Abstract
Here, edible films were prepared by using levan biopolymer and different proportions of powdered ostrich eggshell. These films were characterized, and their bioactivity was measured. Adding ostrich eggshells to the levan films made the film surface smoother. Ostrich eggshell added to the films reduced the water vapor permeability in the films. Levan biopolymer film and ostrich eggshell showed high antioxidant activity when used together (%83.03). The ERL sample without ostrich eggshell has an antimicrobial effect only on bacteria. The highest antimicrobial effect was measured on Pseudomonas aeruginosa with the film sample EROL-6 containing 1.2 g of ostrich eggshell. ERL film samples inhibited the biofilm of Pseudomonas aeruginosa by 68.6%. This is the first study using ostrich eggshells to produce edible film.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Levan is an exopolysaccharide in which β-(2,6) bonds within fructose monomers are linked to form polymers with occasional β-(2,1) branching [1]. Levan is a water-soluble biopolymer that can be produced by various bacteria and plant species [2,3,4]. Levan has been widely applied in medical industry as anti-cholesterol, antidiabetic, antimicrobial, antiviral, antibiofilm, antioxidant, anti-inflammatory and antitumor [3, 5, 6]. In addition to its medical importance, levan has a valuable potential to be used as an edible film or coating in the food industry [7, 8].
Ostriches are the largest living and non-flying winged birds in the world today [9]. Ostrich eggs average 1500–1800 g in weight. This weight is approximately 1.2% of the body weight of the animal. Ovulation occurs every two days [10]. Ostrich eggs are generally 14–18 cm long and 12–15 cm wide and resemble ellipse. The shell is very hard and durable enough to bear a weight of 250 kg. The mechanical resistance of the shell surrounding the egg is approximately 55 kg/cm2 [11]. Eggshell color is bright white and porous in all species. Shell thickness is 2–3 mm, weight is 232–243 g, surface area is 20651 mm2, and its volume is around 3,284,653 mm3 [10]. A female ostrich is able to produce approximately 60 eggs per year [12]. Regarding the composition, 97% of an ostrich eggshell is of mineral origin distributed among 97.4% calcium carbonate-calcite, 1.9% magnesium phosphate and 0.7% tricalcium phosphate [13].
Eggshell is produced several tons per day and also requires high management costs to dispose of. Therefore, it is very important economically to evaluate and dispose of this waste material [14]. Eggshell waste is used as a solid base catalyst in the production of biodiesel, to reduce the production costs of biodiesel, to remove heavy metals from wastewater, in biomaterial compositions designed as bone tissue, as fertilizer and calcium supplement in human, animal and plant nutrition [15,16,17,18]. So far, to the best of our knowledge, no research has been conducted on the use of ostrich eggshells to produce edible biocomposite films.
Ostrich eggshells can be used in levan-based edible films. This study focused on the preparation of edible film from powdered ostrich eggshell with levan synthesized by Pseudomonas mandelii. The films were characterized, and the antimicrobial, antioxidant and antibiofilm properties of the films were evaluated (Fig. 1). For the first time, with this study, a edible film was obtained using powdered ostrich eggshell.
Material and methods
Materials
The broken eggshells of newly hatched juvenile ostriches were obtained from the farm (Ankara, Turkey). Ostrich eggshells were cleaned with sterile distilled water three times, and inner shell membranes were removed. The cleaned eggshells were grounded and powdered at Hacettepe University Geology Engineering Department. Levan biopolymer was microbially produced by Pseudomonas mandelii cultures as described [3]. Glycerol, acetic acid, nutrient broth, nutrient agar, Sabouraud dextrose agar, Sabouraud dextrose broth were all obtained from Sigma-Aldrich. Cultures of bacteria (Escherichia coli 25,922, Staphylococcus aureus subsp. aureus 29,213, Pseudomonas aeruginosa, Klebsiella oxytoca, Klebsiella pneumoniae, Streptococcus pneumoniae 6303, Shigella sonnei, Acinetobacter baumanii calcoaseticus complex, Methicillin sensitive Staphylococcus aureus (MSSA), Klebsiella pneumoniae 1705, Pseudomonas aeruginosa 27,853) and cultures of fungi (Candida albicans 90,028, Candida parapsilosis, Candida krusei, Aspergillus niger, Candida albicans 10,231, Candida glabrata) were obtained from Hacettepe University, Biotechnology Department Culture Collection Laboratory, (Ankara, Turkey).
Film preparation
Powdered ostrich eggshell, levan biopolymer, glycerol and acetic acid were used for the development of film. Firstly, 5 g of levan was dissolved in 1% acetic acid. 2 mL of glycerol was added to the dissolved levan solution, and the mixture was stirred at room temperature for 4 h. Then, different amounts of powdered ostrich eggshell were added to the levan/glycerol solution and mixed again at room temperature for 1 h. Samples are named ERL (role of edible levan) and EROL (role of edible ostrich eggshell and levan).
The ERL sample is a control sample and does not contain powdered ostrich eggshells.
The samples of EROL-1, EROL-2, EROL-3, EROL-4, EROL-5, EROL-6, EROL-7, EROL-8, EROL-9, EROL-10 and EROL-11 contain 0.1 g, 0.3 g, 0.5 g, 0.75 g, 1 g, 1.2 g, 1.5 g, 1.7 g, 2 g, 2.5 g, 3 g powdered ostrich eggshell, respectively.
The ostrich eggshell/levan/glycerol mixtures were then poured into Petri plates and allowed to be dried at room temperature for a week.
Characterization of ostrich eggshell /levan film properties
Thickness (δ)
The film thickness was determined using a digital micrometer (MESEM, Turkey) with a resolution of 0.01 mm. The final thickness was calculated by the arithmetic mean of eight measurements at different points.
Transparency (T)
Film transparency properties of levan/powdered ostrich eggshells were examined in a UV–vis spectrometer (Shimadzu UV-1700, Kyoto, Japan) at the visible light wavelength of 600 nm by the following equation [19]
[T:transparency, Abs600:absorbance of the film at 600 nm, d:thickness of the film (mm).]
Moisture content (ω)
Determination of moisture content of samples was made according to ASTM D644-99 standards [20]. Film samples were cut to 2 × 2 size and weighed. Then, the samples were oven-dried at 105 °C for 24 h. Moisture content values were assessed in triplicate for each film. The weight loss of each sample was determined, and the moisture content was calculated as the percentage of water removed from system.
Mechanical properties
Mechanical properties of the ostrich eggshell/levan film samples were determined by measuring tensile strength (TS) and elongation at break (E) of the films according to the standard method of ASTM 828–88. For this analysis, each film sample was cut into piece with size about 2.5 × 10 cm. The TS and E tests were carried out using ZwickRoell 250kN Universal Testing Machine (Model Z250, METU, Turkey) operated in tensile mode with an initial grip separation and crosshead speed set at 20 mm and 10 mm/min, respectively. Film samples were mounted between two grips on the universal testing machine and stretched until they broke. At least, ten measurements were taken for each sample, and the average values were presented as TS (MPa) and E (%) of the each film [21].
Water vapor permeability (WVP)
Water vapor permeability (WVP) of ostrich eggshell/levan films was determined according to the method of common used protocols with some modifications [22,23,24]. Firstly, 2 g of anhydrous CaCl2 was loaded into each test vial. The test tube was then tightly sealed with each film sample. The test tubes sealed with the films were placed in desiccants filled with distilled water at 25 °C with 100% RH.
Water vapor permeability (WVP) was calculated by following Eq. (2).
[△m:incremental mass of each test vial, t:time interval, A:effective exposed surface area of film (vial mouth area was 1.766 cm2), d:thickness of film, △P:partial pressure difference of water vapor on both sides of films, △P at 25 ℃ = 3.168 kPa].
Thermal analysis
Thermal gravimetric analysis of 8–10 mg ostrich eggshell/levan film samples was performed at a 10 °C min−1 heating rate under nitrogen atmosphere (20 mL min−1) (TA INSTRUMENTS, Q600 SDT).
Scanning electron microscopy (SEM)
The microstructure of the ostrich eggshell/levan film surfaces was imaged using a scanning electron microscope (TESCAN, GAIA 3) after being coated with the Au sputtering layer.
FTIR
The presence of functional groups of ostrich eggshell/levan film was determined by the Fourier transform infrared (FTIR) spectrometer (Thermo Fisher, Nicolet is50) under the spectrum range of 4000–400 cm−1.
Antioxidant activity-DPPH radical scavenging assay
The antioxidant activity of the ostrich eggshell/levan films was evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay with little modification [25, 26]. 2 mL of 0.1 mM ethanolic DPPH solution was added to 2 mL of ostrich eggshell/levan/glycerol mixtures. It was mixed and incubated at room temperature for 30 min in the dark condition. The absorbance was measured at 517 nm. The DPPH radical scavenging activity was measured using the following Eq. (3)
[A0: Control Absorbance, A1: Sample Absorbance].
Ascorbic acid was the positive control.
Antimicrobial activity
Antimicrobial activities of ostrich eggshell/levan films against Escherichia coli 25,922, Staphylococcus aureus subsp. aureus 29,213, Pseudomonas aeruginosa, Klebsiella oxytoca, Klebsiella pneumoniae, Streptococcus pneumoniae 6303, Shigella sonnei, Acinetobacter baumanii calcoaseticus complex, Methicillin sensitive Staphylococcus aureus (MSSA), Candida albicans 90,028, Candida parapsilosis, Candida krusei, Aspergillus niger were tested by well-diffusion assay [27, 28]. The bacteria cultures were grown in nutrient broth (NB, Sigma-Aldrich) at 37 °C, 24 h, and fungal strains were grown in Sabouraud dextrose broth(SDB, Sigma-Aldrich) at 30 °C, 24 h. Nutrient agar plates and Sabouraud dextrose agar plates were prepared and swabbed with 50 µL of broth culture of the bacteria and fungi cultures. 5 mm diameter wells were made in each Petri dish with sterile cork borer. 20 µL samples of ostrich eggshell/levan film were used to determine the antimicrobial activity. Bacterial cultures were incubated for 24 h at 37 °C, and fungal cultures were incubated for 48 h at 30 °C after adding to the wells. The experiment was repeated three times, and the zone diameter was measured in mm [8].
Antibiofilm activity
Antibiofilm activity was analyzed by a crystal violet staining microtiter biofilm formation assay [29]. Escherichia coli 25,922, Staphylococcus aureus subsp. aureus 29,213, Candida parapsilosis, Candida krusei, Candida albicans 10,231, Candida glabrata, Pseudomonas aeruginosa, Candida albicans 90,028, Klebsiella pneumoniae 1705, Pseudomonas aeruginosa 27,853 were used for determining the antibiofilm activity of ostrich eggshell/levan films. The microbial cell cultures that had grown overnight were diluted at a turbidity of 0.05 (OD600) and were used for biofilm formation. 24 wells of a polystyrene plate were filled with different film compositions and microorganisms. Then, the plates with the bacteria were incubated for 24 h at 37 °C, and the plates with the fungi were incubated for 48 h at 30 °C. Thereafter, the wells were gently washed and stained with 1% crystal violet for 30 min. The stained biofilms were then washed with d-water, air-dried and suspended in 96% ethanol to remove the dye. The solubilized crystal violet for each well was calculated by spectrophotometry at 540 nm (Shimadzu UV-1700, Kyoto, Japan). All tests were performed in triplicate.
Statistical analysis
Results were tabulated as mean ± standard deviation (SD). Mean of three replicates was used in calculation.
Results and discussion
Appearance of powdered ostrich eggshell/levan biopolymer films
Levan biofilms prepared using different proportions of powdered ostrich eggs were dried at room temperature. No adhesion was observed on any film that could be easily removed from the Petri dishes (Fig. 2). Depending on the powdered ostrich egg content of the film, it was determined that there were breaks in the EROL-8, EROL-9, EROL-10, EROL-11 film samples starting from the EROL-7 film sample. The study was continued with film samples of ERL, EROL-1, EROL-2, EROL-3, EROL-4, EROL-5 and EROL-6. Characterizations and biological activities were evaluated through these seven films.
Physical and mechanical characterization
The physical and mechanical properties of powdered ostrich eggshell/levan films are shown in Table 1.
The thickness of the powdered ostrich eggshell-free film sample ERL was 0.07 ± 0.08 mm, the lowest among the seven-film groups. Film thickness increased depending on the amount of powdered ostrich eggshell in the films. The highest film thickness was 0.203 ± 0.08 mm in the EROL-6 sample containing 1.2 g powdered ostrich eggshell.
Transparency of powdered ostrich eggshell/levan films is presented in Table 1. The ERL sample with a T value of 2.78 ± 0.04 was the most transparent or, in other words, has the lowest opacity of the films. Increasing the amount of powdered ostrich eggshell in the film content decreased the transparency and increased the opacity.
It is clear from Table 1 that with the increasing amount of powdered ostrich eggshell, the moisture content in the films has decreased. Ostrich eggshell is a biomaterial consisting of 96% crystalline calcite and 4% mostly protein-containing organic material [30, 31]. The agglomeration of CaCO3 particles and filling the gap between levan nanoparticles reduced the moisture content in the films.
Tensile strength (TS) and elongation at break (E) of powdered ostrich eggshell/levan films are shown in Table 1. Studies have shown that films prepared with pure levan have a tensile strength of less than 2 MPa and poor mechanics with an elongation of about 2% [32, 33]. In this study, the amount of levan and glycerol in each film sample prepared is equivalent. The tensile strength and elongation values vary depending on ostrich eggshell ratio. As the ostrich eggshell rate increases, tensile strength increases, and the elongation percentage decreases in the levan films.
Various studies have shown that the pore structures, pigments, protein and calcium amounts and microscopic structures in the eggshells play an important role in water vapor permeability [34,35,36,37]. However, there is no information in the literature about the water vapor permeability of powdered ostrich eggshells. In this study, it has been shown that water vapor permeability decreases when ostrich eggshell is added to films obtained with levan biopolymer (Table 1). The water vapor permeability was 8.14 ± 0.2 in the ERL sample without powdered ostrich eggshell, while the water vapor permeability was 4.05 ± 0.5 in the EROL-6 sample containing 1.2 g powdered ostrich eggshell.
Thermal analysis
The thermal properties of the levan/ostrich eggshell films obtained were measured by TGA (Fig. 3). Powdered ostrich eggshell showed an enhanced thermalstability, resulting in only a 2% weight loss below 500 °C in two degradation step. In the ERL sample without any ostrich eggshells, the mass loss between 175–230 °C is 50%. In the EROL-1 sample containing 0.1 gr ostrich eggshell, mass loss occurred at a rate of 45% between 200 and 260 °C. EROL-2 sample containing 0.3 gr ostrich eggshell lost 40% mass between 200 and 275 °C, and in the EROL-3 sample containing 0.5 gr Ostrich eggshell, this loss of mass at the same rate was between 190 and 250 °C. Furthermore, 30% mass loss between 190 and 250 °C in EROL-4 sample containing 0.75 gr ostrich eggshell, 30% mass loss in EROL-5 sample containing 1 gr ostrich eggshell between 160 and 260 °C, and 20% mass loss between 200 and 275 °C in EROL-6 sample containing 1.2 gr ostrich eggshell were recorded. These results show us that the ostrich eggshell added to the levan-based films increased the thermal stability of the films.
SEM
SEM images of seven powdered ostrich eggshell/levan films are presented in Fig. 4. There is a regular roughness in the ERL film sample, which does not contain powdered ostrich eggshells in its structure. Powdered ostrich eggshells were coated with levan biopolymer, creating a smoother surface. With the increase in the amount of powdered ostrich eggshell, the film surface became smoother. It was observed that pores of different sizes were formed on the surface of the EROL-6 sample.
FTIR
The FTIR spectra of ostrich eggshell/levan films are shown in Fig. 5. As levan and glycerol are hydroxyl-containing hydrocarbons, the FTIR spectra had many similarities.
The spectra of all ostrich eggshell/levan film samples showed a strong absorption band at 3400 cm−1. This is representing O–H stretching vibration [32]. Depending on the furanose ring of the levan biopolymer, a band of 840 cm−1 was observed. The bands near 1300–1400 cm−1 were related to –CH bending vibrational modes [32]. The region in the range of 900–1200 cm−1 appeared in all ostrich eggshell/levan film samples that is typical for carbohydrates. In addition, it can be attributed to the C–O–C glycosidic linkage [38, 39] that occurs in levan biopolymer molecules.
Antioxidant activity-DPPH radical scavenging assay
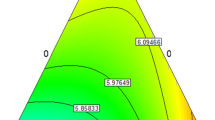
As shown in Fig. 6, the DPPH scavenging activity of ostrich eggshell alone is quite low (3.63%). This is the first study of DPPH activity in ostrich eggshells. The antioxidant activity in ERL, the film sample without ostrich eggshell, is 57.1%. Interestingly, ostrich eggshell added to levan-based films led to a high increase in antioxidant activity. The highest antioxidant activity is seen in EROL-6, which also contains the highest ostrich eggshell (83.03%).
Antimicrobial activity
Antimicrobial effects of film samples consisting of levan and ostrich eggshells were evaluated on different bacterial and fungal cultures (Fig. 7).
The antimicrobial effect of ostrich eggshells in powdered form has not been previously studied. The powdered ostrich eggshell showed antimicrobial effect only on Methicillin sensitive Staphylococcus aureus (MSSA) (6.5 mm) and Candida parapsilosis (10.5 mm). Ostrich eggshell does not have any antimicrobial effect on other microorganisms. Studies have reported that ostrich eggshells have a high contamination rate due to their large pore diameter and large pore area. However, in their natural habitats, the thickness of the eggshells of ostrich eggs, moisture and high-temperature conditions that are not sufficient for bacterial growth prevent bacterial contamination in their natural habitats [40,41,42,43]. The levan film sample ERL, which does not contain ostrich eggshells, showed an antimicrobial effect only on bacteria. The ERL sample does not have an antimicrobial effect on fungi. Ostrich eggshell has an antagonist effect with levan biopolymer. It is seen that the antimicrobial effects of the films to which ostrich eggshells are added decrease. Ostrich eggshell and levan biopolymer had a synergistic effect only on Streptococcus pneumoniae 6303 and Pseudomonas aeruginosa. Ostrich eggshell alone has no antimicrobial effect on Streptococcus pneumoniae 6303 and Pseudomonas aeruginosa. Together with the levan biopolymer and ostrich eggshell, the antimicrobial effect is greater than that of the levan biopolymer alone (Fig. 8).
Antibiofilm activity
Antibiofilm effects of film samples consisting of levan and powdered ostrich eggshells were evaluated on different bacterial and fungal cultures (Fig. 9).
The ERL film sample has the highest antibiofilm effect among the films, with an inhibition of 68.6% on the patient sample Pseudomonas aeruginosa biofilm. The ERL sample performed 51.7% biofilm inhibition in Klebsiella pneumoniae 1705 and 41.7% in Pseudomonas aeruginosa 27,853. ERL sample has no antibiofilm effect on Candida albicans 10,231, Candida albicans 90,028 and Candida glabrata. Powdered ostrich eggs have no antibiofilm effect on any microorganisms used in the study. Although the experiment was repeated 3 times, it is thought that the high differences in OD values measured in the powdered ostrich eggshell may be due to the staining. Powdered ostrich eggshells adhering to the plate wall caused the high OD value.
Conclusion
In this study, the usability of powdered ostrich eggshells in levan-containing biopolymer films was demonstrated. The amount of ostrich eggshell in the film affects the smoothness of the film. The incorporation of powdered ostrich eggshells increased the physical and mechanical properties of levan-based films. A high increase in DPPH scavenging activity was observed when levan and ostrich eggshell were used together. There are synergistic and antagonist antimicrobial effects on different microorganisms between levan and powdered ostrich eggshell.
The results obtained in this study indicate that ostrich eggshells can be used in edible levan-based films. Thus, cheaper materials can be obtained by using waste ostrich eggshells in edible films. In future studies, it is recommended that the applications of these levan/ostrich eggshell films in different foods (fruit or vegetable) need to be investigated due to their remarkable potentials.
Data availability
Patient-related data not included in the paper might be subject to patient confidentiality. All other data are available from the authors upon reasonable request.
References
Yoo SH, Yoon EJ, Cha J, Lee HG (2004) Antitumor activity of levan polysaccharides from selected microorganisms. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2004.01.002
Matsuhira H, Tamura KI, Tamagake H et al (2014) High production of plant type levan in sugar beet transformed with timothy (Phleum pratense) 6-SFT genes. J Biotechnol. https://doi.org/10.1016/j.jbiotec.2014.09.025
Koşarsoy Ağçeli G, Cihangir N (2020) Nano-sized biopolymer levan: its antimicrobial, anti-biofilm and anti-cancer effects. Carbohydr Res. https://doi.org/10.1016/j.carres.2020.108068
Aramsangtienchai P, Kongmon T, Pechroj S, Srisook K (2020) Enhanced production and immunomodulatory activity of levan from the acetic acid bacterium, Tanticharoenia sakaeratensis. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2020.07.001
Srikanth R, Siddartha G, Sundhar Reddy CHSS et al (2015) Antioxidant and anti-inflammatory levan produced from Acetobacter xylinum NCIM2526 and its statistical optimization. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2014.12.079
Öner ET, Hernández L, Combie J (2016) Review of Levan polysaccharide: from a century of past experiences to future prospects. Biotechnol Adv 34:827–844
Srikanth R, Reddy CHSSS, Siddartha G et al (2015) Review on production, characterization and applications of microbial levan. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2014.12.003
Koşarsoy Ağçeli G, Hammamchi H, Cihangir N (2021) Novel levan/bentonite/essential oil films: characterization and antimicrobial activity. J Food Sci Technol. https://doi.org/10.1007/s13197-021-05009-4
Cooper RG (2000) Critical factors in ostrich (Struthio camelus australis) production: a focus on southern Africa. Worlds Poult Sci J. https://doi.org/10.1079/wps20000019
Al-Nasser A, Al-Khalaifa H, Holleman K, Al-Ghalaf W (2003) Ostrich production in the arid environment of Kuwait. J Arid Environ. https://doi.org/10.1006/jare.2001.0876
Gautron J, Bain M, Solomon S, Nys Y (1996) Soluble matrix of hen’s eggshell extracts changes in vitro the rate of calcium carbonate precipitation and crystal morphology. Br Poult Sci. https://doi.org/10.1080/00071669608417914
Ferreira JRM, Louro LHL, Costa AM et al (2016) Ostrich eggshell as calcium source for the synthesis of hydroxyapatite and hydroxyapatite partially substituted with zinc. Ceramica. https://doi.org/10.1590/0366-69132016623642002
Dupoirieux L (1999) Ostrich eggshell as a bone substitute: a preliminary report of its biological behaviour in animals–a possibility in facial reconstructive surgery. Br J Oral Maxillofac Surg. https://doi.org/10.1054/bjom.1999.0041
Faridi H, Arabhosseini A (2018) Application of eggshell wastes as valuable and utilizable products: a review. Res Agric Eng. https://doi.org/10.17221/6/2017-RAE
Rezaei R, Mohadesi M, Moradi GR (2013) Optimization of biodiesel production using waste mussel shell catalyst. Fuel. https://doi.org/10.1016/j.fuel.2013.03.004
Martin-Luengo MA, Yates M, Ramos M et al (2011) Biomaterials from beer manufacture waste for bone growth scaffolds. Green Chem Lett Rev. https://doi.org/10.1080/17518253.2010.544331
Murakami FS, Rodrigues PO, De Campos CMT, Silva MAS (2007) Physicochemical study of CaCO3 from egg shells. Cienc e Tecnol Aliment. https://doi.org/10.1590/S0101-20612007000300035
Kam S-K, Hyun S-S, Lee M-G (2011) Removal of divalent heavy metal ions by Na-P1 synthesized from Jeju scoria. J Environ Sci. https://doi.org/10.5322/jes.2011.20.10.1337
Ss K, Indumathi MP, Rajarajeswari GR (2019) Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2018.11.195
ASTM Committee D06, ASTM D644–99 (2002) Standard test method for moisture content of paper and paperboard by oven drying. American National Standard
Lee JH, Jeong D, Kanmani P (2019) Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115159
Zhang W, Chen J, Chen Y et al (2016) Enhanced physicochemical properties of chitosan/whey protein isolate composite film by sodium laurate-modified TiO2 nanoparticles. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2015.11.031
Giteru SG, Coorey R, Bertolatti D et al (2015) Physicochemical and antimicrobial properties of citral and quercetin incorporated kafirin-based bioactive films. Food Chem. https://doi.org/10.1016/j.foodchem.2014.07.077
Wang C, Chang T, Dong S et al (2020) Biopolymer films based on chitosan/potato protein/linseed oil/ZnO NPs to maintain the storage quality of raw meat. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127375
Maryam Adilah ZA, Jamilah B, Nur Hanani ZA (2018) Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2017.08.017
Siripatrawan U, Harte BR (2010) Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2010.04.003
Kumari M, Mahajan H, Joshi R, Gupta M (2017) Development and structural characterization of edible films for improving fruit quality. Food Packag Shelf Life. https://doi.org/10.1016/j.fpsl.2017.02.003
Valgas C, De Souza SM, Smânia EFA, Smânia A (2007) Screening methods to determine antibacterial activity of natural products. Brazilian J Microbiol. https://doi.org/10.1590/S1517-83822007000200034
O’Toole GA (2010) Microtiter dish biofilm formation assay. J Vis Exp. https://doi.org/10.3791/2437
Feng QL, Zhu X, Li HD, Kim TN (2001) Crystal orientation regulation in ostrich eggshells. J Cryst Growth. https://doi.org/10.1016/S0022-0248(01)01611-6
Texier PJ, Porraz G, Parkington J et al (2010) A howiesons poort tradition of engraving ostrich eggshell containers dated to 60,000 years ago at Diepkloof rock shelter, South Africa. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.0913047107
Chen X, Gao H, Ploehn HJ (2014) Montmorillonite-levan nanocomposites with improved thermal and mechanical properties. Carbohydr Polym 101:565–573. https://doi.org/10.1016/j.carbpol.2013.09.073
Mantovan J, Bersaneti GT, Faria-Tischer PCS et al (2018) Use of microbial levan in edible films based on cassava starch. Food Packag Shelf Life. https://doi.org/10.1016/j.fpsl.2018.08.003
Portugal S, Maurer G, Cassey P (2010) Eggshell permeability: a standard technique for determining interspecific rates of water vapor conductance. Physiol Biochem Zool. https://doi.org/10.1086/656287
Nys Y, Gautron J, Garcia-Ruiz JM, Hincke MT (2004) Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. Comptes Rendus–Palevol. https://doi.org/10.1016/j.crpv.2004.08.002
Mikšík I, Eckhardt A, Sedláková P, Mikulikova K (2007) Proteins of insoluble matrix of avian (Gallus Gallus) eggshell. Connect Tissue Res. https://doi.org/10.1080/03008200601003116
Gorchein A, Lim CK, Cassey P (2009) Extraction and analysis of colourful eggshell pigments using HPLC and HPLC/electrospray ionization tandem mass spectrometry. Biomed Chromatogr. https://doi.org/10.1002/bmc.1158
Romano N, Santos M, Mobili P et al (2016) Effect of sucrose concentration on the composition of enzymatically synthesized short-chain fructo-oligosaccharides as determined by FTIR and multivariate analysis. Food Chem. https://doi.org/10.1016/j.foodchem.2016.02.002
Santos MI, Araujo-Andrade C, Tymczyszyn EE, Gómez-Zavaglia A (2014) Determination of amorphous/rubbery states in freeze-dried prebiotic sugars using a combined approach of near-infrared spectroscopy and multivariate analysis. Food Res Int. https://doi.org/10.1016/j.foodres.2014.07.040
Brown CR, Peinke D, Loveridge A (1996) Mortality in near-term ostrich embryos during artificial incubation. Br Poult Sci. https://doi.org/10.1080/00071669608417838
Horrocks NPC, Hegemann A, Matson KD et al (2012) Immune indexes of larks from desert and temperate regions show weak associations with life history but stronger links to environmental variation in microbial abundance. Physiol Biochem Zool. https://doi.org/10.1086/666988
Horrocks NPC, Hine K, Hegemann A et al (2014) Are antimicrobial defences in bird eggs related to climatic conditions associated with risk of trans-shell microbial infection? Front Zool. https://doi.org/10.1186/1742-9994-11-49
Chen X, Li X, He Z et al (2019) Comparative study of eggshell antibacterial effectivity in precocial and altricial birds using Escherichia coli. PLoS One. https://doi.org/10.1371/journal.pone.0220054
Author information
Authors and Affiliations
Contributions
Gözde Koşarsoy Ağçeli contributed to investigation, resources, writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koşarsoy Ağçeli, G. Development of ostrich eggshell and nano-levan-based edible biopolymer composite films: characterization and bioactivity. Polym. Bull. 79, 11201–11215 (2022). https://doi.org/10.1007/s00289-021-04069-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-04069-y