter and were seen to be high enough to exceed the measuring range (0.00001 Ω ~ 99.9999 MΩ) of the device.
FTIR spectroscopy was used to determine chemical composition and structural configuration [19] of composites prepared by mixing different concentrations (0–30 wt%) of WUF with HDPE and PP polymers. The position and intensities of the transmittance bands give information concerning the interaction between the components of composites. The band at 2920 cm−1 in the FTIR spectrum of powder WUF correspond to the C–H stretching of WUF. The band at 1640 cm−1 indicates the C=O stretching of the primary amide. The band at 1028 cm−1 is attributed to the C–N stretching of the methylene bond (NCH2N) [20, 21]. The FTIR spectrum of PP/WUF composites mixed in different ratios in Fig. 1 was exactly the same as reported literature data. The band seen at 840 cm−1 in the PP spectrum corresponds to the C–CH3 stretching vibration. The absorption band appeared at 972, 997, and 1165 cm−1, respectively, represent –CH3 rocking vibration. The band at 1375 cm−1 in the spectrum corresponds to the symmetric bending vibrational mode of the –CH3 group. The band seen at 2952 cm−1 represents the –CH3 asymmetric stretching vibration. All these peaks indicate the presence of the methyl group in the polypropylene. The peaks correspond to –CH2– symmetric bending, –CH2– symmetric stretching, and –CH2– asymmetric stretching in polypropylene were observed at 1455, 2838, and 2917 cm−1, respectively [22,23,24,25]. As an increasing amount of WUF was mixed into the PP, a broad band of increasing intensity began to appear at 1640 cm−1 correspond to the C=O stretching, and at 3330 cm−1 correspond to the hydroxyl (–OH) stretching of the methylol group. The band at 1551 cm–1 in the FTIR spectrum of 30 wt% WUF mixed PP composite was due to NH bending and CN stretching. The observed band at 2950 cm−1 corresponds to the asymmetrical -CH stretching of –CH2OH. The observed band at 2950 cm−1 and in the regions around 1400–1360 cm–1 correspond to the asymmetrical –CH stretching and CH2OH, CH3, and CN, respectively [11]. As can be seen from the results obtained, mixing WUF to PP at increasing rates changed the intensities of the PP’s bands and led to the emergence of new bands in the spectrum that would be attributed to WUF. This indicates that there is an interaction between PP and WUF.
Figure 2 shows the FTIR spectra of HDPE mixed with different ratios (0–30 wt%) of WUF. The bands observed at 2915 and 2848 cm−1 in the HDPE spectrum correspond to asymmetric and symmetric stretching vibrations of the methylene (–CH2–) groups. The band at 1472 represents the bending deformation of the –CH2– group. Also, the band at 718 cm−1 corresponds to the rocking vibration of the CH2 groups [7] As can be seen from the results obtained, similar to that seen in the FTIR spectrum of PP/WUF composites, the mixing of WUF at increasing ratios to HDPE caused changed the intensity of the bands of HDPE and the emergence of new bands in the spectrum to be attributed to WUF. This indicates an interaction between HDPE and WUF.
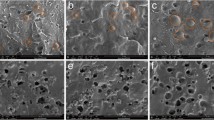
For polymer blends, the study of microscopic morphology plays an important role in determining macroscopic properties. The all composites were frozen in liquid nitrogen for 30 min and then immediately broken and their surfaces examined in SEM. Figure 3a–d show SEM images of WUF and fractures surfaces of PP composites containing 0 wt%, 5 wt%, and 30 wt% WUF. As seen from Fig. 3a, WUF has a granular structure with inhomogeneous dimensions. Dents were observed on the surface of pure PP (Fig. 3b) [26]. As the WUF ratio increased, the number of filled dents in the PP increased (Fig. 3c–d). The improvement of the thermal properties of PP can be attributed to the filling of dents with WUF [27].
Figure 4a–d shows SEM micrographs of WUF and fractures surfaces of HDPE composites containing 0 wt%, 5 wt%, and 30 wt% WUF. Figure 4b shows the surface morphology of pure HDPE. As can be seen from the figure, there are large holes on the surface. Micrographs show that WUF particles fill the holes on the surface of the HDPE polymer. It is seen that as the WUF ratio in the composite increased, the number of filled holes on the surface also increased (Fig. 4c–d) [28]. It has been reported by studies that the thermal properties of polymer matrix composites change with additives [29,30,31,32]. WUF's filling in the holes improved the thermal properties of HDPE as in PP.
XRD was used to examine the effect of the presence of WUF on the structural properties of PP and HDPE. Figure 5 shows the XRD patterns of pure PP, powder WUF and PP composites containing 10 and 30 wt% WUF. The 2θ degrees for the peaks were 22.18°, 24.54°, 31.57° and 40.39°, corresponding to the (110), (011), (020) and (121) planes were observed in the XRD spectrum of the WUF (ICDD Card no: 98-001-5432). The peaks at 2θ = 13.9°, 16.6°, 18.69°, 21.25° and 21.7° corresponding to the (110), (040), (130), (131) and (041) crystal planes of the PP crystal, were observed in the XRD pattern of PP [33]. Mixing WUF into PP caused a shift in peak towards a larger 2θ angle, which means a change in the crystal parameters [34]. In the PP composite mixed with 30 wt% WUF, the peak was observed at 2θ = 21.6°. This shows the presence of WUF in PP [35].
From XRD analysis, crystallite size (D) was calculated using the Scherrer formula in Eqs. (1), as follows:
where β is half width of the crystalline peak, λ is the wave length of the X-ray radiation (1.54 Å with CuKα radiation), and k is the Scherrer constant having a value of 0.9 [36]. Pure PP and 10 wt% WUF/PP composite had the same crystallite size (24.34 nm). The crystallite size of 30 wt% WUF/PP composite was 24.35 nm (Table 3). An increase in crystal size means that some crystal defects are removed [37]. It can be attributed that mixing WUF into PP creates a better crystal structure.
The XRD patterns for pure HDPE, powder WUF and HDPE composites containing 10 and 30 wt% WUF are shown in Fig. 6. The XRD pattern showed peaks appearing at 2θ = 21.5°, 23.9°, and 30.0° correspond to the inter planner spacing of 4.132 Å, 3.707 Å and 2.481 Å, respectively, which correspond to the (110), (200) and (020) lattice planes [38]. Another peak appeared at 36.2° in HDPE’s XRD pattern. Some small peaks appeared at 2θ angles greater than 40 in the amorphous region showing the semi-crystalline nature of HDPE. Mixing WUF into HDPE caused a shift in peak towards a larger 2θ angle as in PP composites means that there was a change in the crystal parameters [39]. Mixing WUF into HDPE reduced the crystallite size (Table 4). However, the crystallite size did not change as the ratio of the mixture concentration increased. In the literature, it has been reported that the crystallite size of HDPE composites is smaller than unfilled HDPE due to multiple nucleation sites in composites. This was in compatible with the results calculated from the XRD spectrum [40].
The crystallinity of materials provides important information for determining their mechanical, electrical and physical properties. The change in percent crystallinity of the produced composites was calculated using the following equation [33]:
Figure 7 shows the percent crystallinity values calculated from Eq. 2. The percentage of crystallinity of WUF calculated was 99.23%. While the calculated percentage of crystallinity of PP was 94.88%, that of 10 wt% WUF blended PP was 97.57% and that of 30 wt% WUF blended PP was 98.18%. The percent crystallinity of HDPE was calculated as 89.50%. With increasing WUF mixing ratio, the percent crystallinity of 10 and 30 wt% WUF mixed HDPE composites was calculated as 94.31% and 94.36%, respectively. The percent crystallinity of the composites is proportional to the crystallinity of the mixed materials [41]. Mixing high crystalline WUF with PP and HDPE increased the percentage crystallinity.
TGA analysis was performed to determine the effect of WUF on the thermal properties of PP and HDPE (Figs. 8 and 9). As can be seen in the TGA curve of WUF, thermal degradation occured in three stages. In the first stage, the weight loss (9.21%) in the range of 25–150° was mainly attributed to the evaporation of the natural moisture of the WUF and the elimination of free formaldehyde monomer. The second degradation stage between 150 and 250 °C is the decomposition of hydroxyl-methyl and methylene group [42]. The mass loss at this stage was 15.08%. In the final stage, the degradation (51.26%) in the range of 250–800° that occurred was due to the dissociation of methylene and methylene ether bonds and removal of the elements N, H, and O [43, 42]. In Fig. 8 while PP showed a single degradation step in the range of 30–500 °C (99.26%), the degradation step increased as the ratio of WUF in the composite increased. About 30 wt% WUF mixed PP showed three degradation steps. In the curve of composite mixed with 30 wt% WUF, the low mass loss around 100 °C (12.99%) and 304 °C (7.76%) is due to the degradation of WUF, while the large mass loss around 368 °C (72.90%) is due to the degradation of PP. The degradation of WUF began with the gradual release of free formaldehyde. It then continued with the separation of carbon-hydrogen (C–H), carbon–oxygen (C–O), carbon–carbon (C–C), and hydrogen–oxygen (O–H) bonds between the composite and UF, respectively [44]. Weight losses with increasing temperature were 99.26% for pure PP, 98.56% for 5.0 wt% PP/WUF composite, 97.08% for 10.0 wt% PP/WUF composite, 96.04% for 20.0 wt% PP/WUF composite and 94.08% for 30.0 wt% PP/WUF composite in the range of 30–500 °C. These results showed that WUF improved the thermal stability of PP.
The TGA measurement result made to determine the effect of WUF on the thermal properties of HDPE is shown in Fig. 9. The pure HDPE showed a single degradation step in the range of 30–500 °C. The composite mixed with 30 wt% WUF showed three degradation steps. While the low mass loss around 120 °C (12.24%) and 312 °C (6.36%) were due to the degradation of WUF, the large mass loss around 400 °C (74.99%) were due to the degradation of HDPE. Weight losses with increasing temperature were 99.07% for pure HDPE, 98.61% for 5.0 wt% HDPE/WUF composite, 97.54% for 10.0 wt% HDPE/WUF composite, 95.87% for 20.0 wt% HDPE/WUF composite and 86.02% for 30.0 wt% HDPE/WUF composite in the range of 30–500 °C. These results showed that WUF improved the thermal stability of HDPE. It is known that the thermal stability of polymers depends on their crystallinity. High crystallinity significantly improves thermal stability [45]. The increased crystallinity by mixing WUF into PP and HDPE resulted in increased thermal stability. Obtained results are compatible with TGA measurements.
Insulation materials used for cable systems should be stabilized as much as possible against oxidation and environmental effects [46]. In order to increase stability, the voids in insulation materials are filled with various fillers. The OIT test, which is a sensitive measure of the level of anti-oxidative additives within the polymer, is accepted as the standard for the stability requirements of insulation materials in industrial applications [47, 48]. OIT test was used to examine the thermo-oxidative performance of the composites. As seen in Fig. 10, the OIT value of PP was 1.07 min, while the OIT value was 3.14 min when 30.0 wt% WUF was added. According to the results obtained, the OIT value of PP increased when WUF was added to the composition. It is known from the literature that an increase in the degree of crystallinity decreases the degradation rate of the material. Degradation occurs in the amorphous regions of semicrystalline polymers. Mixing WUF into PP resulted in an increase in crystallinity, which means a decrease in its amorphous content [49]. Thus, the increase in the crystallinity of the composites prepared by mixing WUF caused an improvement in the degradation of the composites compared to PP.
As seen in Fig. 11, the OIT value of HDPE was 1.09 min, while the OIT value was 1.55 min when 30.0 wt% WUF was added. According to the results obtained, the oxidative stability of the composites improved when WUF was added to the composition. The improvement in oxidative stability was attributed to the filling of the voids in the polymers with WUF. As with PP, mixing WUF into HDPE resulted in an increase in crystallinity. This also improved the degradation time of HDPE. However, the degradation time of PP was longer than HDPE. This may be due to morphological features. The number of voids filled in the polymer can affect the degradation time [48].
Conclusions
This study was carried out to examine the effect of WUF, which is a low-cost material, on the thermal and oxidative stability, structural and morphological properties of PP and HDPE used as cable insulation material. Composites were prepared by mixing 0–30 wt% WUF into PP and HDPE and characterized. XRD and FTIR measurements were used to show the presence of WUF in the prepared composites. As a result of the measurements, peaks belonging to WUF were seen in the FTIR and XRD spectra. It was observed that the peaks of the polymers affected their structure by causing shifts in their intensity and location. Morphological examination using SEM revealed that WUF filled the voids in PP and HDPE. TGA and OIT tests were used to examine the effect of WUF on the thermal and oxidative stability of polymers. In the tests, it was seen that WUF improved the thermal and oxidative stability of the polymers. This result was attributed both to the increased degree of crystallinity and to the improved morphology with the filling of voids in the polymers by WUF. Thus, according to the results obtained, the structural and morphological properties and thermal and oxidative stability improved by mixing WUF allow the production of more fire resistant polymer composites. WUF, which is a waste material, provides a great advantage in terms of production cost of cable insulation materials [49].
References
Zurina M, Ismail H, Ratnam CT (2007) Characterization of unvulcanized and dynamically vulcanized ENR-50/EVA blends. Polym Plast Technol 47:1–12. https://doi.org/10.1080/03602550701575938
Wang W, Yang S, Liu H et al (2022) A novel multifunctional fertilizer derived from wasted straw: synthesis, characteristics and agriculture applications. Ind Crops Prod 176:114308. https://doi.org/10.1016/J.INDCROP.2021.114308
Peydro MA, Parres F, Crespo JE, Navarro R (2013) Recovery of recycled acrylonitrile–butadiene–styrene, through mixing with styrene–ethylene/butylene–styrene. J Mater Process Technol 213:1268–1283. https://doi.org/10.1016/J.JMATPROTEC.2013.02.012
Mohamad Z, Ismail H, Chantara Thevy R (2006) Characterization of epoxidized natural rubber/ethylene vinyl acetate (ENR-50/EVA) blend: effect of blend ratio. J Appl Polym Sci 99:1504–1515. https://doi.org/10.1002/APP.22154
Thomas R, Vijayan P, Thomas S (2012) Recycling of thermosetting polymers: their blends and composites. Recent Dev Polym Recycl 121:153
Panasonic life solutions | About us. https://lstr.panasonic.com/en/corporate/about-us-1/. Accessed 13 Mar 2022
Matuana LM, Jin S, Stark NM (2011) Ultraviolet weathering of HDPE/wood-flour composites coextruded with a clear HDPE cap layer. Polym Degrad Stab 96:97–106. https://doi.org/10.1016/j.polymdegradstab.2010.10.003
Bliznakov ED, White CC, Shaw MT (2000) Mechanical properties of blends of HDPE and recycled urea–formaldehyde resin. J Appl Polym Sci 77:3220–3227. https://doi.org/10.1002/1097-4628
Akpabio UD (2010) Retention of urea–formaldehyde resin finish on cellulosic fabric and its effect on water absorbent capacity of the treated fabric. Int J Adv Sci Tech Res 2(5):31–39
Luongo JP (1960) Infrared study of polypropylene. J Appl Polym Sci 3:302–309. https://doi.org/10.1002/app.1960.070030907
Sakai K, Sobue H (1972) Study of structure and thermal properties of polypropylene and chlorinated polypropylene by infrared spectroscopy and differential scanning calorimetry. J Appl Polym Sci 16:2657–2670. https://doi.org/10.1002/app.1972.070161018
Cran MJ, Bigger SW (2003) Quantitative analysis of polyethylene blends by fourier transform infrared spectroscopy. Appl Spectrosc 57(8):928–932
Karian HG (2003) Handbook of polypropylene and polypropylene composites. Marcel Dekker, New York
Karger-Kocsis J (1995) Polypropylene: structure, blends and composites. Chapman & Hall, London
Kaempfer D, Thomann R, Mülhaupt R (2002) Melt compounding of syndiotactic polypropylene nanocomposites containing organophilic layered silicates and in situ formed core/shell nanoparticles. Polym (Guildf) 43:2909–2916. https://doi.org/10.1016/S0032-3861(02)00113-1
Samaržija-Jovanović S, Jovanović V, Jovanović T et al (2022) Hydrolytic, thermal and radiation stability of modified urea–formaldehyde composites: influence of montmorillonite particle size. Int J Adhes Adhes 115:103131. https://doi.org/10.1016/J.IJADHADH.2022.103131
Dao NL, Lewin PL, Hosier IL, Swingler SG (2010) A comparison between LDPE and HDPE cable insulation properties following lightning impulse ageing. In Proceedings 2010 IEEE international conference on solid dielectrics ICSD
Cao X, Andritsch T (2019) Modification of polypropylene-based cable insulation material. DEStech Trans Eng Technol Res. https://doi.org/10.12783/DTETR/EEEC2018/26871
Benli M, Gümüş BE, Kahraman Y et al (2021) Thermal, structural and morphological characterization of dental polymers for clinical applications. J Prosthodont Res 65:176–185. https://doi.org/10.2186/JPR.JPOR_2019_534
Chiang TC, Hamdan S, Osman MS (2016) Urea formaldehyde composites reinforced with sago fibres analysis by FTIR, TGA, and DSC. Adv Mater Sci Eng. https://doi.org/10.1155/2016/5954636
Luo J, Zhang J, Luo J et al (2015) Effect of melamine allocation proportion on chemical structures and properties of melamine-urea-formaldehyde resins. BioResources 10:3265–3276. https://doi.org/10.15376/BIORES.10.2.3265-3276
Gumu BE, Yagci O, Erdogan DC, Tademir M (2019) Dynamical mechanical properties of polypropylene composites filled with olive pit particles. J Test Eval 47:2551–2561. https://doi.org/10.1520/JTE20180198
Gopanna A, Mandapati RN, Thomas SP et al (2019) Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and wide-angle X-ray scattering (WAXS) of polypropylene (PP)/cyclic olefin copolymer (COC) blends for qualitative and quantitative analysis. Polym Bull 76:4259–4274. https://doi.org/10.1007/s00289-018-2599-0
Andreassen E (1999) Infrared and Raman spectroscopy of polypropylene. Springer, Dordrecht, pp 320–328
Prasad A (1998) A quantitative analysis of low density polyethylene and linear low density polyethylene blends by differential scanning calorimetery and fourier transform infrared spectroscopy methods. Polym Eng Sci 38:1716–1728. https://doi.org/10.1002/pen.10342
Perumal Ramasamy R, Yang K, Rafailovich MH (2014) Polypropylene-graphene-a nanocomposite that can be converted into a meta-material at desired frequencies. RSC Adv 4:44888–44895. https://doi.org/10.1039/C4RA05814C
Kodal M, Üniversitesi K, Mühendisliği Bölümü K (2018) Dinamik olarak vulkanize edilmiş PP/SR harmanlarının mekanik, ısıl ve morfolojik özelliklerinin incelenmesi. Dicle Üniv Mühendis Fak Mühendis Derg 9(1):325–335
Lapčík L, Vašina M, Lapčíková B et al (2020) Study of the material engineering properties of high-density poly(ethylene)/perlite nanocomposite materials. Nanotechnol Rev 9:1491–1499. https://doi.org/10.1515/NTREV-2020-0113/MACHINEREADABLECITATION/RIS
Szczepaniak R, Komorek A, Przybyłek P et al (2022) Research into mechanical properties of an ablative composite on a polymer matrix base with aerogel particles. Compos Struct 280:114855. https://doi.org/10.1016/J.COMPSTRUCT.2021.114855
Shin YK, Lee WS, Yoo MJ, Kim S (2013) CERAMICS Effect of BN filler on thermal properties of HDPE matrix composites. Ceram Int 39:S569–S573. https://doi.org/10.1016/j.ceramint.2012.10.137
Vaggar GB, Sirimani VB, Sataraddi DP, Hiremath NM, Bhajantri F (2021) Effect of filler materials on thermal properties of polymer composite materials: a review. Int J Eng Res Technol (IJERT) 10:08
Abdelrazek EM, Elashmawi IS (2008) Characterization and physical properties of CoCl2 filled polyethyl-methacrylate films. Polym Compos 29:1036–1043. https://doi.org/10.1002/PC.20481
Yağci Ö, Eker Gümüş B, Taşdemir M (2020) Thermal, structural and dynamical mechanical properties of hollow glass sphere-reinforced polypropylene composites. Polym Bull. https://doi.org/10.1007/s00289-020-03257-6
Niu P, Liu B, Wei X et al (2011) Study on mechanical properties and thermal stability of polypropylene/hemp fiber composites. J Reinf Plast Compos 30:36–44. https://doi.org/10.1177/0731684410383067
Liu M, Thirumalai RVKG, Wu Y, Wan H (2017) Characterization of the crystalline regions of cured urea formaldehyde resin. RSC Adv 7:49536–49541. https://doi.org/10.1039/c7ra08082d
Jain K, Madhu G, Bhunia H et al (2015) Physico-mechanical characterization and biodegradability behavior of polypropylene/poly(L-lactide) polymer blends. J Polym Eng 35:407–415. https://doi.org/10.1515/POLYENG-2014-0179/PDF
Chatterjee A, Kumar S, Singh H (2020) Tensile strength and thermal behavior of jute fibre reinforced polypropylene laminate composite. Compos Commun 22:100483. https://doi.org/10.1016/J.COCO.2020.100483
Gümüş BE, Yağci Ö, Taşdemir M (2022) High-density polyethylene/artichoke leaf powder polymer composites: dynamic mechanical, morphological and thermal properties. Iran Polym J 1:1–11. https://doi.org/10.1007/S13726-022-01031-1
Benabid F, Kharchi N, Zouai F et al (2019) Impact of co-mixing technique and surface modification of ZnO nanoparticles using stearic acid on their dispersion into HDPE to produce HDPE/ZnO nanocomposites. Polym Polym Compos 27:389–399. https://doi.org/10.1177/0967391119847353
Olesik P, Godzierz M, Kozioł M et al (2021) Structure and mechanical properties of high-density polyethylene composites reinforced with glassy carbon. Materials 14(14):4024. https://doi.org/10.3390/MA14144024
Prabowo I, Nur Pratama J, Chalid M (2017) The effect of modified ijuk fibers to crystallinity of polypropylene composite. IOP Conf Ser Mater Sci Eng. https://doi.org/10.1088/1757-899X/223/1/012020
Chen K, Cheng X, Chen Y et al (2021) Thermal degradation kinetics of urea–formaldehyderesins modified by almond shells. ACS Omega 6:25702. https://doi.org/10.1021/ACSOMEGA.1C03896
Yunus NYM, Abdullah ZA (2019) Analysıs of urea formaldehyde and urea-formaldehyde-acrylamıde resıns usıng thermogravımetrıc method. Gading J Sci Technol 2:1–8
Wahab R, Dom SMM, Mustafa MT et al (2015) Properties of empty fruit bunch oil palm (Elaeis guineesis) composite boards at different densities and resin contents. J Plant Sci 10:179–190. https://doi.org/10.3923/jps.2015.179.190
Li J, Wang Y, Wang X, Wu D (2019) Crystalline characteristics, mechanical properties, thermal degradation kinetics and hydration behavior of biodegradable fibers melt-spun from polyoxymethylene/poly(l-lactic acid) blends. Polymer (Basel). https://doi.org/10.3390/POLYM11111753
Schmid M, Affolter S (2003) Interlaboratory tests on polymers by differential scanning calorimetry (DSC): determination and comparison of oxidation induction time (OIT) and oxidation induction temperature (OIT). Polym Test 22:419–428. https://doi.org/10.1016/S0142-9418(02)00122-8
Oxidative induction times (OIT) testing by differential scanning calorimetry. https://www.intertek.com/analytical-laboratories/oit-dsc/. Accessed 13 Mar 2022
US7238765B2 - High density polyethylene and insulation compositions for wire and cable - Google Patents. https://patents.google.com/patent/US7238765B2/en. Accessed 12 Mar 2022
Jenkins MJ, Harrison KL (2008) The effect of crystalline morphology on the degradation of polycaprolactone in a solution of phosphate buffer and lipase. Polym Adv Technol 19:1901–1906. https://doi.org/10.1002/PAT.1227
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yağci, Ö., Eker Gümüş, B. & Taşdemir, M. Thermal, structural and morphological properties of polypropylene and high density polyethylene polymer composites filled with waste urea formaldehyde. Polym. Bull. 80, 4005–4022 (2023). https://doi.org/10.1007/s00289-022-04245-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04245-8