Abstract
Molecular imprinting is a technique for providing synthetic polymer with specific cavity toward a target molecule. In this study, as an adsorbent of betanin, molecularly imprinted polymer was synthesized using 4-vinylpyridine as functional monomer, and its ability for separation and detection of betanin from pomegranate juice medium was evaluated. For binding test, 30 mg polymer was mixed with 4 ml stock solution (2 × 10−3 M betanin) for 10 min at room temperature. Batch adsorption experiments of betanin 100 ppm stock solution revealed a binding capacity of 10.67 mg betanin adsorption per gram of the synthesized polymer. The complexation of betanin with 4-vinylpyridine was affirmed by Fourier transform infrared spectrometry analysis. The synthesized polymer exerted high thermal degradation point, and the average diameter of polymer particles was obtained to be 3900 nm by DLS experiment. This study demonstrates that the detection of pomegranate juice adulteration with red beet juice does not need to be difficult, time-consuming or expensive through selective separation techniques such as molecularly imprinted polymers.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Betanin is a naturally derived colorant from water-soluble nitrogen-containing betalains group. One of the main sources of betalains is red beet. Red beet betalains are composed of two major structural groups: the red–violet betacyanins (mainly betanin and isobetanin) and the yellow betaxanthins (mainly vulgaxanthin ǀ and ǁ). Betanin is the dominant and most important pigment in red beet (300–600 mg/kg). It has a strong color as it produces high excellent hue values in low quantity [1, 2]. Betanin is a betanidin 5-O-β-glucoside containing a phenolic group and a cyclic amine group, functioning as very good electron donors. It is stable in pH ranges of 3–7 [1,2,3,4].
Red beet betacyanins (betanin) have found practical applications in beverages and food processing such as jellies, candies, ice cream, some fruit chews, juices. A common method of fruit juice adulteration is the usage of other juices to comply with the juice requirements such as color. Betanin (E-number E162) is the only betalain approved for use in food, and it is almost entirely obtained from red beet crops. Moreover, betalains are antioxidative pigments, which are used in the food industries as natural colorants [5]. Because of its high price and short harvest season, red beet juice addition to valuable juices like pomegranate juice can be a research subject. Therefore, developing a fast, selective and accurate analytical technique to detect this type of adulteration by natural or synthetic dyes in fruit juices is needed. Hereon, betanin can be used as an indicator of beet juice presence in other juices.
Common measurements and detection methods of betanin are spectrophotometric [2] and high-performance liquid chromatography (HPLC) analysis [3]. A new method applied in this study for the detection and selective extraction of betanin is molecularly imprinted polymer (MIP) technique. In this method, a synthetic polymer is formed around a target molecule (template). After template removal by solvents, a template-shaped cavity in polymer matrix is created with affinity and is complementary in size, shape and position with the functional groups toward target molecule. High affinity, selectivity, stability and simplicity of preparation are several advantages of MIPs [6, 7]. MIPs are also stable to extremes of pH, organic solvents and temperature which allows for more flexibility in the analytical methods [8, 9]. These properties demonstrate the potentials of MIPs for utilization as an adsorbent in MIP-based mimetic sensors with good accuracy, a simple pretreatment procedure, antibody receptor mimics and sample preparation, mainly in solid-phase extraction in complicated food matrices [10, 11].
Based on our knowledge, there are scarce publications about synthesis of betanin imprinted polymer. Recently, Nestora et al. [12] have studied synthesizing a molecularly imprinted polymer solid-phase extraction for selective cleanup of betanin and its stereoisomer isobetanin from red beet extracts. They applied dipicolinic acid as a dummy template for MIP preparation.
Components required for MIP synthesis are template, monomer, cross-linker, porogen solvent and initiator. Radical polymerization of functional monomers with the target molecule occurs via covalent or non-covalent interactions, and then monomers arrangement takes place by using a cross-linking agent at a porogenic solvent [13]. The two functional monomers, 4-vinylpyridine (4-VP) and methacrylic acid (MAA), are commonly used in imprinting process as 4-VP interacts with the carboxyl group, and MAA forms a hydrogen bond with the carbonyl moiety. In the group of basic functional monomers, 4-VP is frequently used for imprinting of acidic templates because of its low cost and good solubility in common solvents [14]. In their basic form, it represents electron-rich k-electron ring systems, which allows them to interact strongly with electron-deficient aromatic rings, as well as through acid–base interactions and H-bond acceptance [15]. Nowadays, quantification of adulteration in fruit juices via molecularly imprinted polymers is a burgeon approach which has been used frequently. In a research by Ghasempour et al. (2017), 4-Vp was used to synthesize a highly selective imprinted polymer of carmoisine (a synthetic food colorant) in order to detect fruit juice authentications [16].
This study aimed at fabricating an adsorbent polymer for betanin extraction from pomegranate juice as authentication marker of pomegranate juice. Dimethyl sulfoxide is considered as a solvent due to the good solubility of betanin in this porogenic solvent. Moreover, 4-vinylpyridine is assumed as a monomer. As templates carrying carboxylic acid moieties, 4-vinylpyridine is the monomer of preference. Ethylene glycol dimethacrylate (EGDMA) and 2,2-azobisisobutyronitrile are commonly used as a cross-linker and initiator in MIP technique. The method of polymerization applied in this research for polymer synthesis was precipitation polymerization which is performed in a more dilute polymerization mixture and results in submicron-sized polymers in comparison with other techniques [7, 8]. Polymer evaluation and characterization were taken through a binding test by HPLC, Fourier transform infrared spectrometry (FT-IR), thermogravimetric and particle size analysis methods. The potential of the synthesized polymer in betanin extraction from juice medium is also assayed.
Experimental
Reagents, solutions and apparatus
All polymerization reagents containing ethylene glycol dimethacrylate (EGDMA; 98%), 2, 2-azobisisobutyronitrile (AIBN; 98%), HPLC grade acetonitrile, dimethyl sulfoxide (DMSO; 99%), formic acid and methanol were supplied by Merck (Germany) and the template betanin and 4-vinylpyridine (4-VP; 95%) by Sigma Aldrich (Spain). Methanol (CH3OH; 99.9%) and glacial acetic acid (CH3COOH; 100%) as a washing elution were purchased from Scharlau (Barcelona, Spain) and CDH (New Delhi, India), respectively. Double distilled water was used in all solutions and experiments.
Applied devices in this study were: centrifuge (Hettich, eba 270, Germany 4000 rpm), ultrasonic equipment (Panasonic 2600s, Iran), microcentrifuge (Eppendorf, MiniSpin, Germany 12,000 rpm), laboratory balance (Acculab sartorius group, Atilon, USA), magnetic heater–stirrer (Heidolph, mr hei-standard, Germany), HPLC (CECIL, CE4900, UK), FT-IR spectrometer (Perkin Elmer, Spectrum Two, USA), oven (Memmert, EFB 400, Germany), deionizer (Millipore, Direct Q UV-3, France), horizontal tube shaker (Behdad, Iran), particle size analyzer (Malvern Zetasizer, Nano-ZS, UK), scanning electron microscope (SEM LEO 1430VP, Germany–England) and thermogravimetric analyzer (Linseis, STA PT-1000, Germany), Brunauer-Emmett-Teller (BET, Belsorp mini II, Japan).
Betanin imprinted polymer synthesis through precipitation polymerization
The template betanin at 0.1 mmol was added into a 100-ml flask containing 10 ml dimethyl sulfoxide as a porogen solvent and was mixed by sonication until it reached a complete dissolution. Afterward, 0.4 mmol 4-VP was added to the mixture and it was allowed to stay for 12 h for pre-polymerization under stirring. Cross-linker EGDMA (2 mmol) and initiator AIBN (0.29 mmol) were then added to pre-polymerized mixture. After sonication for 5 min, degassing of the mixture was carried out by purging N2 for 10 min and then sealed completely. Polymerization was initiated by heating the mixture at 60 °C in a water bath. After 24 h, the obtained polymer was washed with the solution of methanol: acetic acid (90:10) to remove polymerization residuals and then with water: acetic acid (90:10) for the complete elution of betanin. Eventually, it was washed three times with deionized water to elute the acetic acid from the polymer. Drying of the solid polymers was performed at 55 °C in an oven for 24 h, and then it was ground carefully and sieved. As a control polymer, non-imprinted polymer (NIP) was also prepared following the same procedure as MIP with the exclusion of betanin.
Surface morphological analysis of polymers
Scanning electron microscopy (SEM) instrument was applied to investigate the shape and surface morphology of the produced MIP and NIP. The polymeric particles were sputter coated with gold-palladium for a few minutes before SEM analysis. SEM micrographs of the materials were obtained by a backscattered electron detector (BSD) at an accelerating voltage of 15 kV.
Spectroscopic analysis of polymers
Fourier transform infrared spectrometry was utilized to analyze the molecular structure of the MIP. This end, spectra of MIP before and after washing, NIP, betanin and 4-VP were recorded by FT-IR spectrometer. To prepare the samples for FT-IR spectroscopic analysis, 1 mg of truly dried sample was mixed with about 150 mg of dry KBr [17]. FT-IR KBr pellets were prepared by compressing the mixture with about 60 kPa within 10 min in a compress instrument. FT-IR spectroscopy was run at a wavenumber range from 400 to 4000 cm−1 with a resolution of 0.5 cm−1.
Binding test of the synthesized polymers in pomegranate juice and aqueous medium
At first, betanin stock solution 2 × 10−3 M was prepared with deionized water. Batch binding test has been developed to evaluate the binding capacity of imprinted polymers toward betanin. For binding test, 30 mg of polymer and 4 ml of stock solution were mixed in a 10-ml tube and stirred in ultrasonic equipment for 10 min to reach equilibrium between polymer and template. The same procedure was implemented in juice medium. Before adjusting the betanin concentration, the juice samples betanin concentration was evaluated through HPLC. Just before any experiment, the juice was diluted twice, and its concentration was adjusted to be 2 × 10−3 M betanin solution. Each mixture was prepared three times and then centrifuged at 10,000 RPM for 10 min at room temperature. After syringe filtering (PTFE, 0.22 μm) of the supernatant, the free betanin concentration was analyzed by HPLC-UV. The adsorbed amount of betanin to binding sites of the polymer was obtained from subtracting initial concentration and free concentration in the supernatant.
HPLC conditions applied for betanin detection were as follows: C18 Hichrom 250 mm × 4.6 mm × 5 μm column, %0.5 formic acid in deionized water: acetonitrile (80:20) as a mobile phase with a flow rate of 1 ml/min, UV detector at the wavelength of 538 nm and water–methanol as a washing solution [18]. Betanin peak was evaluated by its retention time. Given the sample and standard spectra, retention times were found to be at 3.1 min.
Particle size and zeta potential analysis
Particle size of synthesized polymer (diameter and width) was determined by dynamic light scattering (DLS) technique, which measures the random changes in the intensity of light scattered from a suspension or solution. Both particle size and zeta potential experiments were performed in Zetasizer. Considering ISO 14887-2000, methanol was utilized as a dispersant solvent. About 10 mg of dried powder of betanin imprinted polymer was diluted with 1 ml dispersing solvent. After sonication and filtration, the suspension was poured into a cuvette for the analyses.
Brunauer–Emmett–Teller analysis
The analyses of surface area, pore volume and mean pore diameter of the betanin MIP and NIP were investigated through the nitrogen adsorption–desorption technique at 77 K, using the Brunauer–Emmett–Teller (BET) method. Polymers treatment was performed as follows: About 0.1 g of powdered samples was heated and degassed at 393 K for 24 h to remove adsorbed gases and moisture.
Thermogravimetric analysis
The thermal properties of MIP (TGA, and derivative thermo-gravimetry (DTG)) were measured through thermogravimetric analysis instrument. Approximately 10 mg of sample was heated at a constant rate of 10 °C/min in the thermal range of 0–700 °C. Before scanning the changes, the atmosphere was made inert by purging with nitrogen gas.
Results and discussion
Scanning electron microscopy of imprinted polymers
SEM images of betanin imprinted polymer and NIP are illustrated in Fig. 1 which were taken at a magnification of 100,000. The polymer was obtained using 4-VP, EGDMA at a ratio of 4:1 and 20:1 to the template in a diluted medium with dimethyl sulfoxide. As it can be seen in Fig. 1, the particles have rough spherical surface. Comparison of the synthesized MIP and NIP reveals that betanin imprinted polymer comprises interconnected seams which seems to be aggregations of tightly attached cavities; however, NIP is comprised of rough irregular unshaped cavities which are not porous and probably enable a poor selectivity rate for betanin.
FT-IR spectrometry analysis
FT-IR spectroscopy was applied to analyze the molecular structure of the imprinted polymer. The FT-IR spectra of MIP, NIP, functional monomer (4-VP) and the template (betanin) are shown in Fig. 2.
Both MIP and NIP have similar IR spectra indicating resemblance in backbone structure. However, template and monomer complexation caused differences in their spectra. Vinylpyridine assignation of bands is presented in Table 1. The sharp adsorption peaks at 1500–1700 cm−1 are assigned to the C=C and C=N stretching groups of 4- VP, which are not present in betanin spectrum.
It can be seen that complexation form of betanin imprinted polymer has broader peaks. The band at 3300–3600 cm−1, which is broad, corresponds to the stretching vibration of hydrogen-bonded –OH and –NH group, while free –OH and –NH group gives sharp peak in this region as seen in NIP spectrum. The peak at about 2900 cm−1 corresponds to symmetric and asymmetric C–H stretching. Considering the peak transformation and sharpness shifting, FT-IR results indicate that the template could successfully interact with the functional monomer.
Binding experiment
Washing stage in template removal from polymer has crucial importance in MIP synthesis and its binding properties, whereas incomplete rinsing of the template makes rebinding difficult into the polymer interaction sites. Betanin removal from polymer was investigated through HPLC for investigating betanin residual in washing solution.
HPLC analyses were used to evaluate the adsorption capacity of betanin imprinted polymer which indicates effectiveness of the imprinting process. The HPLC results revealed that 4-VP-based polymer presented 80 percent adsorption toward betanin in 2 × 10−3 M betanin stock binding medium (pH = 4.8). Calculated from Eq. 1, the adsorption capacity of 4-VP polymer toward betanin showed 10.67 mg betanin adsorption per gram of synthesized betanin imprinted polymer. It is reported that VP monomers are preferred for interaction with templates bearing aromatic rings [15]. The betanin imprinted polymer showed 80% adsorption toward betanin from juice medium, the same as what was observed in the 10 ppm betanin stock solution. It can be demonstrated that there is no matrix interference in the separation of betanin from juice medium. The NIP of the mentioned polymer showed very low affinity to the template (Fig. 3). Distribution coefficients for betanin imprinted polymer and NIP were calculated according to Eq. 2 as 3.5 and 0.08 L/g, respectively. Therefore, betanin imprinted polymer has 43-fold imprinting factor (IF) in binding medium in comparison with NIP. This obviously shows that the cavities of betanin imprinted polymer have greater affinity for the betanin molecule, in comparison with NIP.
Adsorption capacity of betanin imprinted polymer was calculated according to Eq. 1:
where V is the solution volume (L), m is the polymer mass (g) and Ci and Cf are the initial and final (supernatant) betanin concentrations in the binding medium (ppm).
Distribution coefficients (kd) were calculated using the following equation:
Imprinting factors of the polymers were obtained using the following equation [19]:
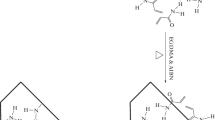
Our earlier studies showed that adsorption of betanin to polymer binding sites occurs in first 10 min and raising time intervals did not alter the amount of adsorption. Schematic representation of betanin, 4-VP and EGDMA complexation via polymerization and binding test is displayed in Fig. 4.
Quantitative parameters including the limit of detection (LOD) and relative standard deviation (RSD) were determined for the HPLC for betanin to be 0.69 mg/L and 9.2%, respectively.
Betalains are more soluble in water than in nonpolar solvents, and methanol has generally been used to extract betacyanins. Since methanol has toxic characteristics, other extraction systems like solid-phase extraction (SPE) and molecularly imprinted polymers are preferred by food scientists [20].
Particle size and zeta potential of polymer
As expected in precipitation polymerization, the particle size was about 3900 and 700 nm in length and in width, respectively. Polydispersity index (PDI), which shows the particle size distribution and ranges from 0 to 1, was about 0.2. Based on zeta potential data, the polymer was positively (60 mV) charged, indicating a poor tendency of polymer particles for aggregation [21].
Polymers pore size and volume
BET analysis gives substantial data about specific surface area, pore volume and pore size of the MIP and NIP through N2 adsorption–desorption analysis. Table 2 represents the evaluated data which show that comparing the imprinted and the non-imprinted polymer, pore volume and surface area of the MIP were greater than those of NIP. These findings reveal that a higher number of selective binding sites were distributed throughout the cavity. Also higher pore volume enables higher loading capacities. Therefore, MIP could provide more accessible cavities and binding sites for target molecule than NIP. The synthesized polymers could be remarked as mesoporous particles as their mean pore diameters were in the range of 2–100 nm. So, the synthesized MIP showed clearly larger specific surface area and more multi-porous structure compared with the NIP, implying a facile and rapid adsorption of betanin [16, 22].
Thermal analysis of polymer
Thermogravimetric analysis measures amount of change in the mass of a sample as a function of temperature or time under a controlled atmosphere to determine the properties of polymeric materials like thermal stability. Thermal analysis data depend on the molecular weight, polymeric architecture, synthesis route and moisture content [16]. Obtained results showed that the betanin imprinted polymer underwent thermal degradation beginning at 270 °C and with a total mass loss of about 10% that could be attributed to the release of solvent residues. Maximum polymer degradation peaks can be seen in Fig. 5, which are about 370 and 500 °C, showing a mass loss of 75 and 88%, respectively. High degradation temperature can be attributed to 4-VP structured polymer.
Conclusions
Molecularly imprinted polymer technique was applied to fabricate a selective adsorbent of betanin for the detection of red beet juice addition into pomegranate juice known as an adulteration type in beverage industry. The synthesized polymer with 4-VP as a monomer, EGDMA as a cross-linker and AIBN as an initiator showed a good affinity for betanin separation (10.67 mg betanin adsorption per gram of synthesized MIP) from juice medium. This polymer showed high thermal resistance. The prepared betanin imprinted polymer can be utilized as an adsorbent for the separation of betanin in sensors or in SPEs to improve/optimize sample preparation via selectivity increment. Synthesizing imprinted polymer with new surface imprinting techniques was proposed to facilitate the adsorption and desorption stages for target molecule detection in fruit juices.
References
Neelwarne B (2012) Red beet biotechnology: food and pharmaceutical applications. Springer, New York
Gliszczyńska-Świgło A, Szymusiak H, Malinowska P (2005) Betanin, the main pigment of red beet: molecular origin of its exceptionally high free radical-scavenging activity. Food Addit Contam 23:1079–1087. https://doi.org/10.1080/02652030600986032
Cai Y, Sun M, Corke H (2005) HPLC characterization of betalains from plants in the Amaranthaceae. J Chromatogr Sci 43:454–460. https://doi.org/10.1093/chromsci/43.9.454
Esatbeyoglu T, Wagner AE, Schini-Kerth VB, Rimbach G (2015) Betanin-a food colorant with biological activity. Mol Nutr Food Res 59:36–47. https://doi.org/10.1002/mnfr.201400484
Leong HY, Show PL, Lim MH, Ooi CW, Ling TC (2018) Natural red pigments from plants and their health benefits: a review. Food Rev Int 34:463–482. https://doi.org/10.1080/87559129.2017.1326935
Lok CM, Son R (2009) Application of molecularly imprinted polymers in food sample analysis-a perspective. Int Food Res J 16:127–140
Subrahmanyam S, Piletsky SA (2009) Computational design of molecular imprinted polymers. In: Potyrailo AR, Mirsky VM (eds) Combinatorial methods for chemical and biological sensors. Springer, New York, pp 135–172
He K, Qiu F, Qin J, Yan J, Yang D (2014) Selective adsorption of L-TA/D-TA by β-cyclodextrin derivative modified with l-tryptophan: isotherm, kinetic and thermodynamics studies. J Ind Eng Chem 20:1293–1300. https://doi.org/10.1016/j.jiec.2013.07.008
Tan J, Li R, Jiang ZT (2014) Discrimination of fresh fruit juices by a fluorescent sensor array for carboxylic acids based on molecularly imprinted titania. Food Chem 165:35–41. https://doi.org/10.1016/j.foodchem.2014.05.104
Wang P, Sun X, Su X, Wang T (2016) Advancements of molecularly imprinted polymers in the food safety field. Analyst 141:3540–3553. https://doi.org/10.1039/C5AN01993A
Moghaddas Kia E, Alizadeh M, Vardast MR, Rezazad M (2017) Separation of stigma sterol using magnetic molecularly imprinted nanopolymer fabricated by sol-gel method. Urmia Med J 28:44–53. https://doi.org/10.18869/acadpub.umj.28.4.44
Nestora S, Merlier F, Prost E, Haupt K, Rossi C, Bui BTS (2016) Solid-phase extraction of betanin and isobetanin from beetroot extracts using a dipicolinic acid molecularly imprinted polymer. J Chromatogr A 1465:47–54. https://doi.org/10.1016/j.chroma.2016.08.069
Tadi KK, Motghare R (2013) Potentiometric selective recognition of oxalic acid based on molecularly imprinted polymer. Int J Electrochem Sci 8:3197–3211
Mattiasson B, Ye L (eds) (2015) Molecularly imprinted polymers in biotechnology (vol. 150). Springer, Heidelberg
Mayes AG, Whitcombe MJ (2005) Synthetic strategies for the generation of molecularly imprinted organic polymers. Adv Drug Deliv Rev 57:1742–1778. https://doi.org/10.1016/j.addr.2005.07.011
Ghasempour Z, Alizadeh-Khaledabad M, Vardast MR, Rezazad-Bari M (2017) Synthesis of a molecularly imprinted polymer for the selective recognition of carmoisine (Azorubin E122) from pomegranate juice. J Sep Sci 40:962–970. https://doi.org/10.1002/jssc.201600855
Lauten EH (2006) Configurationally imprinted biomimetic polymers with specific recognition for oligopeptides. Dissertation, University of Texas
Ravichandran K, Saw NM, Mohdaly AA, Gabr AM, Kastell A, Riedel H, Cai Z, Knorr D, Smetanska I (2013) Impact of processing of red beet on betalain content and antioxidant activity. Food Res Int 50:670–675. https://doi.org/10.1016/j.foodres.2011.07.002
Clausen DN, Pires IM, Tarley CR (2014) Improved selective cholesterol adsorption by molecularly imprinted poly (methacrylic acid)/silica (PMAA–SiO2) hybrid material synthesized with different molar ratios. Mater Sci Eng, C 44:99–108. https://doi.org/10.1016/j.msec.2014.08.008
Naderi N, Ghazali HM, Hussin ASM, Amid M, Manap MYA (2012) Characterization and quantification of dragon fruit (Hylocereus polyrhizus) betacyanin pigments extracted by two procedures. Pertanika J Trop Agric Sci 35:33–40
Deshmukh K, Tanwar YS, Shende P, Cavalli R (2015) Biomimetic estimation of glucose using non-molecular and molecular imprinted polymer nanosponges. Int J Pharmaceut 494:244–248. https://doi.org/10.1016/j.ijpharm.2015.08.022
Saad EM, Madbouly A, Ayoub N, El Nashar RM (2015) Preparation and application of molecularly imprinted polymer for isolation of chicoric acid from Chicorium intybus L. medicinal plant. Anal Chim Acta 877:80–89. https://doi.org/10.1016/j.aca.2015.03.047
Acknowledgements
The authors are grateful to Mr. Naser Ranjkeshzadeh (Urmia University of Medical Sciences, Reference Laboratory) who helped in the HPLC and FT-IR analysis of polymers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasempour, Z., Alizadeh Khaled-Abad, M., Vardast, M.R. et al. Fabrication of betanin imprinted polymer for rapid detection of red beet adulteration in pomegranate juice. Polym. Bull. 76, 1793–1805 (2019). https://doi.org/10.1007/s00289-018-2444-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2444-5