Abstract
The linear low-density polyethylene (LLDPE) fibers were sulfonated in 95 % concentrated sulfuric acid. The structure composition and thermal stability of the polyethylene (PE) fibers sulfonated for various times were determined by Fourier transform infrared spectroscopy (FTIR), resonance Raman spectra, elemental analysis, X-ray photoelectron spectroscopy (XPS) and thermogravimetric analysis (TGA). It was confirmed that substitution, oxidation and desulfonation took place in succession during sulfonation. The initial increase of sulfonic acid and hydroxyl groups, as an indication of the substitution reaction, led to a proposal that the reaction was initiated by the abstraction of hydrogen by H2SO4 to give PE radical, which reacted with H2SO4 to give HSO3· and HO· radical, and terminated by the formation of PE–SO3H and PE–OH. The subsequent decrease of hydroxyl groups and generation of carbonyl groups were attributed to the oxidation reaction. The formation of C=C double bonds and the decrease of sulfur and sulfonic acid groups later on implied that the desulfonation reaction proceeded to induce intermolecular cross-linking. The cross-linked and conjugated polyenes derived from the desulfonation probably attributed to the high char yield for sulfonated LLDPE fibers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon fiber (CF) has been successfully used to make high-performance composites for aerospace (airplanes and missiles) and racing cars due to its high specific strength and light weight [1–5]. Unfortunately, more widespread applications of CF are limited because of its high price, especially in the area of civilian uses. To obtain widespread acceptance in high-volume markets, CF with reasonable mechanical properties and low cost is in urgent need.
With polyacrylonitrile (PAN) being the dominating precursor in the CF industries, several other materials such as polyolefin [6–8] and lignin [9–11] have been under consideration as a less expensive precursor. Polyethylene (PE) is one of the best candidates, due not only to the mature synthesizing and melt-spinning technologies at a large scale and low cost, but also to its high carbon content. PE fiber could be highly oriented during spinning and improved performance was achieved [12]. PE-based CF with acceptable mechanical property might be obtained by maintaining the initial high orientation of the molecular chains in the subsequent processes of stabilization and carbonization [13, 14].
The manufacturing of CF usually involves an initial thermal stabilization stage, which prepares the precursor to withstand the high temperatures up to 1000 °C for the following carbonization. As a thermoplastic material, PE fiber unavoidably softens or melts at around 130 °C and on subjecting to pyrolysis at around 490 °C, consequently, loses its fibrous shape. The thermal stability must be improved thereof to render the fiber infusible and carbonizable. The stabilization of the PE fiber was performed via sulfonation treatment with highly concentrated sulfuric acid, fuming sulfuric acid, or chlorosfor specific applicationsulfonic acid [15].
Recent researches on the sulfonation of PE were focused on surface modification to improve its printability, hydrophilicity, hardness, roughness, antithrombogenicity and adhesion of micro-organisms for specific applications [16, 17]. As a traditional commercial material for making tube, membrane and fiber, PE has not gained considerable importance as speciality material due to its inert surface. The surface property and structure of the sulfonated PE mainly depend on the attached functional groups. As a result of attachment of the −SO3 groups onto the surface of polymers, the sulfonated PE membrane showed much better ion-exchange capacity and water permeability in comparison with the unmodified one [18]. Increase of surface hardness was found owing to selective attachment of −SO3 groups in interlamellar regions and crystal surface to form linkages [19]. The attachment of the bulky groups also imposed a gauche conformation of the adjacent CH2 group, leading to a buildup of internal stresses to distort the lattice in crystallites adjacent to sulfonated regions. Thus, crystallinity was gradually lost during the reaction [20]. However, deep sulfonation in the bulk of PE membrane only led to the decrease of mechanical properties, without giving any more improvement of the surface hydrophilicity. Therefore, little did the bulky sulfonation be cared about [18]. As sufficient sulfonation is a prerequisite for preparing high-performance PE-based carbon fiber, bulky sulfonation is investigated by comparison with the surface sulfonation in this paper.
Up to now, few researches on the thermal degradation of sulfonated PE have been done. The reports about the thermal degradation of synthetic vinyl polymers, such as poly (vinyl fluoride) (PVF) and poly (vinyl chloride) (PVC), can be used for reference [21, 22]. Generally, there were two competitive actions in their thermal degradation process: (1) formation of polyene sequences by HX (X is for the substituted groups) elimination and (2) formation of halogenated or oxygenated compounds by main-chain cleavage. A portion of the polyene would undergo further molecular rearrangements to form char. About 25 % higher yield of char was obtained by adding metal oxides in PVC by inducing cross-linking to suppress main-chain cleavage [23]. The characteristic of the thermal degradation of sulfonated PE that is different from the above synthetic vinyl polymer is also discussed in this paper.
Although sulfonation plays a key role in the formation of the resultant CF for PE fiber, the relationship between the molecular structures formed in sulfonation and its ability for carbonization remain unclear. The aim of the present investigation is to understand the underlying reaction mechanisms and the effect of sulfonation on composition variations including elements and functional groups, to discern the role of the reactions on the thermal stability of PE fibers. This work serves to control the sulfonation process to obtain precursors with better qualities prior to the carbonization stage.
Materials and methods
Fiber preparation
LLDPE fibers were chosen for its good orientation and spinnability. The fibers were produced via bicomponent extrusion through the combination of a designed orifice and flow path, to provide thinner fibers than usual for reducing the sulfonation time. SECCO LL0220KJ LLDPE granules with a melt flow index of 2 g/10 min and NATUREWORKS 2003D polylactic acid (PLA) granules with a melt flow index of 6 g/10 min were used to produce the bicomponent fibers. The melt spinning of the bicomponent fibers were performed at an extrusion temperature of 250 °C for both LLDPE and PLA, using a spinneret of 48 holes with a diameter of 0.3 mm. Fibers of different crystallinity were obtained by hot drawing the as-spun fibers under different temperatures with a draw ratio of λ = 1.5. The PLA component was dissolved by immersing the bicomponent fibers in 2.5 M/L NaOH (aq) at 60 °C. Subsequently, LLDPE fibers with an average diameter of ca. 10 μm were prepared for further sulfonation treatment. The ultrahigh molecular weight polyethylene (UHMWPE) fibers were also used for comparison.
Sulfonation procedure
Sulfonation treatment was carried out by using highly concentrated sulfuric acid (95 % conc.) at 120 ± 0.5 °C in a glass flask using an oil bath. The fibers were wound onto a polyflon fixture to prevent shrinkage. The surface of the fibers changed initially to pale green and then from yellowish brown to dark brown, ultimately to dark black with the proceeding of sulfonation. After a predetermined time, the samples were taken out and rinsed by pure water until a constant pH was reached. The pH value was measured using a commercial microglass electrode after submerging the sulfonated sample in 20 mL of distilled water for 10 min. Then the rinsed samples were dried at 80 °C for 1–2 h until the weight was constant.
Bromination
The sulfonated fibers were kept in a solution of 0.2 M/L Br2 (aq) for 24 h at 35 °C in the dark for bromination treatment. Then the fibers were repeatedly washed and cleaned ultrasonically in pure water at 25 °C to ensure that they were free from adsorbed bromine.
Characterization methods
A Fourier transform infrared spectroscopy (FTIR) microscope (Varian610-IR, USA) equipped with an x–y motorized micropositioning stage (precision 1 μm) and an attenuated total reflection (ATR) objective (germanium, incidence angle of the IR beam 45) was used for mapping samples. ATR spectra were collected (40 × 40 μm2 surface area) using 32 scans and 4 cm−1 resolution as default conditions. The scanning range of the spectrometer was 480–4000 cm−1. Each sample spectrum was auto-corrected against a corresponding background scan using the same instrument settings. Finally, all the spectra were analyzed using the Resolutions Pro software.
The resonance Raman spectra were measured with a microscopic confocal laser spectrometer (Renishaw Invia, UK), using the 532 nm lines of an argon ion laser source in the range of 500–3500 cm−1. All spectra were collected in the extended mode under 50× objective with exposure time of 1 s and 0.1 % laser power for ten accumulations each. The laser was calibrated by the use of the silicon chip before running tests for samples.
X-ray photoelectron spectroscopy experiments (XPS) were carried out using AXIS UTLTRADLD (Shimadzu Corporation, Japan), operated at 12 kV under a current of 10 mA. Samples were placed in an ultrahigh vacuum chamber with electron collection. In all the cases, C (1 s) levels were recorded. The C (1 s) levels were very broad and possessed multiple peaks. The XPS peaks were assumed to have Gaussian line shape and were resolved into individual components after proper subtraction of baseline using the linear background subtraction method. Relative atomic concentrations were determined by subtracting a Shirley-type background. The spectra peak shift due to the surface charging was corrected by setting the binding energy of C (1 s) to 284.8 eV.
Thermogravimetric analysis (TGA) of the fibers was done using with a Mettler Toledo thermal analysis system. The heating rate of 10 °C was used with a temperature range from 50 to 900 °C in nitrogen environments.
Quantitative elemental analysis for carbon, hydrogen and sulfur was carried out in a Perkin-Elmer 2400 organic element analyzer.
The morphologies of the fibers were characterized by S-4300 SE/N scanning electron microscope (Hitachi, Japan).
Results and discussion
Assessment of the FTIR results
FTIR spectra were firstly used to identify the functional group changes for LLDPE fibers after the sulfonation treatment. Figure 1 shows the FTIR spectra of LLDPE fibers treated with highly concentrated sulfuric acid for different times. The absorbance bands assignable to the bending of methylene groups (700, 1460, 2840, 2920 cm−1) are visible from the spectrum of neat LLDPE (Curve A). Such bands gradually descend as the reaction proceeds and disappear for fibers sulfonated from 2 to 8 h (Curves C–E). Two new bands appear at 1040 cm−1 assignable to νs(O=S=O) and 1175 cm−1 assignable to νas(O=S=O) of the sulfonic acid group. Meanwhile, the strong band at 1630 cm−1 assigned to C=C double bonds and another strong band at 1710 cm−1 assigned to carbonyl group (C=O) gradually appear with longer time, concurrent with the appearance of a broad band within 3000–3800 cm−1 assigned to hydroxyl groups which may be from alcohol, carboxyl or moisture. On comparing the spectra, it can be observed that the absorption bands for νs(O=S=O) and ν (C=O) shift toward higher wave number. The S=O and C=O double bonds could be in conjugation with the neighboring C=C double bonds. The sulfonic acid group and carbonyl group should have an enhanced double bond character owing to the resonance effect, when they are conjugated to the polyenes structure. The more the conjugation of double bonds with the sulfonic acid or carbonyl groups is enhanced, the more is the absorptions shift toward higher wave number. A wavenumber shift of 30 cm−1 for νas(O=S=O) was observed on comparing the sulfonated PE with unconjugated polyvinylsulfonate [24]. The broadening of the bands is due to the distribution of absorptions by different sequence lengths of conjugated polyenes. The absorption peak position variations of νas(O=S=O) and ν(C=O) are listed in Table 1. An increase of the two absorption peak positions is observed until 4 h, when the slight falling back at 8 h is considered to be ascribed to the decrease of conjugated sequence length. A narrowing of the bands is also observed for long periods of time.
Assessment of the Raman results
Figure 2 shows the resonance Raman spectra of LLDPE for different sulfonation times. It is revealed from the spectra that a new band appears at around 1560 cm−1, ν, which is assigned to conjugated C=C double bonds. The average conjugated polyenes sequence length, n of (–CH=CH–) n , of the sulfonated PE fibers can be estimated according to the equation reported by Baruya et al. for polyacetylene (ν = 1461 + 151.24exp−0.07808n) [25]. The calculation results are shown in Table 2. After a slight increase at 2 h, a dramatic decrease of the conjugated sequence length is observed. The initial increase of the sequence length degree indicates the formation of conjugated polyenes, and the decrease is due to the chemical cross-linking in long sulfonation [26, 27]. A similar decrease of sequence length of polyenes for polyenesulfonic acids exposed to UV was also observed [28]. It is noteworthy that the decrease of conjugated sequence length here appears earlier than those measured by FTIR. The sulfonation is a diffusion-controlled process as only the penetrated region of fibers can interact chemically with the acid molecules, which means that the reaction on the surface progresses faster than the bulk, so the decrease of the conjugated sequence length is measured earlier by Raman which just has a nanoscale test depth. The sulfonated HDPE by SO3 had much longer sequence lengths from 8 to 26, which was consistent with the greater IR absorption shift of νas(O=S=O) mentioned above [26]. In addition, the bands at near 2850 and 2880 cm−1 assigned to the methylene groups gradually vanished with time. Only a little model of the absorptions bands was visible at 1 h (curve B).
Assessment of the XPS data
The recorded XPS spectra of C (1 s) levels for all the sufonated fibers were resolved into three components: C1, C2 and C3 shown in Fig. 3. The C1 peak near 284.8 eV is assigned to the presence of C–C, C–S and C=C bonds. Since the difference among the BE values of these bonds is very small, it is hard to differentiate them under the present instrumental conditions. The C2 peak near 286.0 eV is assigned to C–OH, and the C3 peak near 288.3 eV is assigned to C=O from ketone (287.8 eV) and carboxyl (288.6 eV). The content change of C2 and C3 component on the surface is plotted in Fig. 4. The C–OH groups initially increase and then gradually decrease, while the C=O groups increase steadily during sulfonation, indicating the formation of alcohol functionalities probably due to substitution reaction and the formation of carboxyl groups by oxidation of the hydroxyl groups.
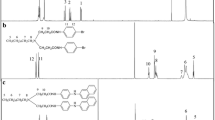
The presence of unsaturation in sulfonated PE fibers was confirmed by the XPS spectra of brominated and sulfonated PE as shown in Fig. 5 (curve C), which presents the characteristic bond of Br–C [Br (3d), 70.5 eV] [29]. The increase of the ratio of Br/C shown in Fig. 6d with sulfonation indicates the steady increase of unsaturation. The presence of a little oxygen on the surface of virgin LLDPE fiber is due to its susceptibility to slow environmental oxidation [30]. The presence of sulfur is visible in the spectra of sulfonated PE.
XPS was also used to investigate the changes of elements on the surface of fibers, when elemental analysis was used for the bulk. It is clear from Fig. 6a that the ratio of S/C similarly shows an initial steady increase and a subsequent slow decline for both the surface and bulk. But the S/C on the surface reaches its maximum much more quickly, because the reaction of the surface progresses faster than the bulk which has been mentioned above. The sulfonation on the surface is just an epitome of the bulk. When the S/C ratio reaches its maximum value, the surface O/S ratio [Fig. 6b] approaches nearly 3 (the O/S ratio of the −SO3H group) almost at the same time, indicating that the highest sulfonic group concentration is reached on the surface of LLDPE fibers sulfonated for about 1 h. The decline of sulfur or sulfonic group and the increase of unsaturation demonstrate that the formation of C=C derived from the loss of sulfonic acid groups, namely desulfonation, could have happened in the sulfonation process. Furthermore, the surface O/C ratio [Fig. 6c] also shows similar variation with the S/C ratio, except a slower reach to the maximum value, due to the substitution of alcoholic groups which supplement oxygen instead.
Assessment of the TGA results
Figure 7a shows the TGA scans of the samples under study. The weight retention increases with sulfonation time, and 43.3 % residual weight is obtained at 900 °C for the fibers sulfonated for 4 h (curve E). A slight decline of the weight retention is observed for the fibers sulfonated for 8 h (curve F), which become very fragile probably due to over-sulfonation. The SEM images of cross section and surface of the carbonized fibers with different sulfonation time are shown in Fig. 8. Porous hollow carbon fibers were obtained for the fibers sulfonated for 2 h as the insufficiently sulfonated core decomposed under high temperature, resulting in low residual weight. Then, dense carbon fibers were obtained for the fully sulfonated fibers for 4 h. Although a dense structure was also observed for the fibers sulfonated for 8 h, the surface became very coarse, because the surface construction was destroyed by the etching of strong acid after a very long treatment. A negative effect of under- and over-sulfonation on the mechanical performance was also found for UHMWPE-based carbon fibers [14]. By comparison of TGA curves (Fig. 9) of the fibers with different crystallinity, it can be found that the weight retention increases with the improvement of crystallinity, especially for UHMWPE fibers which have little branching, and the residual weight is as high as 67 %. High crystallinity and low branching, which always mean dense aggregation structure, would significantly improve the thermal stability and favor charring. Therefore, a decline of char yield appeared for the etched fibers with loose structure.
DTA curves of the partially and completely sulfonated PE fibers are shown in Fig. 7b. Initial derivative weight loss peak at about 180 °C corresponds to the loss of sulfonic acid groups. Figure 7c enlarges the DTA curves from 120 to 400 °C to show the desulfonation more clearly. The DTA curve at 0.5 h is dominated by a broad weight loss peak, which is separated into double peaks at 1 h, indicating that different bonding form of the sulfonic acid groups may be generated in sulfonation; then the peak at higher temperature evolves into a shoulder with prolonged time. The second derivative weight loss peak at about 480 °C corresponds to the degradation of PE which can be confirmed with TGA measurement of neat LLDPE fibers (curve A). The weight loss within 120–400 °C was used to calculate the content of sulfonic acid group, while the weight loss within 400–600 °C was used to calculate the content of unreacted PE molecule for partially sulfonated LLDPE fibers. Their weight fractions of the samples were, respectively, plotted in Fig. 10. An S-shaped curve was fitted for the content change of sulfonic acid group, and correspondingly a reversed S-shaped curve was fitted for the content change of unsulfonated PE molecule. A decline of sulfonic acid group is also observed in long sulfonation, which further confirms the desulfonation reaction. Figure 7d enlarges the DTA curves from 400 to 600 °C to show the degradation of PE more clearly. The pyrolysis peak temperature and the corresponding weight loss speed are listed in Table 3. The peak temperature rises slightly at the beginning of sulfonation and then drops significantly until it disappears. The rise of temperature is due to the cross-linking which causes the increase of molecular weight and makes the decomposition more difficult. On the contrary, the dropping of temperature with sulfonation indicates the decrease of the decomposition difficulty. This is because the cross-linked molecule has priority to transform into char with the increase of conjugation degree, separating the original long chains into short ones.
Reaction mechanisms
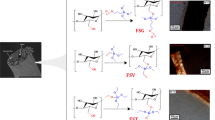
There were mainly two different viewpoints on the sulfonation mechanisms of PE. As proposed by Ihata, the reaction of PE with SO3 was initiated by the abstraction of hydrogen atoms by SO3 to give the PE radical, which reacted with SO3 to give the −SO3H groups, then an elimination of a sulfurous acid took place to give conjugated double bonds, resulting in polyenes with the −SO3H groups [Eq. (1)]. [24]. However, Kaneko et al. argued that the reaction of PE with fuming sulfuric acid was initiated by the abstraction of the hydride ion by SO3 to give carbocation, and the loss of a proton from the adjacent methylene carbon occurred to give double bonds. Then, the double bonds reacted with SO3 to form β-sultone and rearranged to yield polyenes with −SO3H groups also [Eq. (2)] [31]. It seems that the reaction process depends on the sulfonation agent being used (Scheme 1).
It can be found that the above proposed reactions were both initiated by the abstraction of hydrogen by SO3. However, there is little SO3 in 95 % concentrated sulfuric acid normally. As shown in Scheme 2, it could be assumed that the abstraction of hydrogen is initiated by sulfuric acid at elevated temperatures to give the PE radical [Eq. (3)], which reacts with sulfuric acid to form the HO· and HSO3· radicals [Eqs. (4), (5)]. The radical transfer could be terminated by the reaction of PE· radical with HO· or HSO3· radical as a result of yielding PE–SO3H and PE–OH [Eqs. (6), (7)]. A significant generation of carbonyl groups was observed in the long reaction time by IR and XPS measurement, which was probably derived from the oxidation of PE–OH. As a result, LLDPE was continually converted into sulfonated or hydrolyzed PE as the reaction proceeded.
Considering the shift of IR absorption and the variation of the content of sulfur and sulfonic acid, it is concluded that the desulfonation reaction results in cross-linked and conjugated polyene structure as indicated in Scheme 3. The desulfonation preferentially happened at the neighboring carbon atoms within the chain to form conjugated polyenes with sulfonic groups linking to double bonds [Eq. (8)]. Then the desulfonation continued to progress between the molecular chains, resulting in an intermolecular chemical cross-linking to give a cross-linked alkene sulfonic acid [Eq. (9)]. Another evidence was that the final product was insoluble in decalin (a strong solvent for PE), which indicated an intrinsic structure change of PE by sulfonation. The evolution of sulfur dioxide during the sulfonation of low-density polyethylene, isotactic polypropylene and atactic polypropylene by concentrated sulfuric acid at 100–125 °C was confirmed by Cameron et al. [32]. It was reported that conjugated polyacetylene would lose considerable weight at around 490 °C, and only 30 % residual char was obtained at 600 °C in the TG experiment and no residue was observed for both the chemically cross-linked HDPE with peroxide [21, 33]. In other words, neither the conjugation nor the cross-linking can separately satisfy the carbonization demand for PE fibers. However, cross-linking with maleimides effected a significant stabilization of polyvinyl alcohol [34]. It is generally accepted that the ability of certain polymers to cyclize and form ladder polymers increases their thermal stability. So it can be deduced that the conjugation structure provided the ability to cyclize, while the cross-linking greatly improved this ability. The cross-linked and conjugated polyenes structure formed by desulfonation is exactly what make the sulfonated PE fibers have much higher char yield (>40 %) than the above-mentioned synthetic vinyl polymers (<28 %) [21].
Conclusion
The characterization of sulfonation of LLDPE fibers with concentrated sulfuric acid by FTIR and resonance Raman spectroscopy indicated the possibility of the formation of sulfonic acid groups and C=C double bonds, which demonstrated that both substitution and elimination of sulfonic acid groups took place to result in polyenes structure and chemical cross-linking during sulfonation. It is assumed that the reaction could be initiated by the abstraction of hydrogen by H2SO4 to give the PE radical, which reacted with H2SO4 to give the HSO3· and HO· radical and terminated by the formation of PE–SO3H and PE–OH. Then, oxidation was followed on transforming hydroxyl groups into carboxyl groups, while desulfonation was followed to form cross-linked and conjugated polyenes which contributed to the PE fibers’ good carbonizability. More works need to be done on making clear the effect of oxidation on its carbonizability, which may have relations with over-sulfonation, to guide the optimization of sulfonation to provide superior quality precursors for making CFs.
References
Soutis C (2009) Carbon fiber reinforced plastics in aircraft construction. Mat Sci Eng A 412:171–176
Windhorst T, Blount G (1997) Carbon-carbon composites: a summary of recent developments and applications. Mater Des 18:11–15
Immarigeon J, Holt R, Koul A, Zhao L, Wallace W, Beddoes J (1995) Lightweight materials for aircraft applications. Mater Charact 35:41–67
Malquarti G, Berruet RG, Bois D (1990) Prosthetic use of carbon fiber-reinforced epoxy resin for esthetic crowns and fixed partial dentures. J Prosthet Dent 63:251–257
Feraboli P, Masini A (2004) Development of carbon/epoxy structural components for a high performance vehicle. Compos Part B Eng 35:323–330
y Leon CL, Schimpf W, Hansen B, Herren C, Frame A, Heatherly P (2002) Low cost carbon fiber from non-acrylic based precursors: polyethylene. Int SAMPE Tech Conf 34:506–519
y Leon CL (2001) Polyethylene and polypropylene as low cost carbon fiber (LCCF) precursors. Int SAMPE Tech Conf 33:1289–1296
Liu Y, Kumar S (2012) Recent progress in fabrication, structure, and properties of carbon fibers. Polym Rev 52:234–258
Baker DA, Rials TG (2013) Recent advances in low-cost carbon fiber manufacture from lignin. J Appl Polym Sci 130:713–728
Baker DA, Gallego NC, Baker FS (2012) On the characterization and spinning of an organic-purified lignin toward the manufacture of low-cost carbon fiber. J Appl Polym Sci 124:227–234
Compere A, Griffith W, Leitten C, Shaffer J (2001) Low cost carbon fiber from renewable resources. Int SAMPE Tech Conf 33:1306–1314
Kitao T, Ohya S, Furukawa J, Yamashita S (1973) Orientation of polymer molecules during melt spinning. II. Orientation of crystals in as-spun polyolefin fibers. J Polym Sci Pol Phys 11:1091–1109
Penning JP, Lagcher R, Pennings AJ (1991) The effect of diameter on the mechanical properties of amorphous carbon fibres from linear low density polyethylene. Polym Bull 25:405–412
Zhang D (1993) Carbon fibers from oriented polyethylene precursors. J Thermoplast Compos Mater 6:38–48
Horikiri S, Iseki J, Minobe M (1978) Process for production of carbon fiber. US Patent No. 4,070,446
Fonseca C, Perena JM, Fatou JG, Bello (1985) Sulphuric acid etching of polyethylene surfaces. J Mater Sci 20:3283–3288
Fischer D, Eysel HH (1994) Analysis of polyethylene surface sulfonation. J Appl Polym Sci 52:545–548
Dimov A, Islam MA (1991) Sulfonation of polyethylene membranes. J Appl Polym Sci 42:1285–1287
Baltá Calleja FJ, Fonseca C, Pereña JM, Fatou JG (1984) Surface hardening of polyethylene through sulphuric acid exposure. J Mater Sci Lett 3:509–511
Bikson B, Jagu-Grodzinski J, Vofsi D (1981) Distortion of crystallites caused by heterogeneous chlorosulfonation of polyethylene. J Polym Sci Pol Phys 19:23–32
Montaudo G, Puglisi C, Scamporrino E, Vitalini D (1986) Correlation of thermal degradation mechanisms: polyacetylene and vinyl and vinylidene polymers. J Polym Sci Pol Chem 24:301–316
Collins S, Yoda K, Anazawa N, Birkinshaw C (1999) The thermal stability of some vinylidene chloride copolymers. Polym Degrad Stabil 66:87–94
Ballistreri A, Montaudo G, Puglisi C, Scamporrino E (1981) Mechanism of smoke suppression by metal oxides in PVC. J Polym Sci Pol Chem 19:1397–1408
Ihata J (1998) Formation and reaction of polyenesulfonic acid. I. Reaction of polyethylene films with SO3. J Polym Sci Pol Chem 26:167–176
Baruya A, Gerrard D, Maddams W (1983) Resonance Raman spectrum of degraded poly (vinyl chloride). 4. Determination of conjugated polyene sequence lengths. Macromolecules 16:578–580
Gerrard D, Maddams W (1977) The resonance Raman spectrum of degraded poly (vinyl chloride). 2. γ-irradiated samples. Macromolecules 10:1221–1224
Martinez G, Mijangos C, Gerrard DL, Maddams WF (1979) Polyene sequence distribution in degraded poly (vinyl chloride) as a function of the tacticity. Makromol Chem 180:2937–2945
Ihata J (1988) Formation and reaction of polyenesulfonic acid. II. Photoreaction of polyenesulfonic acids. J Polym Sci Pol Chem 26:177–185
Wagner C (1978) X-ray photoelectron spectroscopy with x-ray photons of higher energy. J Vac Sci Technol 15:518–523
Bonhomme S, Cuer A, Delort A, Lemaire J, Sancelme M, Scott G (2003) Environmental biodegradation of polyethylene. Polym Degrad Stab 81:441–452
Kaneko M, Kumagai S, Nakamura T, Sato H (2004) Study of sulfonation mechanism of low-density polyethylene films with fuming sulfuric acid. J Appl Polym Sci 91:2435–2442
Cameron GG, Main BR (1983) The action of concentrated sulphuric acid on polyethylene and polypropylene: Part 1—Evolution of sulphur dioxide and carbon dioxide. Polym Degrad Stab 5:215–225
Khonakdar HA, Morshedian J, Wagenknecht U, Jafari SH (2003) An investigation of chemical crosslinking effect on properties of high-density polyethylene. Polymer 44:4301–4309
Gilman JW, VanderHart DL, Kashiwagi T (1995) Thermal decomposition chemistry of poly (vinyl alcohol). Acs Sym Ser 599:161–185
Acknowledgments
The assistance and cooperation of Mr. Qingpeng Zhang in the Fiber Preparation Technology Team of CAS is gratefully acknowledged for the extrusion of the multifilaments.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xie, B., Hong, L., Chen, P. et al. Effect of sulfonation with concentrated sulfuric acid on the composition and carbonizability of LLDPE fibers. Polym. Bull. 73, 891–908 (2016). https://doi.org/10.1007/s00289-015-1525-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1525-y