Abstract
A novel class of hydrophobic associated copolymers (PASC) with nonionic surfmer was synthesized. These copolymers are the copolymerization product of acrylamide (AM), 2-(acrylamido)-2- methyl propane sulfonic acid (AMPS) and different contents of nonionic surfmer (CP). Macroscopic and microscopic self-assembly associative properties in solutions of PASC, as well as the effects of salt and temperature on the associative behavior were studied via viscosimetry, scanning electron microscope (SEM), atomic force microscope (AFM), rheology and fluorescence spectroscopy (FS). The results show that the introduced nonionic surfmer along polymer chain can lead to a strong intermolecular hydrophobic association in water. The viscosity and surface activity increased with the increase of polymer concentration and nonionic surfmer concentration. Additionally, the polymer showed a good thermal stability and good salt tolerance. Contact angle studies shows that PASC solution has good potential on change of wettability from oil-wet to water-wet. All of these properties indicate that the polymer is an excellent chemical for chemical enhanced oil recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the recovery of the reservoirs all over the world, most crude oil is trapped in the reservoirs after using the conventional oil production methods. To deal with the sharp consumption of fossil energy and the new round of energy crisis, enhanced oil recovery technologies are required and have been explored to recover the remaining oil in the mature oil fields [1]. Chemical recovery is the most widely applied EOR ways for squeezing the additional oil in the underground [2].
In chemical recovery, polymers, surfactants, alkalis, and the combination of these chemicals are often used as efficient technologies of chemical EOR to reduce the mobility between water and crude oil and improve the oil recovery in the old oil fields in harsh reservoirs such as higher temperature and salinity reservoirs, offshore oil fields, and heavy oil pools [3, 4].
However, the chemical flooding systems still have some faults. For example, the viscosity retention rate of HPAM is low under harsh conditions (high salinity, high temperature, and high shear rate) in polymer flooding [5]. For surfactants flooding, the surfactant may be incompatible with the reservoir, which will lead to the decline of solution performances such as aggregation, phase separation, and diffusion performance in porous medium [6]. The alkali can form scale on pipes and equipments and decrease the permeability of formation as it can react with metal ions (like Ca2+). It also can react with polymer that contains acrylamide units and degrade it, which can significantly reduce the viscosity and the thermal stability of polymer solution. There are also some problems about the technique of polymer-surfactant (SP) flooding and polymer-surfactant-alkali (ASP) flooding [7], the chromatographic separation occurs because of the migration speed difference among different components in SP flooding and ASP flooding, and lead to poor synergy of different displacing agents and poor flooding effect.
To solve these problems, a new polymer with excellent thickening property and interfacial activity has been studied, which might increase the oil displacement efficiency and sweep efficiency in enhanced oil recovery processes. Li et al. [8] synthesized a cationic fluorinated polyacrylamide via free-radical polymerization using acrylamide (AM), diallyl dimethyl ammonium chloride (DADMAC), and 2-(perfluorooctyl)ethyl acrylate (FEA) as raw materials. They observed that the polymer solution had an excellent capability of shear resistance, remarkable temperature tolerance and surface activity. Yahaya et al. [9] synthesized a hydrophobically modified polyacrylamide by micellar copolymerization of acrylamide, and hydrophobic monomer (N-benzylacrylamide) and showed that the polymer exhibited excellent surface activity and the properties of pseudoplastic fluid which can meet the property requirements for EOR polymer. In most of the previous researches, micellar mechanism [10,11,12,13] has been employed for polymerization of AM, hydrophobic monomer, which had a complicated post-treatment of the product owning to elimination of large amounts of micromolecule surfactants. And if the micromolecule surfactants displaced by surface active monomers, the synthesis of hydrophobic associating polyacrylamide will be more convenient.
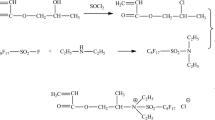
In this work, the nonionic surfmer (CP) was prepared using polyoxythylene (10) octylphenyl ether (OP-10) to react with allyl chloride at first [14]. Then, a new hydrophobically associating polyacrylamide was prepared by radical polymerization with AM, AMPS and CP. A schematic illustration of the chemical structure of this polymer is displayed in Scheme 1.
The aim of this study is to examine the effects of macro-monomer addition and salt on the self-assembly of aqueous solutions of the novel class of hydrophobic associated polymers through apparent viscosity, surface tension, fluorescence spectra, SEM and AFM to develop a viscosifying agent with the view of enhancing oil recovery (EOR) applications.
Experimental
Materials
Acrylamide (AM, 98% purity) was purchased from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China). 2-Acrylamido-2-methyl propane sulfonic acid (AMPS, 99% purity) was from Zhonghai Chemical Industry (Shandong, China). Pyrene (99% purity) was purchased from Aladdin CO., Ltd. (NH4)2S2O8, Na2SO3, NaOH, NaCl and CaCl2 were obtained from the Sinopharm Chemical Reagent Co. Ltd and 99% purity.
All chemicals were of analytical grade and were used as received without further purification. Distilled water was used in all experiments.
Elemental analysis
The carbon, hydrogen, nitrogen, and sulfur contents of the polymer were determined with a Vario EL-III elemental analyzer (Germany).
Apparent viscosity
The apparent viscosities of polymer solutions were measured at room temperature (25 °C) with a Brookfield DV-III viscometer, and the measuring time is 5 min for every sample. The conductivity value of the water is less than 0.1 us/cm, and the 0.01 mol/L KCl solution used for calibration at 25 °C, the conductivity value is about 1408.3 us/cm.
SEM
The morphology of the polymer solution was detected by S-3000 N SEM (Hitachi, Japan). The samples were kept at room temperature without stirring for another 48 h in the room temperature for 24 h before testing. Then, the samples were prepared by the vacuum sublimation freezing–drying technique.
Fluorescence measurement
Fluorescence spectra were measured with a Hitachi F4500 fluorescence spectrophotometer using 1.0 cm quartz cell. The pyrene concentration was fixed at 1.0 × 10−6 mol/L in the measurements. Pyrene spectra were recorded with fixed excitation wavelength at 335 nm, and the slit widths of excitation and emission were fixed at 2.5 and 5 nm, respectively. The emission spectra were scanned at the range of 350–600 nm. I 1 and I 3 were obtained from the emission intensities at 373 and 384 nm, and the ratio between the fluorescence intensities of peaks I 1 and I 3 was used to evaluate the polarity of the local microenvironment of pyrene. The experiments were measured at 25 °C.
AFM
Atomic force microscopic (AFM) images were taken by a commercial Nanoscope III (Digital Instruments, Santa Barbara, CA, USA) using Si3N4 probe to analyze the apparent morphology of the polymer solution.
Surface tension
The surface tension of the copolymer solution were measured by a surface tensiometer (JK99B, ShangHai) at 313 ± 0.1 K. Solutions of polymer of different concentrations were made 24 h prior to experiment in Mili-Q water.
Contact angle measurement
The contact angle measurements were performed with the sessile drop method. The clean glass is put into different polymer solution for 12 h, and dried under vacuum for at least 20 h. For, this, accurately measured 10 μL solution was carefully dropped on the top surface of the glass. The contact angle between synthesized polymeric solutions and water were measured by contact angle goniometer (JC2000, ShangHai) at 10 °C to evaluate wettability alteration, and variations of contact angle with time were measured for wettability alteration study.
Results and discussion
Elemental analysis
C, H, N, S analysis (Table 1) results reveal that there is obvious increase in C and H concentration with the increase of CP. This clearly indicates that CP has been grafted onto polymer backbone.
Apparent viscosity
Influence of polymer concentration on apparent viscosity
The thickening ability of the hydrophobically associating polymer is greatly dependent on both the length and number of hydrophobic blocks in the polymer chains as determined by the amount of hydrophobe. Figure 1 shows the influence of the polymer concentration on the thickening properties of the PASC polymers while the other reaction conditions are the same. The molar compositions of the synthesized polymers are shown in Table 1.
For the six polymers, the apparent viscosities enhance with the polymer concentration, but they have difference all the same. As the CP feed amount is only 0.2 mol% (PASC-1) and 0.4 mol% (PASC-2), the apparent viscosities of the polymer solutions are the lowest and slowly vary with polymer concentration, because of the weak intermolecular hydrophobic associations. As the CP amount beyond 0.6% (PASC-3), the apparent viscosities of the PASC solutions increased rapidly, especially after the concentration of 1.5 or 2 g/L. This suggested that about 1.5 or 2 g/L is a critical association concentration for the copolymers with PASC-3, PASC-4, PASC-5 and PASC-6, at which intramolecular association transforms into intermolecular association [15], the hydrophobic interaction makes the linear molecules entangle with each other and augments the viscosity of the copolymer solution. When the PASC concentration increases further, the intermolecular association degree is strengthened because of the shortening of the distance between macromolecules. This leads to an intermolecular physical network structure that contributes significantly to the thickening, and the viscosity enhances rapidly, as indicated in Fig. 1.
Larger and denser intermolecular physical networks in the aqueous solution will be formed for the copolymer with a higher hydrophobic content [16,17,18], which results in a higher apparent viscosity of the solution. However, when CP content exceeds 1 mol%, intramolecular interactions between hydrophobic segments might enhance and the water solubility of the hydrophobic associative copolymer becomes poor.
Figure 2a showed associative morphologies of 0.3 g/L PASC-4 in aqueous solution, and the three-dimensional networks are formed via micro-crosslink because of intramolecular hydrophobic association. The SEM image of the 1.8 g/L PASC-4 in aqueous solution is shown in Fig. 2b. We can see that, the solution emerged with strong cross linking properties, and the three-dimensional network structure become closed, leading to a sharp increase in apparent viscosity.
Influence of temperature on apparent viscosity
The effect of temperature on the apparent viscosity of a polymer is an important factor to be taken into account for its application. Figure 3 shows the dependence of apparent viscosity of the PASC-5 solution on temperature. There were two kinds of different trends of solution viscosity with the increasing concentrations of PASC.
For the concentration of 1 g/L of polymer solution, which was below critical association concentration (cac), apparent viscosity of the PASC-5 solution decreases rapidly initially and then levels off with increasing temperature, which resulted from a weakening intermolecular association from the beginning.
For the concentration of PASC was above the cac, the trend was totally different from the first one. The viscosity was initially on the rise since the temperature increased from 20 to 40 °C and then decreased from 40 to 90 °C. The hydrophobic interaction is an endothermic process driven by entropy, therefore, rising temperature is favorable for intermolecular hydrophobic association and results in enhancement of the solution viscosity [19]. However, rising temperature can also result in destroying the hydrophobic association and dehydration of the hydrophilic groups which favors the coiling loose structure of molecular chains, so the viscosity decreased when the temperature increased from 40 to 90 °C.
Surface tension
Influence of polymer concentration on surface tension
Figure 4 showed the relationship between surface tension and concentration of PASC. The following features are observed: (1) the surface tension drops with the concentration of PASC increase, and drops remarkably for the polymer PASC-2 to PASC-6. A decrease in the values of surface tension could be due to the increased adsorption of the molecules of PASC at the aqueous/air interface in the solution. For PASC-4, an excess of the copolymer had formed at the interface due to an increase in the concentration of PASC-4. At lower copolymer concentrations before 1.5 g/L, PASC-4 molecules were absorbed at the interface such that the hydrophobic blocks could avoid the unfavorable aqueous environment and escape into the air phase because of the unavailability of an adequate number of polymer molecules to form intermolecular aggregates. As a result, a sharp decrease in the surface tension was observed at lower PASC-4 concentrations. In contrast, at higher copolymer concentrations after 1.5 g/L, the interface became saturated with PASC-4 molecules and the intermolecular aggregates provided a favorable environment for the hydrophobic groups. Consequently, a little decrease in the surface tension was observed at higher polymer concentrations. So, the cac was determined to be 1.5 g/L. (2) The surface tension drops with the concentration of CP monomer increase. When the CP content is 0.2 mol% (PASC-1), the polymer PASC-1 can dissolve easily and the weak hydrophobic interaction reduce the surface tension a little, as show in Fig. 5a. When the CP amount is 0.4 mol% (PASC-2), the hydrophobic groups increase and can form intramolecular association, so the surface tension reduces, as show in Fig. 5b. When the CP amount is 0.6 mol% and beyond 0.6 mol%, a large number of associating structures are formed via the intermolecular hydrophobic associations, as show in Fig. 5c.
The ability to decrease the surface tension of water was determined, to a great extent, by the degree of replacement of water molecules by PASC at the water/air interface, which in turn was dependent on the measure of saturated adsorption of PASC.
Influence of electrolytes on surface tension
Figure 6 shows the influence of the NaCl and CaCl2 amount on the surface tension of the 1 g/L PASC-4 which was before cac. The surface tension reduces continuously with an increase in NaCl and CaCl2 concentration from 0.2 to 2%.
First, as the NaCl and CaCl2 concentration increased from 0.2 to 1%, the surface tension reduces which could be due to the charge shielding of the electrostatic repulsion of –SO3− groups and the formation of coiled polymer chains.
As electrolytes concentration keeps increasing from 1 to 2%, electrolytes will break the hydrogen bond between water and the C–O–C bonds, and the Na+ or Ca2+ will complex with the C–O bonds in the PEO side chains and so the hydrophilicity of the PEO chains decreases [20]. However, the decreased trend of Na+ was more obvious than Ca2+, the potential explanation was that the Ca2+ can be complex with the C–O bonds in the PEO side chains and formed crown ether, which lead the hydrophilicity of the PEO chains increases.
Fluorescence spectroscopy
Pyrene fluorescence is known to be sensitive to changes in the microenvironment. The intensity ratio of the first to the third peaks, I 1/I 3, is often used as a measure of the polarity of the microenvironment [21,22,23].
Figure 7 is the relationship curve between the values of I 1/I 3 and polymer concentrations at 25 °C. We found that the fluorescence intensity gradually became stronger as the concentration of PASC increased. This illustrated that the dissolution of the pyrene probe, in the micelle containing the hydrophobic blocks, increased with increasing concentration of PASC. In other words, the higher the concentration of the hydrophobic blocks, the better was the dissolution of the pyrene probe in the hydrophobic groups, thereby resulting in stronger fluorescence intensity, except PASC-6. It is found that the polymer of PASC-6 is difficult to be soluble at high concentration, so the nonpolarity of hydrophobic microdomains for PASC-5 solution is the strongest, resulting in the lowest value of I 1/I 3. cac were estimated from the plots in Fig. 8 by drawing tangents to the curves passing through their respective inflection points, the intersection of these tangents were taken as the cac.
Atomic force microscope (AFM)
The microstructure of hydrophobically associating polyacrylamide solution could be directly observed by AFM, which was an effective supplement to the method of viscosity, fluorescence and so on [24, 25]. The images of PASC solutions with different concentration are shown in Fig. 9.
Learned from Fig. 9a, there were no aggregate structures appearing in PASC at the low concentration (0.04 g/L) and it was just a flat surface. However, with increasing PASC concentration but still being kept lower than cac (0.50 g/L), the crosslinking network had already appeared in the PASC solution (Fig. 9b), we can see many big bumps which is discontinuous, it means the intramolecular association formed gradually. When the concentration increased to 1.5 g/L, the sizes of the network structure augmented significantly and the bumps become smaller and denser as indicated in Fig. 9c. It presented that the PASC could spontaneously form more and larger hydrophobic associative structures. All that was in accordance with the results obtained from both methods of viscosity measurement and FS method, which indicated the strong self-assembly effect occurred in PASC solution.
Wettability alteration
Effect of polymer concentration on contact angle
Application of surfactants largely affects the wettability of solid surface, which is very important in the context of oil recovery, as the change of wettability of rock from oil-wet to water-wet increases the oil recovery. The interfacial crude oil/aqueous solution/solid substrate interactions are characterized by the formation of a contact angle on a solid surface. The knowledge of contact angle behavior is one of the challenging problems in surface science due to its role in all processes involved in three-phase interfacial phenomena. The contact angle is related to solid substrate structure and its surface roughness, whereas the second is associated with the mutual interactions of immiscible liquids in contact with the solid substrate.
The relationship between contact angle and the concentration of polymers are displayed in Fig. 10. Initially, contact angles sharply dropped with small additions of polymer. This response was expected since polymer can promote wetting by collecting at the liquid interface where they act to reduce the liquid surface tension, and ultimately lower the contact angle on any solid surface.
Effect of contact time on contact angle
A schematic of contact angle between water and polymer morphology at start and after 200 s is shown in Fig. 11. The concentration of polymer was 2.5 g/L for PASC-5. The results indicated that the contact angle was not constant, instead it was decreasing initially over the contact time and then reaching an equilibrium, suggesting the potential effect of the polymer on wettability alteration from oil-wet to water-wet.
Conclusions
PASC is a surface active copolymer, which was successfully synthesized by free-radical polymerization. The solution properties of these polymers such as apparent viscosity, surface tension and fluorescence were investigated. In addition, the wettability alteration property was also evaluated. The apparent viscosity, surface tension and fluorescence results showed that the introduction of nonionic surfmer groups into polymer chain resulted in a strong intermolecular hydrophobic association in water.
PASC exhibited an excellent thickening property with the increasing of the addition of CP and polymer concentration, especially beyond the cac concentration. The cac of these polymers were about 1.5 or 2 g/L by viscosimetry, and furthermore, the fluorescent probe measurements showed that the cac of polymers were 1.718, 1.17, 1.158, 0.75 g/L corresponding to PASC-3, PASC-4, PASC-5 and PASC-6. The PASC also displayed fine surface activity, while the CP amount above 0.2 mol%, and the surface activity increased with the increasing of polymer concentration and CP monomer concentration. The polymer chain bundles were found by AFM for PASC in water, suggesting that the incorporation of CP units lead to the formation of expanded PASC chains. Contact angle studies showed that PASC solution has good potential on change of wettability from oil-wet to water-wet. Overall, the introduction of nonionic surfmer groups in the polymer chain was helpful in increasing the oil displacement efficiency and sweep efficiency in enhanced oil recovery processes.
References
Wang W, Liu YZ, Gu YA (2003) Application of a novel polymer system in chemical enhanced oil recovery (EOR). Colloid Polym Sci 281:1046–1054
Chen QS, Wang Y, Lu ZY, Feng YJ (2013) Thermoviscosifying polymer used for enhanced oil recovery: rheological behaviors and core flooding test. Polym Bull 70:391–401
Manrique EJ, Thomas CP, Ravikiran R et al (2010) SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, SPE 130113
Bondor PL, Hite JR, Avasthi SM (2005) SPE Latin American and Caribbean Petroleum Engineering Conference, Rio de Janeiro, Brazil. SPE 94637
Levitt DB, Pope GA (2008) SPE improved oil recovery symposium. SPE 113845
Seright RS, SeheultM Talashek T (2009) Injectivity characteristics of EOR polymers. SPE Reserv Eval Eng 12(5):783–792
Liu PD, Zhang S, Yang N (2012) A novel surface active polymer oil displacement agent. Pet Explor Dev 39:580–584
Li PZ, Shen YD, Yang XW (2011) Solution properties and flocculation of hydrophobically associating cationic fluorinated polyacrylamide. Polym Bull 67:961–973
Yahaya GO, Ahdabb AA, Ali SA, Abu-Sharkh BF, Hamad EZ (2001) Solution behavior of hydrophobically associating water-soluble block copolymers of acrylamide and N-benzylacrylamide. Polymer 42:3363–3372
Li TT, Liu HR, Zeng L, Miao WF, Wu Y (2011) Study of emulsion polymerization stabilized by amphiphilic polymer nanoparticles. Colloid Polym Sci 289:1543–1551
Wang C, Li XR, Li PZ (2012) Study on preparation and solution properties of hydrophobically associating polyacrylamide by emulsifier-free ultrasonic assisted radical polymerization. J Polym Res 19:9933
Yang XW, Liu JP, Li PZ, Liu CD (2015) Self-assembly properties of hydrophobically associating perfluorinated polyacrylamide in dilute and semi-dilute solutions. J Polym Res 22:103
Yang ZL, Gao BY (2010) Synthesis and characterization of hydrophobically associating cationic polyacrylamide. Chem Eng J 161:27–33
Renil Manat, Meidal Morten (1996) POEPOP and POEPS: inert polyethylene glycol crosslinked polymeric supports for solid synthesis. Tetrahedron Lett 37:6185–6188
Lu HS, Feng YJ (2008) Study on associative polymerizable inverse microemulsion. J Macromol Sci A 45:372–380
Ma JT, Cui P, Zhao L, Huang RH (2002) Synthesis and solution behavior of hydrophobic association water-soluble polymers containing arylalkyl group. Eur Polym J 38:1627–1633
Shashkina YA, Zaroslov YD, Smirnov VA, Philippova OE, Khokhlov AR, Pryakhina TA, Churochkina NA (2003) Hydrophobic aggregation in aqueous solutions of hydrophobically modified polyacrylamide in the vicinity of overlap concentration. Polymer 44:2289–2293
Feng YJ, Billon L, Grassl B, Khoukh A, Francoise J (2002) Hydrophobically associating polyacrylamides and their partially hydrolyzed derivatives prepared by post-modification: synthesis and characterization. Polymer 43:2055–2064
Zhong CR, Huang RH, Xu JY (2008) Characterization, solution behavior, and microstructure of a hydrophobically associating nonionic copolymer. J Solution Chem 37:1227–1243
Zhong CR, Zhang H, Feng LM (2014) Solution behavior and associating structures of a salt-tolerant tetra-polymer containing an allyl-capped macromonomer. J Polym Res 21:604
Glenn K, Van Bommel A, Bhattacharya SC, Palepu RM (2005) Self aggregation of binary mixtures of sodium dodecyl sulfate and polyoxyethylene alkyl ethers in aqueous solution. Colloid Polym Sci 283:845–853
Dutta P, Dey J, Ghosh G, Nayak RR (2009) Self-association and microenvironment of random amphiphilic copolymers of sodium N-acryloyl-l-valinate and N-dodecylacrylamide in aqueous solution. Polymer 50:1516–1525
Chen J, Jiang M, Zhang YX, Zhou H (1999) (1999) Fluorescence studies on hydrophobic associations of fluorocarbon-modified poly(acrylic acid) solutions. Macromolecules 32:4861–4866
Li GH, Shen YD, Li PZ (2010) Study of solution properties of copolymer of fluorinated surface active monomer and acrylamide. Acta Polymerica Sinica 3:347–351
Chen H, Han LJ, Xu P (2003) The thickening mechanism study of hydrophobically modified polyacrylamide. Acta Phys- Chem Sin 19(11):1020–1024
Acknowledgements
We acknowledge the financial support received from the National Natural Science Foundation of China (Grant Number: 51304029), the National Science and Technology Major Project of China (Grant Number: 2011ZX05046) and the Science and Technology Innovation foundation of CNPC Nos. 2012D-5006-0104.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, F., Luo, Y., Hu, P. et al. Study on the self-assembly properties of surface active hydrophobically associating polyacrylamide with nonionic surfmer units. Polym. Bull. 74, 2873–2886 (2017). https://doi.org/10.1007/s00289-016-1871-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1871-4